High-Salt Diets, Intestinal Barrier, and Hypertension: A Mechanistic Review and the Promise of Dietary Therapy
Abstract
1. Introduction
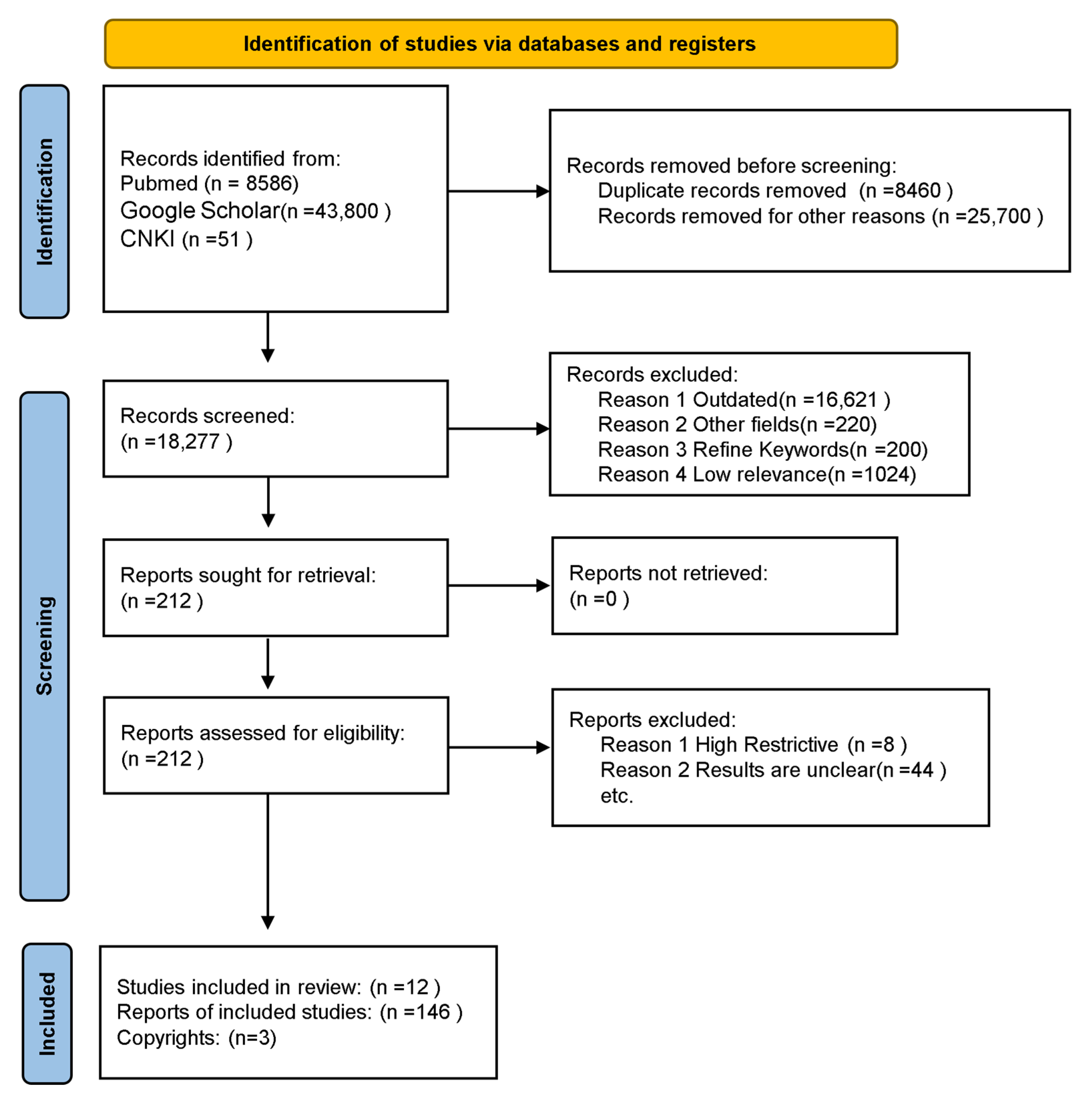
2. Materials and Methods
3. High-Salt Diets and Hypertension
4. Intestinal Barrier and Gut Microbiota
5. Intestinal Barrier Mechanisms of High-Salt-Diet-Induced Hypertension
5.1. Disruption of the Intestinal Barrier-Induced Hypertension
5.1.1. Disruption of the Intestinal Mechanical Barrier
5.1.2. Disruption of the Intestinal Chemical Barrier
5.1.3. Disruption of the Intestinal Biological Barrier Induced Hypertension
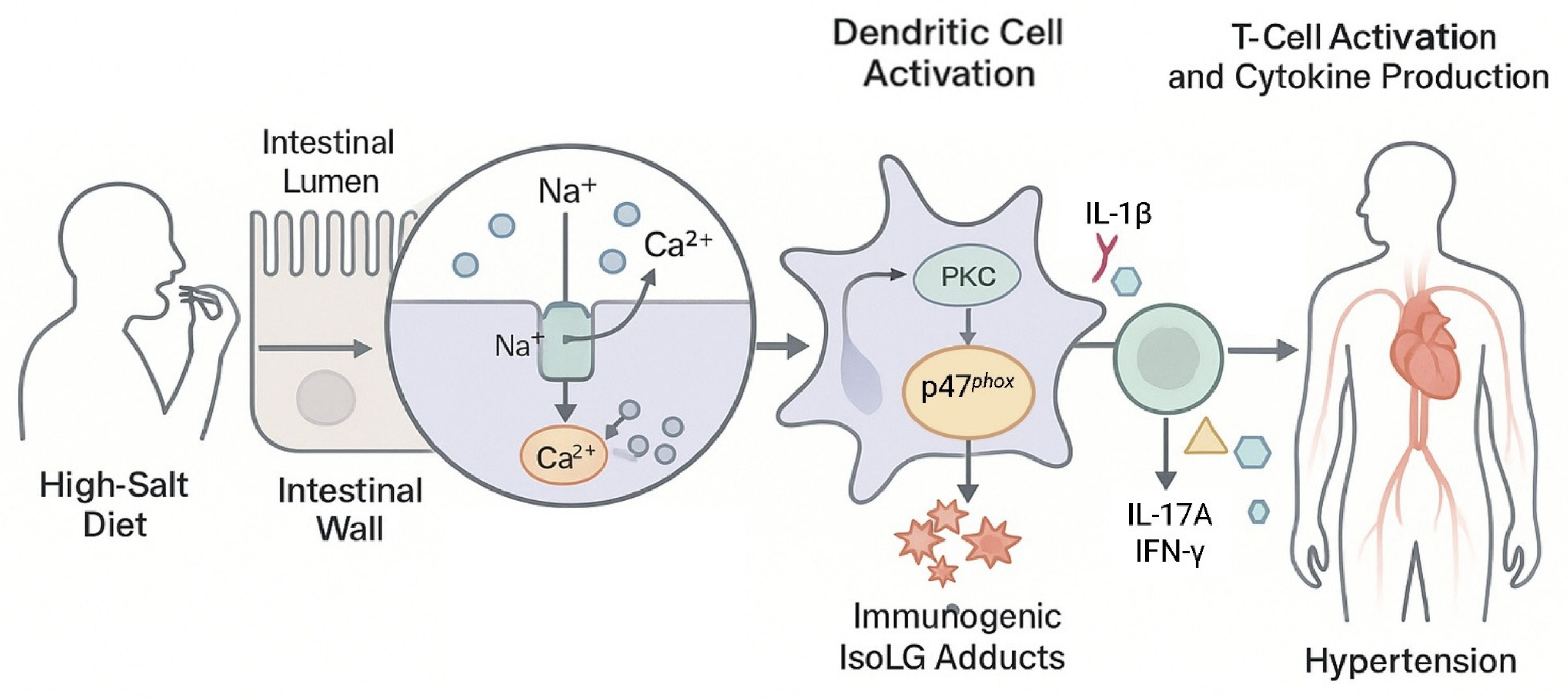
5.2. Accumulation of Intestinal IsoLG-Protein Adducts Induces Hypertension
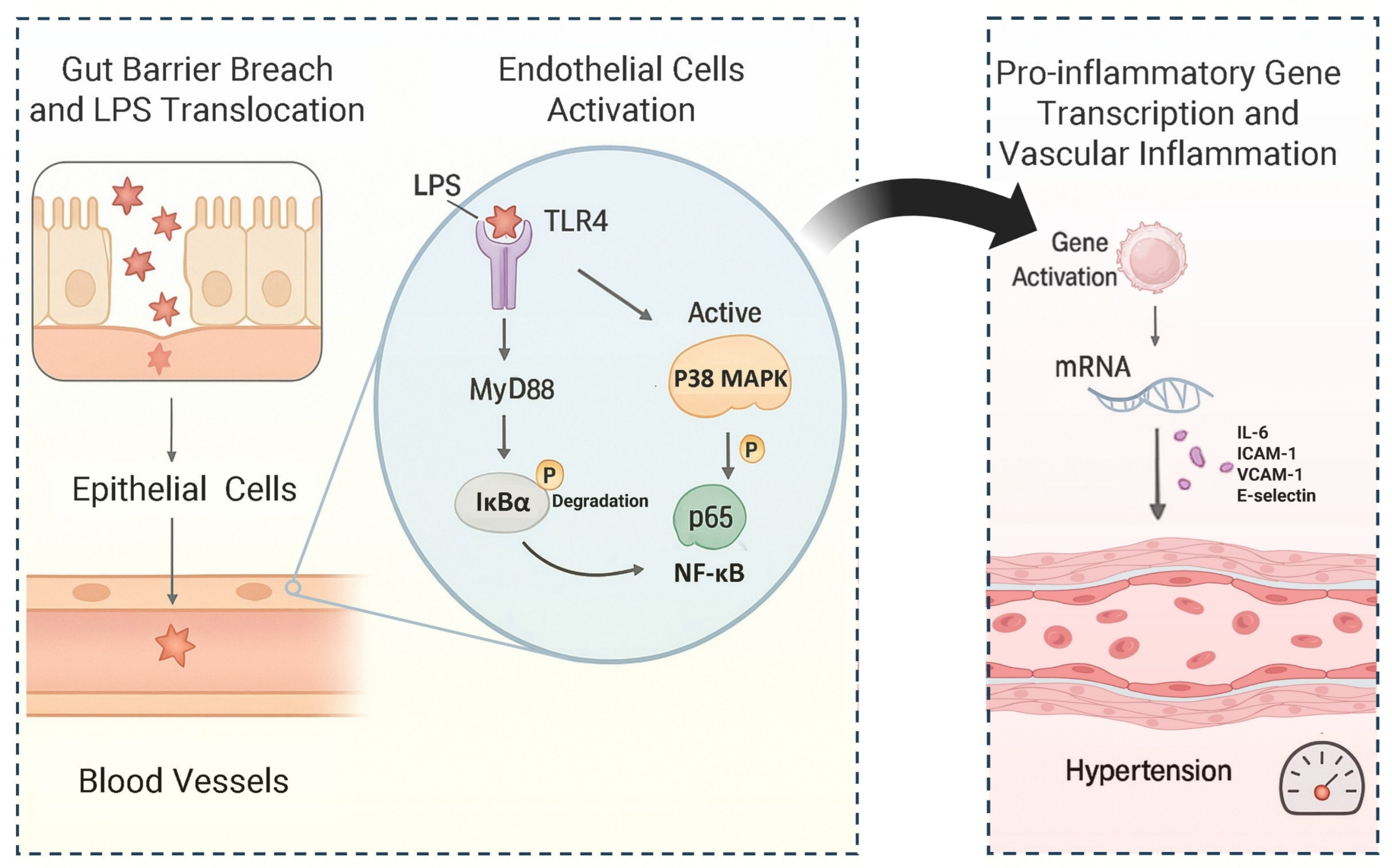
5.3. Promoting LPS Activation of the MAPK/NF-κB Pathway Induces Hypertension
6. Dietary Therapy for Hypertension
6.1. Supplement with Natural Blood Pressure-Lowering Food Resources
6.1.1. Supplement with Plant-Based Foods
6.1.2. Supplement with PFAs
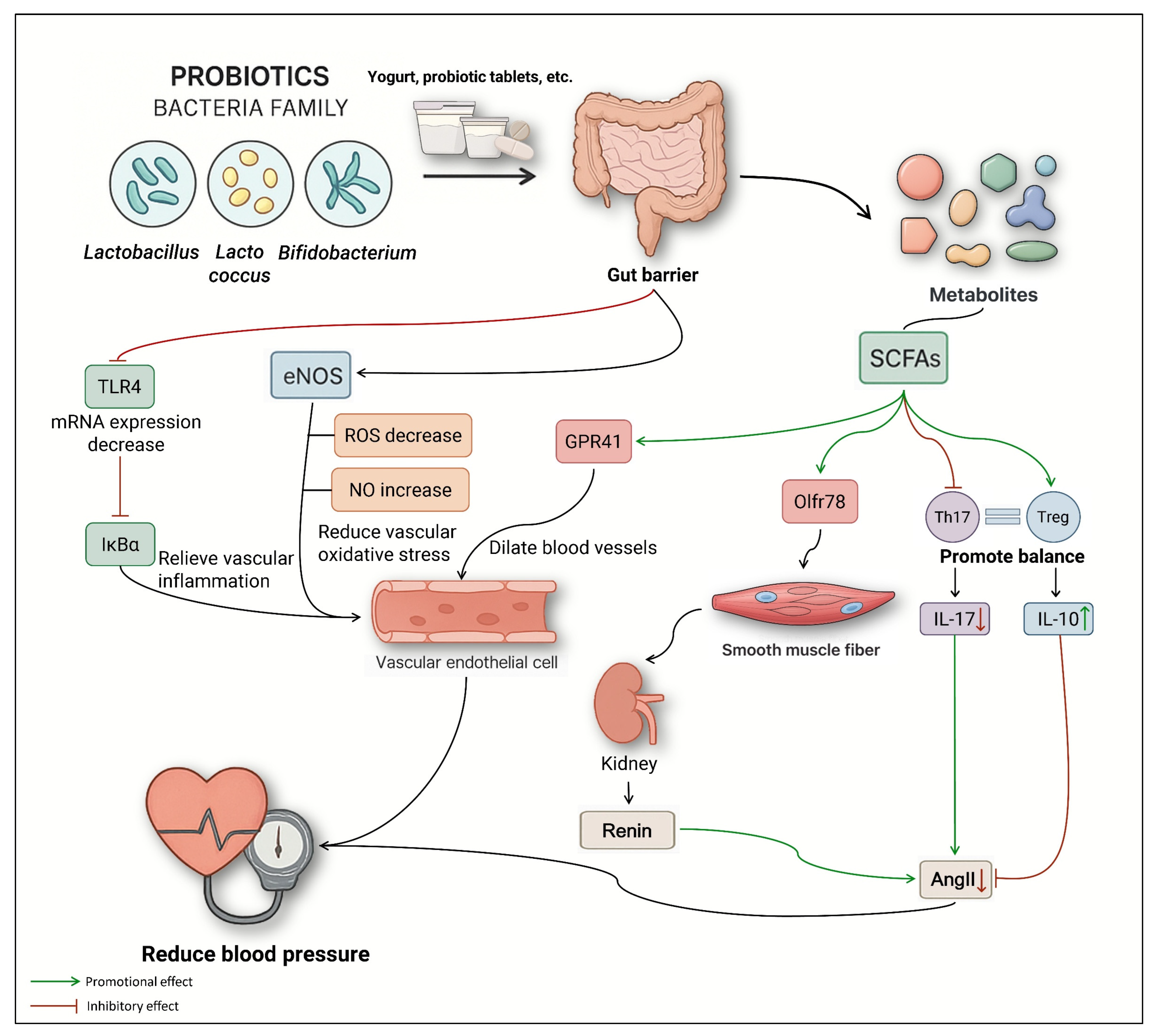
6.1.3. Supplement with Probiotics and Prebiotics
6.1.4. Supplement with FMHS
6.1.5. Supplementation with Minerals and Vitamins
6.2. Changing Dietary Patterns
6.2.1. DASH
6.2.2. MD
6.2.3. KD
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, S.; Chong, F.; Xu, H. Advances in understanding the relationship between the dietary inflammatory index and metabolic diseases. Electron. J. Metab. Nutr. Cancer 2021, 8, 232–239. [Google Scholar]
- Phillips, C.M.; Chen, L.; Heude, B.; Bernard, J.Y.; Harvey, N.C.; Duijts, L.; Mensink-Bout, S.M.; Polanska, K.; Mancano, G.; Suderman, M. Dietary inflammatory index and non-communicable disease risk: A narrative review. Nutrients 2019, 11, 1873. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Sodium Intake Reduction; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Society, C.N. Chinese Dietary Guidelines; People’s Medical Publishing House: Beijing, China, 2022. [Google Scholar]
- Tan, M.; He, F.J.; Wang, C.; MacGregor, G.A. Twenty-four-hour urinary sodium and potassium excretion in China: A systematic review and meta-analysis. J. Am. Heart Assoc. 2019, 8, e012923. [Google Scholar] [CrossRef]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shi, Y.; Zhou, B.; Huang, Z.; Zhao, Z.; Li, C.; Zhang, X.; Han, G.; Peng, K.; Li, X. Prevalence, awareness, treatment, and control of hypertension in China, 2004–2018: Findings from six rounds of a national survey. Bmj 2023, 380, e071952. [Google Scholar] [CrossRef] [PubMed]
- Klag, M.J.; Whelton, P.K.; Randall, B.L.; Neaton, J.D.; Brancati, F.L.; Ford, C.E.; Shulman, N.B.; Stamler, J. Blood pressure and end-stage renal disease in men. N. Engl. J. Med. 1996, 334, 13–18. [Google Scholar] [CrossRef]
- Stamler, J. Blood pressure and high blood pressure: Aspects of risk. Hypertension 1991, 18, I95–I107. [Google Scholar] [CrossRef]
- Novello, M.F.; Rosa, M.L.G.; Ferreira, R.T.; Nunes, I.G.; Jorge, A.J.L.; Correia, D.M.d.S.; Martins, W.d.A.; Mesquita, E.T. Compliance with the prescription of antihypertensive medications and blood pressure control in primary care. Arq. Bras. De Cardiol. 2017, 108, 135–142. [Google Scholar] [CrossRef]
- Ambard, L. Causes de l’Hypertension anterielle. Arch. Gén. De Méd. 1904, 1, 520. [Google Scholar]
- Mente, A.; O’Donnell, M.J.; Rangarajan, S.; McQueen, M.J.; Poirier, P.; Wielgosz, A.; Morrison, H.; Li, W.; Wang, X.; Di, C. Association of urinary sodium and potassium excretion with blood pressure. N. Engl. J. Med. 2014, 371, 601–611. [Google Scholar] [CrossRef]
- Tesfaye, F.; Byass, P.; Wall, S.; Berhane, Y.; Bonita, R. Association of smoking and khat (Catha edulis Forsk) use with high blood pressure among adults in Addis Ababa, Ethiopia, 2006. Prev. Chronic Dis. 2008, 5, A89. [Google Scholar] [PubMed]
- Aburto, N.J.; Ziolkovska, A.; Hooper, L.; Elliott, P.; Cappuccio, F.P.; Meerpohl, J.J. Effect of lower sodium intake on health: Systematic review and meta-analyses. Bmj 2013, 346, f1326. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, F.; Saadat, Y.R.; Barzegari, A.; Ardalan, M.; Vahed, S.Z. The impact of gut microbiota on kidney function and pathogenesis. Biomed. Pharmacother. 2017, 93, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Qian, Z.; Yin, J.; Xu, W.; Zhou, X. The role of intestinal microbiota in cardiovascular disease. J. Cell. Mol. Med. 2019, 23, 2343–2350. [Google Scholar] [CrossRef]
- Ahmadmehrabi, S.; Tang, W.H.W. Gut microbiome and its role in cardiovascular diseases. Curr. Opin. Cardiol. 2017, 32, 761–766. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Liang, L.; Saunders, C.; Sanossian, N. Food, gut barrier dysfunction, and related diseases: A new target for future individualized disease prevention and management. Food Sci. Nutr. 2023, 11, 1671–1704. [Google Scholar] [CrossRef]
- Kanbay, M.; Onal, E.M.; Afsar, B.; Dagel, T.; Yerlikaya, A.; Covic, A.; Vaziri, N.D. The crosstalk of gut microbiota and chronic kidney disease: Role of inflammation, proteinuria, hypertension, and diabetes mellitus. Int. Urol. Nephrol. 2018, 50, 1453–1466. [Google Scholar] [CrossRef]
- Mashaqi, S.; Gozal, D. Obstructive sleep apnea and systemic hypertension: Gut dysbiosis as the mediator? J. Clin. Sleep Med. 2019, 15, 1517–1527. [Google Scholar] [CrossRef]
- Ma, J.; Ji, J.; Lu, M. Research progress on diet therapy for hypertension. Chin. Foreign Med. Res. 2019, 17, 186–188. [Google Scholar] [CrossRef]
- Toft, U.; Riis, N.L.; Lassen, A.D.; Trolle, E.; Andreasen, A.H.; Frederiksen, A.K.S.; Joergensen, N.R.; Munk, J.K.; Bjoernsbo, K.S. The effects of two intervention strategies to reduce the intake of salt and the sodium-to-potassium ratio on cardiovascular risk factors. a 4-month randomised controlled study among healthy families. Nutrients 2020, 12, 1467. [Google Scholar] [CrossRef] [PubMed]
- Bigiani, A. Salt taste, nutrition, and health. Nutrients 2020, 12, 1537. [Google Scholar] [CrossRef]
- Li, K.; Zhou, N.; Tian, L.; Zhang, Z. Historical evolution of Chinese medicinal salt and its processing method. J. Chin. Med. Mater. 2018, 41, 1757–1762. [Google Scholar] [CrossRef]
- Moss, M. Salt, Sugar, Fat: How the Food Giants Hooked Us; Random House: New York, NY, USA, 2013. [Google Scholar]
- He, F.; Brinsden, H.C.; MacGregor, G.A. Salt reduction in the United Kingdom: A successful experiment in public health. J. Hum. Hypertens. 2014, 28, 345–352. [Google Scholar] [CrossRef]
- Xu, W.; Xu, J.; Dai, D.; Zhu, J.; He, Q.; Xing, X.; Chen, Y.; Liu, Z. Estimation of dietary salt intake in adult residents in Anhui province, 2019. Chin. J. Epidemiol. 2021, 42, 823–826. [Google Scholar] [CrossRef]
- Tian, M.; Luo, X.; Zhang, C.; Niu, B.; Miao, R.; Zhou, Y.; Li, H.; Liu, C. Dietary sodium intake levels and food sources of adult residents in Hebei. Mod. Prev. Med. 2022, 49, 1590–1594. [Google Scholar]
- Hu, X. Tudy on Salt Intake and Related Factors Among Adult Residents in Six Counties of China. Master’s Thesis, Chinese Center for Disease Control and Prevention, Beijing, China, 2019. [Google Scholar]
- Wang, H.; He, Y.; Li, W.; Yang, F.; Sun, N. Relationship between salt intake and blood pressure level in hypertensive patients in Beijing. Chin. J. Hypertens. 2019, 27, 1036–1040. [Google Scholar] [CrossRef]
- Zeng, H.; Lyu, C.; Lin, H.; Wang, L.; Tan, Y.; Wang, J.; Liu, W.; Yao, Y.; Luo, J.; Huang, Y.; et al. Association between salt intake and hypertension in rural areas of Southwest China: A cohort study. In Proceedings of the 14th National Conference on Nutritional Science, the 11th Asia Pacific Clinical Nutrition Conference, and the 2nd Global Chinese Nutrition Scientists Conference, Nanjing, China, 20–22 September 2019; p. 1. [Google Scholar]
- Zheng, P.; Shu, L.; Zhang, X.; Si, C.; Yu, X.; Gao, W.; Tong, X.; Zhang, L. Association between dietary patterns and the risk of hypertension among Chinese: A cross-sectional study. Nutrients 2016, 8, 239. [Google Scholar] [CrossRef]
- Nan, X.; Lu, H.; Wu, J.; Xue, M.; Qian, Y.; Wang, W.; Wang, X. The interactive association between sodium intake, alcohol consumption and hypertension among elderly in northern China: A cross-sectional study. BMC Geriatr. 2021, 21, 135. [Google Scholar] [CrossRef]
- He, F.J.; Tan, M.; Ma, Y.; MacGregor, G.A. Salt reduction to prevent hypertension and cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 75, 632–647. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Yin, L.; Li, L.; Silva-Nash, J.; Tan, J.; Pan, Z.; Zeng, J.; Yan, L.L. Hypertension in China: Burdens, guidelines and policy responses: A state-of-the-art review. J. Hum. Hypertens. 2022, 36, 126–134. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Z.; Fan, J.; Hu, S. Epidemiology and Management of Hypertension in China: An Analysis Using Data from the Annual Report on Cardiovascular Health and Diseases in China (2021). Chin. Gen. Pract. 2022, 25, 3715–3720. [Google Scholar] [CrossRef]
- Fan, W.; Xie, F.; Wan, Y.; Campbell, N.R.C.; Su, H. The impact of changes in population blood pressure on hypertension prevalence and control in China. J. Clin. Hypertens. 2020, 22, 150–156. [Google Scholar] [CrossRef]
- Sanchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, Z.; Yu, K.; Ding, R.; Ye, K.; Dai, C.; Xu, X.; Zhou, G.; Li, C. High-salt diet has a certain impact on protein digestion and gut microbiota: A sequencing and proteome combined study. Front. Microbiol. 2017, 8, 1838. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Guyenet, P.G.; Stornetta, R.L.; Souza, G.M.; Abbott, S.B.; Brooks, V.L. Neuronal networks in hypertension: Recent advances. Hypertension 2020, 76, 300–311. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Zhou, X.; Cai, J. The role and mechanism of intestinal flora in blood pressure regulation and hypertension development. Antioxid. Redox Signal. 2021, 34, 811–830. [Google Scholar] [CrossRef]
- Zheng, T.; Wu, Y.; Guo, K.; Tan, Z.; Yang, T. The process of hypertension induced by high-salt diet: Association with interactions between intestinal mucosal microbiota, and chronic low-grade inflammation, end-organ damage. Front. Microbiol. 2023, 14, 1123843. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Jin, J.; Su, X.; Yin, X.; Gao, J.; Wang, X.; Zhang, S.; Bu, P.; Wang, M.; Zhang, Y. Intestinal flora modulates blood pressure by regulating the synthesis of intestinal-derived corticosterone in high salt-induced hypertension. Circ. Res. 2020, 126, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.G.; Fusieger, A.; Milião, G.L.; Martins, E.; Drider, D.; Nero, L.A.; de Carvalho, A.F. Weissella: An emerging bacterium with promising health benefits. Probiotics Antimicrob. Proteins 2021, 13, 915–925. [Google Scholar] [CrossRef]
- Huang, Q. High-Salt-Diet-Induced Gut Microbiota Contributes to the Development of Hypertension. Master’s Thesis, Southern Medical University, Guangzhou, China, 2020. [Google Scholar]
- Abt, M.C.; McKenney, P.T.; Pamer, E.G. Clostridium difficile colitis: Pathogenesis and host defence. Nat. Rev. Microbiol. 2016, 14, 609–620. [Google Scholar] [CrossRef]
- Jia, B. Commentary: Gut microbiome–mediated bile acid metabolism regulates liver cancer via NKT cells. Front. Immunol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Insenser, M.; Murri, M.; Del Campo, R.; Martinez-Garcia, M.A.; Fernandez-Duran, E.; Escobar-Morreale, H.F. Gut microbiota and the polycystic ovary syndrome: Influence of sex, sex hormones, and obesity. J. Clin. Endocrinol. Metab. 2018, 103, 2552–2562. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Huo, D.; Xu, C.; Hu, Q.; Zhang, J. Intestinal microbiota in Li cohort and its correlation with their diets. Microbiology 2017, 44, 2624–2633. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Suda, W.; Luo, C.; Kawaguchi, T.; Motoo, I.; Narushima, S.; Kiguchi, Y.; Yasuma, K.; Watanabe, E.; Tanoue, T. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 2017, 358, 359–365. [Google Scholar] [CrossRef]
- Huo, R.; Wang, Y.; Hou, S.; Wang, W.; Zhang, C.; Wan, X. Gut mucosal microbiota profiles linked to colorectal cancer recurrence. World J. Gastroenterol. 2022, 28, 1946. [Google Scholar] [CrossRef]
- Kaplan, M.M. Novosphingobium aromaticivorans: A potential initiator of primary biliary cirrhosis. Am. J. Gastroenterol. 2004, 99, 2147–2149. [Google Scholar] [CrossRef] [PubMed]
- Bernardet, J.F.; Hugo, C.J.; Bruun, B. Chryseobacterium. Bergey’s Man. Syst. Archaea Bact. 2015, 1–35. [Google Scholar] [CrossRef]
- Mukerji, R.; Kakarala, R.; Smith, S.J.; Kusz, H.G. Chryseobacterium indologenes: An emerging infection in the USA. Case Rep. 2016, 2016, bcr2016214486. [Google Scholar] [CrossRef] [PubMed]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef]
- Murakami, M.; Iwamoto, J.; Honda, A.; Tsuji, T.; Tamamushi, M.; Ueda, H.; Monma, T.; Konishi, N.; Yara, S.; Hirayama, T. Detection of gut dysbiosis due to reduced Clostridium subcluster XIVa using the fecal or serum bile acid profile. Inflamm. Bowel Dis. 2018, 24, 1035–1044. [Google Scholar] [CrossRef]
- Wang, Y.; Ouyang, M.; Gao, X.; Wang, S.; Fu, C.; Zeng, J.; He, X. Phocea, Pseudoflavonifractor and Lactobacillus intestinalis: Three potential biomarkers of gut microbiota that affect progression and complications of obesity-induced type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 2020, 13, 835–850. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.; Rodriguez-Palacios, A. The genus Alistipes: Gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Das, C.; Mande, S.S. In silico analysis of putrefaction pathways in bacteria and its implication in colorectal cancer. Front. Microbiol. 2017, 8, 2166. [Google Scholar] [CrossRef] [PubMed]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef] [PubMed]
- Miranda, P.M.; De Palma, G.; Serkis, V.; Lu, J.; Louis-Auguste, M.P.; McCarville, J.L.; Verdu, E.F.; Collins, S.M.; Bercik, P. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 2018, 6, 57. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira—A candidate for the next-generation probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A beneficial gut organism from the discoveries in genus and species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef]
- Bier, A.; Braun, T.; Khasbab, R.; Di Segni, A.; Grossman, E.; Haberman, Y.; Leibowitz, A. A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt-sensitive hypertension rat model. Nutrients 2018, 10, 1154. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.S.; Hansson, G.C. Intestinal mucus and their glycans: A habitat for thriving microbiota. Cell Host Microbe 2023, 31, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Solís-Urra, P.; Rodríguez-Rodríguez, F.; Olivares-Arancibia, J.; Navarro-Oliveros, M.; Abadía-Molina, F.; Álvarez-Mercado, A.I. The gut barrier, intestinal microbiota, and liver disease: Molecular mechanisms and strategies to manage. Int. J. Mol. Sci. 2020, 21, 8351. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Seo, J.; Yeun, J.; Choi, H.; Kim, Y.; Chang, S.Y. The role of mucosal barriers in human gut health. Arch. Pharmacal Res. 2021, 44, 325–341. [Google Scholar] [CrossRef]
- Wada, M.; Tamura, A.; Takahashi, N.; Tsukita, S. Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterology 2013, 144, 369–380. [Google Scholar] [CrossRef]
- Holmes, J.L.; Van Itallie, C.M.; Rasmussen, J.E.; Anderson, J.M. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr. Patterns 2006, 6, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; Vanuytsel, T.; Marques, F.Z. Breaking the barrier: The role of gut epithelial permeability in the pathogenesis of hypertension. Curr. Hypertens. Rep. 2024, 26, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Goel, R.; Kumar, A.; Qi, Y.; Lobaton, G.; Hosaka, K.; Mohammed, M.; Handberg, E.M.; Richards, E.M.; Pepine, C.J. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin. Sci. 2018, 132, 701–718. [Google Scholar] [CrossRef]
- De Angelis, M.; Garruti, G.; Minervini, F.; Bonfrate, L.; Portincasa, P.; Gobbetti, M. The Food-gut Human Axis: The Effects of Diet on Gut Microbiota and Metabolome. Curr. Med. Chem. 2019, 26, 3567–3583. [Google Scholar] [CrossRef]
- Ye, S.; Deng, Y.; Su, H. Research Progress in Psoriasis and Intestinal Barrier. Chin. J. Dermatovenereol. 2025, 39, 807–812. [Google Scholar] [CrossRef]
- Ling, B.; Jing, H.; Zhang, Q.; Wang, Y.; Qi, S.; Liu, S.; Si, M.; He, Y. The Role of the Gut Microbiota-Bile Acid Axis in the Pathogenesis of Hypertension. Chin. J. Hypertens. 2023, 31, 1043–1051. [Google Scholar] [CrossRef]
- Kida, T.; Tsubosaka, Y.; Hori, M.; Ozaki, H.; Murata, T. Bile acid receptor TGR5 agonism induces NO production and reduces monocyte adhesion in vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, R.X.; Colgan, S.P. Physiologic hypoxia in the intestinal mucosa: A central role for short-chain fatty acids. Am. J. Physiol.-Cell Physiol. 2024, 327, C1087–C1093. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, H.; Tu, X.; Gao, Z. The role of short-chain fatty acids of gut microbiota origin in hypertension. Front. Microbiol. 2021, 12, 730809. [Google Scholar] [CrossRef]
- Zheng, T.; Wu, Y.; Peng, M.; Xiao, N.; Tan, Z.; Yang, T. Hypertension of liver-yang hyperactivity syndrome induced by a high salt diet by altering components of the gut microbiota associated with the glutamate/GABA-glutamine cycle. Front. Nutr. 2022, 9, 964273. [Google Scholar] [CrossRef]
- Inotsuka, R.; Uchimura, K.; Yamatsu, A.; Kim, M.; Katakura, Y. γ-Aminobutyric acid (GABA) activates neuronal cells by inducing the secretion of exosomes from intestinal cells. Food Funct. 2020, 11, 9285–9290. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Wong, M.H.; Thelin, A.; Hansson, L.; Falk, P.G.; Gordon, J.I. Molecular analysis of commensal host-microbial relationships in the intestine. Science 2001, 291, 881–884. [Google Scholar] [CrossRef]
- Yao, P.; Cui, M.; Li, Y.; Deng, Y.; Wu, H. Effects of rhubarb on intestinal flora and toll-like receptors of intestinal mucosa in rats with severe acute pancreatitis. Pancreas 2015, 44, 799–804. [Google Scholar] [CrossRef]
- Durbán, A.; Abellán, J.J.; Jiménez-Hernández, N.; Ponce, M.; Ponce, J.; Sala, T.; D’Auria, G.; Latorre, A.; Moya, A. Assessing gut microbial diversity from feces and rectal mucosa. Microb. Ecol. 2011, 61, 123–133. [Google Scholar] [CrossRef]
- Ferguson, J.F.; Aden, L.A.; Barbaro, N.R.; Van Beusecum, J.P.; Xiao, L.; Simons, A.J.; Warden, C.; Pasic, L.; Himmel, L.E.; Washington, M.K. High dietary salt–induced DC activation underlies microbial dysbiosis-associated hypertension. JCI Insight 2019, 4, e126241. [Google Scholar] [CrossRef] [PubMed]
- Kirabo, A.; Fontana, V.; De Faria, A.P.; Loperena, R.; Galindo, C.L.; Wu, J.; Bikineyeva, A.T.; Dikalov, S.; Xiao, L.; Chen, W. DC isoketal-modified proteins activate T cells and promote hypertension. J. Clin. Investig. 2014, 124, 4642–4656. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Hoch, N.E.; Brown, K.A.; McCann, L.A.; Rahman, A.; Dikalov, S.; Goronzy, J.; Weyand, C.; Harrison, D.G. Role of the T cell in the genesis of angiotensin II–induced hypertension and vascular dysfunction. J. Exp. Med. 2007, 204, 2449–2460. [Google Scholar] [CrossRef] [PubMed]
- Madhur, M.S.; Lob, H.E.; McCann, L.A.; Iwakura, Y.; Blinder, Y.; Guzik, T.J.; Harrison, D.G. Interleukin 17 promotes angiotensin II–induced hypertension and vascular dysfunction. Hypertension 2010, 55, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Itani, H.A.; Xiao, L.; Saleh, M.A.; Wu, J.; Pilkinton, M.A.; Dale, B.L.; Barbaro, N.R.; Foss, J.D.; Kirabo, A.; Montaniel, K.R. CD70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ. Res. 2016, 118, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Saleh, M.A.; Kirabo, A.; Itani, H.A.; Montaniel, K.R.C.; Xiao, L.; Chen, W.; Mernaugh, R.L.; Cai, H.; Bernstein, K.E. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J. Clin. Investig. 2016, 126, 50–67. [Google Scholar] [CrossRef]
- Wu, J.; Thabet, S.R.; Kirabo, A.; Trott, D.W.; Saleh, M.A.; Xiao, L.; Madhur, M.S.; Chen, W.; Harrison, D.G. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ. Res. 2014, 114, 616–625. [Google Scholar] [CrossRef]
- Barbaro, N.R.; Foss, J.D.; Kryshtal, D.O.; Tsyba, N.; Kumaresan, S.; Xiao, L.; Mernaugh, R.L.; Itani, H.A.; Loperena, R.; Chen, W. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep. 2017, 21, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Grylls, A.; Seidler, K.; Neil, J. Link between microbiota and hypertension: Focus on LPS/TLR4 pathway in endothelial dysfunction and vascular inflammation, and therapeutic implication of probiotics. Biomed. Pharmacother. 2021, 137, 111334. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, J.; Liu, H.; Li, W.; Yu, C. Tetramethylpyrazine suppresses interleukin-8 expression in LPS-stimulated human umbilical vein endothelial cell by blocking ERK, p38 and nulear factor-κB signaling pathways. J. Ethnopharmacol. 2009, 125, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Hippenstiel, S.; Soeth, S.; Kellas, B.; Fuhrmann, O.; Seybold, J.; Krüll, M.; Eichel-Streiber, C.v.; Goebeler, M.; Ludwig, S.; Suttorp, N. Rho proteins and the p38-MAPK pathway are important mediators for LPS-induced interleukin-8 expression in human endothelial cells. Blood J. Am. Soc. Hematol. 2000, 95, 3044–3051. [Google Scholar] [CrossRef]
- Bomfim, G.F.; Echem, C.; Martins, C.B.; Costa, T.J.; Sartoretto, S.M.; Dos Santos, R.A.; Oliveira, M.A.; Akamine, E.H.; Fortes, Z.B.; Tostes, R.C. Toll-like receptor 4 inhibition reduces vascular inflammation in spontaneously hypertensive rats. Life Sci. 2015, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Frey, R.S.; Malik, A.B. TLR4 signaling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J. Clin. Investig. 2003, 112, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Behm, D.J.; Nerurkar, S.; Eybye, M.E.; Haimbach, R.E.; Olzinski, A.R.; Douglas, S.A.; Willette, R.N. p38 MAPK inhibitors ameliorate target organ damage in hypertension: Part 1. p38 MAPK-dependent endothelial dysfunction and hypertension. J. Pharmacol. Exp. Ther. 2003, 307, 932–938. [Google Scholar] [CrossRef]
- Sayer, M.; Webb, D.J.; Dhaun, N. Novel pharmacological approaches to lowering blood pressure and managing hypertension. Nat. Rev. Cardiol. 2025, 22, 649–663. [Google Scholar] [CrossRef]
- Shetty, S.A.; Boeren, S.; Bui, T.P.; Smidt, H.; de Vos, W.M. Unravelling lactate-acetate and sugar conversion into butyrate by intestinal Anaerobutyricum and Anaerostipes species by comparative proteogenomics. Environ. Microbiol. 2020, 22, 4863–4875. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Huangdi Neijing Suwen; People’s Medical Publishing House: Beijing, China, 1963. [Google Scholar]
- Hossen, I.; Wu, H.; Luo, T.; Mehmood, A.; Song, J.; Xu, D.; Cao, Y.; Wu, H.; Gao, Z.; Zhang, K. Phytochemicals and inflammatory bowel disease: A review. Crit. Rev. Food Sci. Nutr. 2022, 60, 1321–1345. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the cardiovascular system in health and in aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef]
- He, H.; Chen, W.; Yang, A.; Jiang, C.; Zhang, R. Effect of blackcurrant polyphenols on lowering blood pressure. Food Ferment. Ind. 2020, 46, 97–103. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y. Antihypertensive Effect of Pomegranate Polyphenols in Spontaneously Hypertensive Rats. China Pharm. 2016, 19, 255–258. [Google Scholar]
- Hua, Y. Study on Antioxidant Activity, Blood Pressure, Blood Lipid and Liver of Blueberry Leaves Polyphenols. Master’s Thesis, College of Food Science and Technology, Nanjing Agricultural University, Nanjing, China, 2016. [Google Scholar]
- Amit, K.S.; Célia, C.; Ramesh, K.; Risha, G.; Harvesh, K.R.; Ashutosh, G.; Maria, R.L.; Claudia, C.; Flávio, R.; Pandey, A.K. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Colussi, G.; Catena, C.; Novello, M.; Bertin, N.; Sechi, L.A. Impact of omega-3 polyunsaturated fatty acids on vascular function and blood pressure: Relevance for cardiovascular outcomes. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, T.; Wissuwa, B.; Tian, Y.; Tajima, N.; Xu, R.; Bauer, M.; Heinemann, S.H.; Hou, S. Omega-3 fatty acids lower blood pressure by directly activating large-conductance Ca2+-dependent K+ channels. Proc. Natl. Acad. Sci. USA 2013, 110, 4816–4821. [Google Scholar] [CrossRef]
- Robertson, R.C.; Oriach, C.S.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Stanton, C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav. Immun. 2017, 59, 21–37. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tatsuno, I. Omega-3 polyunsaturated fatty acids for cardiovascular diseases: Present, past and future. Expert Rev. Clin. Pharmacol. 2017, 10, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Bai, C. Mechanism and clinical application prospects of omega-3 polyunsaturated fatty acids in hypertension. Int. J. Cardiovasc. Dis. 2024, 51, 77–80. [Google Scholar] [CrossRef]
- Ballan, R.; Battistini, C.; Xavier-Santos, D.; Saad, S.M.I. Interactions of probiotics and prebiotics with the gut microbiota. Prog. Mol. Biol. Transl. Sci. 2020, 171, 265–300. [Google Scholar] [CrossRef]
- Aslam, H.; Green, J.; Jacka, F.N.; Collier, F.; Berk, M.; Pasco, J.; Dawson, S.L. Fermented foods, the gut and mental health: A mechanistic overview with implications for depression and anxiety. Nutr. Neurosci. 2020, 23, 659–671. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Kim, Y.A.; Keogh, J.B.; Clifton, P.M. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr. Res. Rev. 2018, 31, 35–51. [Google Scholar] [CrossRef]
- Bell, V.; Ferrão, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One health, fermented foods, and gut microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Li, Y.; Chen, M.; Xue, L.; Wang, J.; Ding, Y.; Gu, Q.; Zhang, J.; Yang, R.; Zhao, H. Effects of probiotics on hypertension. Appl. Microbiol. Biotechnol. 2023, 107, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liang, W.; Liang, J.; Dou, J.; Guo, F.; Zhang, D.; Xu, Z.; Wang, T. Probiotics: Functional food ingredients with the potential to reduce hypertension. Front. Cell. Infect. Microbiol. 2023, 13, 1220877. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Touyz, R.M. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and its biological activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Toral, M.; de la Visitación, N.; Sánchez, M.; Gómez-Guzmán, M.; Romero, M.; Yang, T.; Izquierdo-Garcia, J.L.; Jiménez, R.; Ruiz-Cabello, J. Probiotics prevent dysbiosis and the rise in blood pressure in genetic hypertension: Role of short-chain fatty acids. Mol. Nutr. Food Res. 2020, 64, 1900616. [Google Scholar] [CrossRef]
- Malik, M.; Suboc, T.M.; Tyagi, S.; Salzman, N.; Wang, J.; Ying, R.; Tanner, M.J.; Kakarla, M.; Baker, J.E.; Widlansky, M.E. Lactobacillus plantarum 299v supplementation improves vascular endothelial function and reduces inflammatory biomarkers in men with stable coronary artery disease. Circ. Res. 2018, 123, 1091–1102. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Kasahara, K.; Yamashita, T.; Sasaki, N.; Yodoi, K.; Matsumoto, T.; Emoto, T.; Hayashi, T.; Kitano, N.; Yoshida, N. Oral administration of the lactic acid bacterium Pediococcus acidilactici attenuates atherosclerosis in mice by inducing tolerogenic dendritic cells. Heart Vessel. 2017, 32, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.A.; Pagán, A.; García-Estañ, J.; Atucha, N.M. Evaluation of the Effects of Mulberry Leaf Extracts Morus alba L. on Cardiovascular, Renal, and Platelet Function in Experimental Arterial Hypertension. Nutrients 2024, 17, 49. [Google Scholar] [CrossRef]
- Gao, L.; Li, H.; Li, B.; Shao, H.; Yu, X.; Miao, Z.; Zhang, L.; Zhu, L.; Sheng, H. Traditional uses, phytochemistry, transformation of ingredients and pharmacology of the dried seeds of Raphanus sativus L. (Raphani Semen), A comprehensive review. J. Ethnopharmacol. 2022, 294, 115387. [Google Scholar] [CrossRef]
- Du, W.; Fan, H.; Zhang, Y.; Jiang, X.; Li, Y. Effect of flavonoids in hawthorn and vitamin C prevents hypertension in rats induced by heat exposure. Molecules 2022, 27, 866. [Google Scholar] [CrossRef]
- Zu, W.; Sang, Z.; Zhu, L. Study on Antihypertensive Effect of Ginseng, Pueraria Lobata and Other Medicinal and Edible Chinese Herbal Medicines. Clin. Med. Nursing 2023, 1, 25–29. [Google Scholar] [CrossRef]
- Ye, Q.; Chen, Q.; Li, Y.; Wang, T.; Chen, X.; Yue, Y. Effect of cassia seed aqueous extract on blood pressure level in N-nitro-L-arginine-methyl ester induced hypertensive rats. Chin. J. Tissue Eng. Res. 2021, 25, 1705. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Guo, W.; Yang, X.; Qu, J.; Gao, M.; Chen, S.; Dong, J.; Li, Q.; Wang, T. Comparison of the chemical components, efficacy and mechanisms of action of chrysanthemum morifolium flower and its wild relative chrysanthemum indicum flower against liver-fire hyperactivity syndrome of hypertension via integrative analyses. Int. J. Mol. Sci. 2022, 23, 13767. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Niu, J.; Bu, L.; Li, C.; Liu, Y.; Jiang, J.; Xu, Q.; Ma, P.; Zhou, R. Exploring the Mechanism of Influence of Wolfberry (Lycium barbarum L.) on Pulmonary Arterial Hypertension Based on Network Pharmacology and Molecular Docking. Lett. Drug Des. Discov. 2024, 21, 4005–4025. [Google Scholar] [CrossRef]
- Mukohda, M.; Mizuno, R.; Ozaki, H. Emerging evidence for a cardiovascular protective effect of concentrated Japanese plum juice. Hypertens. Res. 2023, 46, 2428–2429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Yu, D.; Yang, Z.; Shen, Z.; Meng, Y.; Ding, Y.; Li, Y. Botany, chemistry, bio-activity, and application of Polygonatum odoratum (Mill.) Druce: A comprehensive review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 13545–13566. [Google Scholar] [CrossRef] [PubMed]
- Halima, O.A.; Mebarka, B.; Nasser, B.; Khaled, S.; Kawther, A.S.C. Study of Chemical Composition and Evaluation of Anti-hypertensive Effect of A Fixed Oil Extracted from Linum usitatissimum grains. J. Food Pharm. Sci. 2024, 12, 38–51. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Li, B.; Sun, H.; Hao, Y.; Gao, L.; Zhang, L. Study on the effects of ginkgolide on arterial blood pressure in rats. Shaanxi J. Tradit. Chin. Med. 2011, 32, 491–492. [Google Scholar]
- Ishimitsu, A.; Tojo, A.; Satonaka, H.; Ishimitsu, T. Eucommia ulmoides (Tochu) and its extract geniposidic acid reduced blood pressure and improved renal hemodynamics. Biomed. Pharmacother. 2021, 141, 111901. [Google Scholar] [CrossRef] [PubMed]
- Farré, R.; Fiorani, M.; Abdu Rahiman, S.; Matteoli, G. Intestinal permeability, inflammation and the role of nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food components and dietary habits: Keys for a healthy gut microbiota composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Amasheh, M.; Andres, S.; Amasheh, S.; Fromm, M.; Schulzke, J.D. Barrier effects of nutritional factors. Ann. N. Y. Acad. Sci. 2009, 1165, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Challa, H.J.; Ameer, M.A.; Uppaluri, K.R. DASH diet to stop hypertension. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Theodoridis, X.; Chourdakis, M.; Chrysoula, L.; Chroni, V.; Tirodimos, I.; Dipla, K.; Gkaliagkousi, E.; Triantafyllou, A. Adherence to the DASH diet and risk of hypertension: A systematic review and meta-analysis. Nutrients 2023, 15, 3261. [Google Scholar] [CrossRef] [PubMed]
- Tarray, R.; Saleem, S.; Yousuf, I.; Gulnar, A.; Laway, B.; Verma, S. Role of insulin resistance in essential hypertension. Cardiovasc. Endocrinol. Metab. 2014, 3, 129–133. [Google Scholar] [CrossRef]
- Francisco, S.C.; Araújo, L.F.; Griep, R.H.; Chor, D.; Molina, M.D.C.B.; Mil, J.G.; Bensenor, I.M.; Matos, S.M.A.; Barreto, S.M.; Giatti, L. Adherence to the Dietary Approaches to Stop Hypertension (DASH) and hypertension risk: Results of the Longitudinal Study of Adult Health (ELSA-Brasil). Br. J. Nutr. 2020, 123, 1068–1077. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Green, T.; Harrison, T.N.; Reynolds, K. Dietary approaches to prevent hypertension. Curr. Hypertens. Rep. 2013, 15, 694–702. [Google Scholar] [CrossRef]
- Lin, P.; van Vliet, S.; Lin, C.; Svetkey, L.; Tyson, C.; Scialla, J. Impact of the DASH Diet on Intestinal Permeability and Inflammation Markers. Curr. Dev. Nutr. 2020, 4, 542. [Google Scholar] [CrossRef]
- Lin, P.H.; Allen, J.D.; Li, Y.J.; Yu, M.; Lien, L.F.; Svetkey, L.P. Blood Pressure-Lowering Mechanisms of the DASH Dietary Pattern. J. Nutr. Metab. 2012, 2012, 472396. [Google Scholar] [CrossRef] [PubMed]
- Filippou, C.D.; Thomopoulos, C.G.; Kouremeti, M.M.; Sotiropoulou, L.I.; Nihoyannopoulos, P.I.; Tousoulis, D.M.; Tsioufis, C.P. Mediterranean diet and blood pressure reduction in adults with and without hypertension: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2021, 40, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, X.; Jia, P.; You, Y.; Cheng, Y.; Deng, H.; Luo, S.; Huang, B. Ketogenic diet aggravates hypertension via NF-κB-mediated endothelial dysfunction in spontaneously hypertensive rats. Life Sci. 2020, 258, 118124. [Google Scholar] [CrossRef] [PubMed]
- Almnifi, A.H.M.L.; Elsayed, A.G.; Elgendy, L.M. Ketogenic diet and physical activity for prevention of hypertension. MISJ-Int. J. Med. Res. Allied Sci. 2023, 1, 1–6. [Google Scholar]
- Barrea, L.; Verde, L.; Santangeli, P.; Lucà, S.; Docimo, A.; Savastano, S.; Colao, A.; Muscogiuri, G. Very low-calorie ketogenic diet (VLCKD): An antihypertensive nutritional approach. J. Transl. Med. 2023, 21, 128. [Google Scholar] [CrossRef]
- Linsalata, M.; Russo, F.; Riezzo, G.; D’Attoma, B.; Prospero, L.; Orlando, A.; Ignazzi, A.; Di Chito, M.; Sila, A.; De Nucci, S.; et al. The Effects of a Very-Low-Calorie Ketogenic Diet on the Intestinal Barrier Integrity and Function in Patients with Obesity: A Pilot Study. Nutrients 2023, 15, 2561. [Google Scholar] [CrossRef]
- Xia, H.; Guo, J.; Shen, J.; Jiang, S.; Han, S.; Li, L. Butyrate ameliorated the intestinal barrier dysfunction and attenuated acute pancreatitis in mice fed with ketogenic diet. Life Sci. 2023, 334, 122188. [Google Scholar] [CrossRef]
- Agita, A.; Thaha, M. Inflammation, immunity, and hypertension. Acta Medica Indones. 2017, 49, 158–165. [Google Scholar]
- Kono, H.; Fujii, H.; Asakawa, M.; Yamamoto, M.; Matsuda, M.; Maki, A.; Matsumoto, Y. Protective effects of medium-chain triglycerides on the liver and gut in rats administered endotoxin. Ann. Surg. 2003, 237, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Kirpich, I.A.; Feng, W.; Wang, Y.; Liu, Y.; Barker, D.F.; Barve, S.S.; McClain, C.J. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol. Clin. Exp. Res. 2012, 36, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Toivio, L.; Launonen, H.; Lindén, J.; Lehto, M.; Vapaatalo, H.; Salmenkari, H.; Korpela, R. Ketogenic Diet High in Saturated Fat Promotes Colonic Claudin Expression without Changes in Intestinal Permeability to Iohexol in Healthy Mice. Nutrients 2023, 16, 18. [Google Scholar] [CrossRef] [PubMed]

| Age | Sex | Mean Sodium Excretion (mmol/24 h) | Mean Salt Intake (g/24 h) |
|---|---|---|---|
| 3~6 | male/female | 86.99 | 5.09 |
| 6~16 | male/female | 151.09 | 8.84 |
| >16 | male | 194.76 | 11.39 |
| female | 181.54 | 10.62 |
| Microorganism (Genus/Species) | Variation Tendency | Observed or Proposed Host Effect | References |
|---|---|---|---|
| Prevotella | Increased | Increased Prevotella abundance was positively correlated with intestinal mucosal inflammation and could stimulate Th17 cell-mediated immune responses. | [46,47] |
| Weissella | Decreased | Reduced Weissella abundance might impair lactic acid production, which typically inhibits foodborne pathogens. | [46,48] |
| Clostridium (e.g., C. perfringens, C. difficile) | Increased; Clostridium species converted primary bile acids into secondary bile acids. | Increased abundance of Clostridium species can cause severe infections and has been linked to liver cancer progression through the conversion of primary to secondary bile acids, which might inhibit immune response. | [49,50,51] |
| Catenibacterium | Increased | Higher Catenibacterium abundance was observed in polycystic ovary syndrome patients and was associated with a more diverse gut microbiota, with variations among ethnic minority populations (e.g., Li ethnic group) in China. | [49,52,53] |
| Klebsiella | Increased | Increased Klebsiella was prevalent in hypertensive patients; its cell wall LPS could regulate the immune system and contribute to intestinal inflammation. | [49,54,55] |
| Mogibacteriaceae | Increased | Changes in the abundance of intestinal mucosa Mogibacteriaceae in rectal cancer patients might be related to disease onset. | [49,56] |
| Novosphingobium | Increased; Its dihydrolipoamide acetyltransferase component (PDC-E2) protein showed high homology to human PDC-E2. | The subordinate strain’s PDC-E2 protein had high homology with the immunodominant region of human PDC-E2, suggesting a possible role in primary biliary cirrhosis. | [49,57] |
| Chryseobacterium | Increased | Most strains were drug-resistant; could cause severe infections (e.g., bacteremia, pneumonia, meningitis) in immunocompromised individuals. Indole-producing strains, while less virulent, also contributed to these diseases. | [49,58,59] |
| Lactobacillus | Decreased | Decreased Lactobacillus abundance may impair intestinal flora balance, reduce protease secretion (neutralizing bacterial toxins), and compromise intestinal barrier function. | [60] |
| Clostridium XIVa | Decreased | Reduced Clostridium XIVa abundance, a probiotic with properties aiding intestinal microecological balance, was less prevalent in ulcerative colitis patients. | [60,61] |
| Pseudoflavonifractor | Decreased | Reduced Pseudoflavonifractor abundance, a core gut microbiota, has been considered a biomarker for obesity in recent years. | [60,62] |
| Alistipes | Increased | Subordinate strains were isolated from patients with appendicitis, abdominal and rectal abscesses, and rectal cancer, indicating a key role in inflammation. | [60,63,64] |
| Parasutterella | Increased | Changes in Parasutterella abundance were linked to metabolic disorders; it helped maintain bile acid homeostasis and regulated cholesterol metabolism. | [60] |
| Akkermansia | Increased | Akkermansia, a probiotic, could degrade mucoprotein substrates produced by the host. | [60] |
| Ruminococcus (e.g., R. gnavus) | Increased; R. gnavus produced a substance that causes DC cells to produce inflammatory cytokines. | This bacterial community, including R. gnavus, was capable of degrading resistant starch and cellulose. R. gnavus produced inflammatory cytokines (e.g., TNF-α), linking the intestinal bacterial community to extraintestinal inflammatory diseases. | [41,65] |
| Oscillospira | Increased; The strain was less abundant in patients with inflammatory bowel disease. | Increased Oscillospira abundance might aid in the formation of secondary bile acids and resistance to Clostridium difficile infections, despite being less abundant in patients with inflammatory bowel disease. | [41,66,67] |
| Roseburia (e.g., R. intestinalis) | Increased | Roseburia, including probiotic strains like R. intestinalis, produced butyric acid in the colon. | [41,67,68] |
| Anaerostipes | Decreased | Reduced Anaerostipes abundance, a probiotic that converted lactic acid, acetic acid, and sugar into butyric acid in the intestines. | [69] |
| Substances | Active Ingredients | Antihypertensive Mechanism | References |
|---|---|---|---|
| Mori folium | / | Increased NO activity. | [130] |
| Semen raphani | / | Regulation of NOS expression. | [131] |
| Crataegi fructus | Hawthorn flavonoids | Inhibited oxidative stress in blood vessels. | [132] |
| Puerariae lobatae radix | Flavonoids, puerarin | Expanded blood vessels and improved microcirculation. | [133] |
| Panax ginseng | Ginsenoside | Regulation of NOS expression. | [133] |
| Semen cassiae | / | Promoted eNOS expression, antioxidant activity, and inhibition of angiotensin-converting enzyme (ACE) activity. | [134] |
| Dendranthema morifolium | Luteolin, etc. | Inhibit AngII and NF-κB pathways. | [135] |
| Lycium chinese Miller | Quercetin, betaine, etc. | Interfered with multiple signaling pathways (AKT1, EGFR, MYC, etc.). | [136] |
| Fructus mume | / | Acted on vascular smooth muscle cells(VSMC) to protect blood vessels. | [137] |
| Polygonati odorati | Flavonoids | Inhibited vascular oxidative stress. | [138] |
| Cannabisfructus | Hemp seed oil | / | [139] |
| Ginkgo biloba L. | Bilobalide | Increased the activity of superoxide dismutase in the serum and reduced the concentration of malondialdehyde in the serum. | [140] |
| Eucommia ulmoides Oliv. | Chlorogenic acid | Improved endothelial cell function. Inhibited oxidative stress; regulated mitochondrial dysfunction, etc. | [141] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, W.; Zhao, Y.; Wu, Y.; Jiang, J.; Zheng, H.; Yang, Y.; Zheng, T. High-Salt Diets, Intestinal Barrier, and Hypertension: A Mechanistic Review and the Promise of Dietary Therapy. Nutrients 2025, 17, 3688. https://doi.org/10.3390/nu17233688
Si W, Zhao Y, Wu Y, Jiang J, Zheng H, Yang Y, Zheng T. High-Salt Diets, Intestinal Barrier, and Hypertension: A Mechanistic Review and the Promise of Dietary Therapy. Nutrients. 2025; 17(23):3688. https://doi.org/10.3390/nu17233688
Chicago/Turabian StyleSi, Wenhao, Yan Zhao, Yuhang Wu, Jiani Jiang, Hui Zheng, Yong Yang, and Tao Zheng. 2025. "High-Salt Diets, Intestinal Barrier, and Hypertension: A Mechanistic Review and the Promise of Dietary Therapy" Nutrients 17, no. 23: 3688. https://doi.org/10.3390/nu17233688
APA StyleSi, W., Zhao, Y., Wu, Y., Jiang, J., Zheng, H., Yang, Y., & Zheng, T. (2025). High-Salt Diets, Intestinal Barrier, and Hypertension: A Mechanistic Review and the Promise of Dietary Therapy. Nutrients, 17(23), 3688. https://doi.org/10.3390/nu17233688






