Association Between Over-the-Counter Magnesium Supplement Use and Health Outcomes in Veterans with Newly Diagnosed Heart Failure
Abstract
1. Introduction
2. Material & Methods
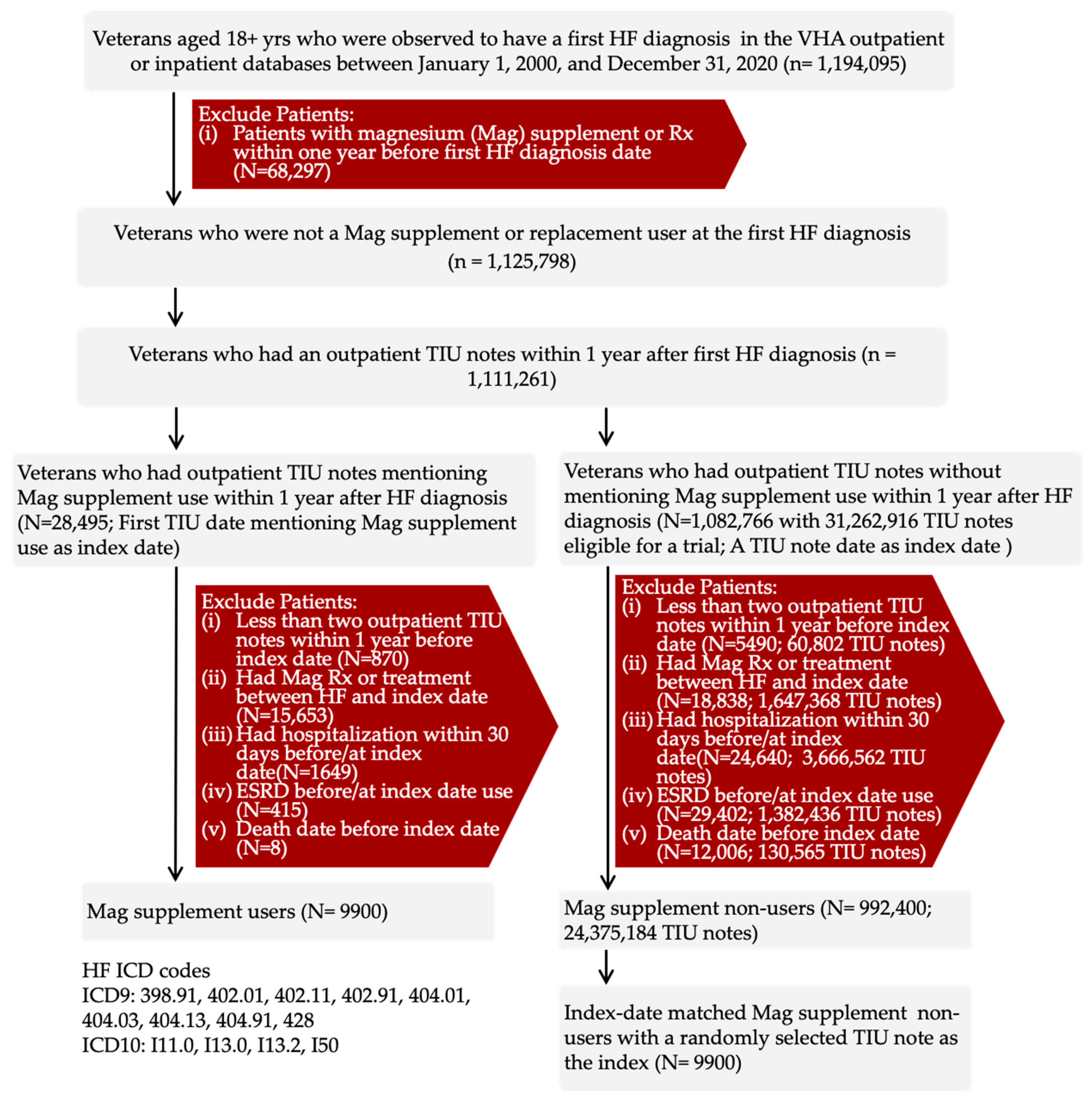
2.1. Study Population
2.2. Study Design and Ethics
2.3. Target Trial Emulation
2.4. Exposure
2.5. Study Outcomes and Follow-Up
2.6. Study Covariates
2.7. Variables with Missing Values
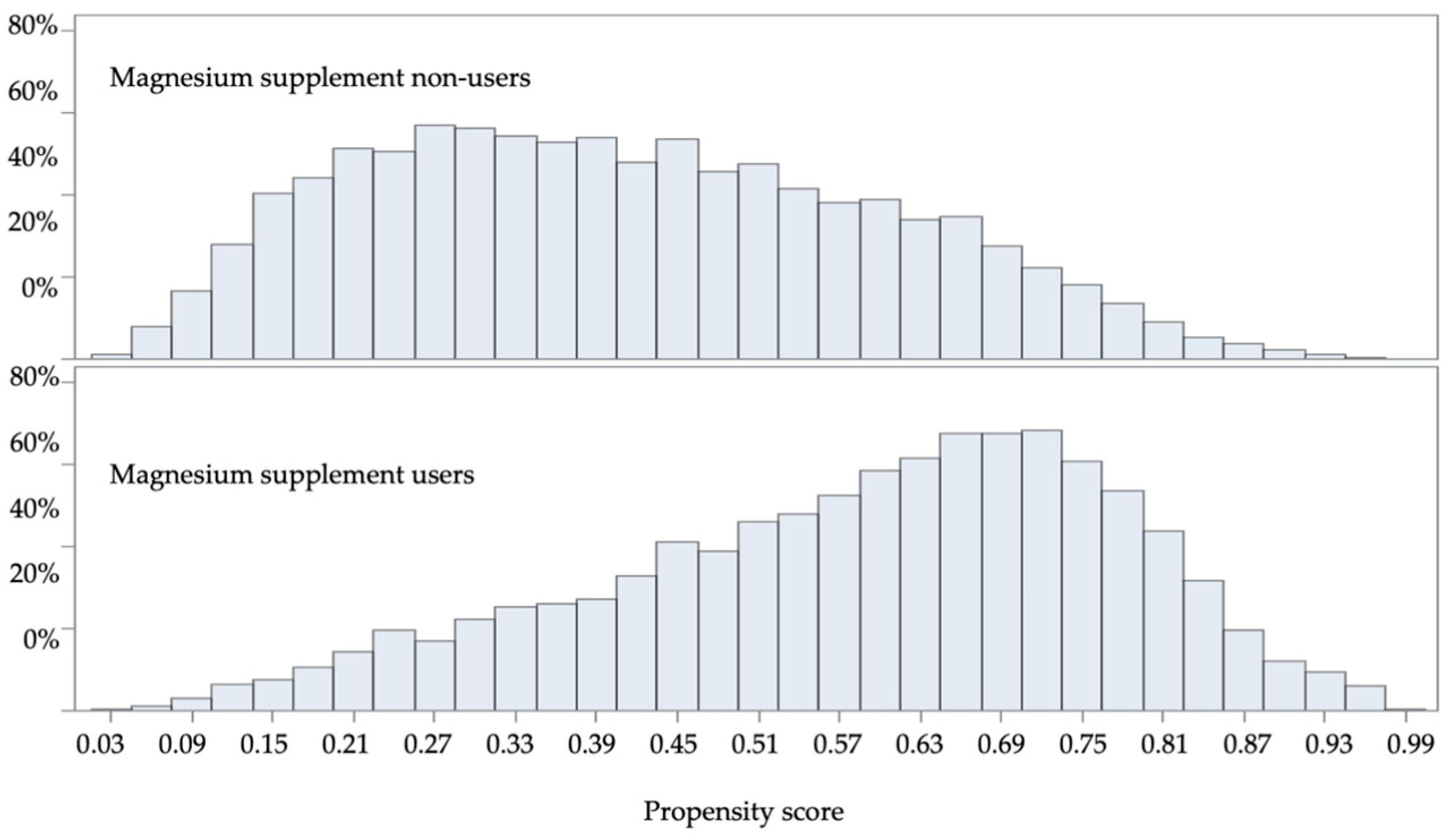
2.8. Statistical Analysis
2.9. Sensitivity Analyses
2.10. Target Trial Assumption Evaluation
3. Results
3.1. Baseline Characteristics
3.2. Magnesium Supplement Use and Adverse Outcomes
3.2.1. Descriptive Analysis
3.2.2. Main and Sensitivity Analyses
3.2.3. Interactions and Stratified Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paolisso, G.; Scheen, A.; D’Onofrio, F.; Lefebvre, P. Magnesium and glucose homeostasis. Diabetologia 1990, 33, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Douban, S.; Brodsky, M.A.; Whang, D.D.; Whang, R. Significance of magnesium in congestive heart failure. Am. Heart J. 1996, 132, 664–671. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Liu, J.; O’Keefe, J.H. Magnesium for the prevention and treatment of cardiovascular disease. Open Heart 2018, 5, e000775. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, S.S. Importance of magnesium in congestive heart failure. Am. J. Cardiol. 1989, 63, G39–G42. [Google Scholar] [CrossRef]
- Nishihara, T.; Yamamoto, E.; Sueta, D.; Fujisue, K.; Usuku, H.; Oike, F.; Takae, M.; Arima, Y.; Araki, S.; Takashio, S.; et al. Clinical significance of serum magnesium levels in patients with heart failure with preserved ejection fraction. Medicine 2019, 98, e17069. [Google Scholar] [CrossRef]
- Taveira, T.H.; Ouellette, D.; Gulum, A.; Choudhary, G.; Eaton, C.B.; Liu, S.; Wu, W.C. Relation of Magnesium Intake with Cardiac Function and Heart Failure Hospitalizations in Black Adults: The Jackson Heart Study. Circ. Heart Fail. 2016, 9, e002698. [Google Scholar] [CrossRef]
- Adamopoulos, C.; Pitt, B.; Sui, X.; Love, T.E.; Zannad, F.; Ahmed, A. Low serum magnesium and cardiovascular mortality in chronic heart failure: A propensity-matched study. Int. J. Cardiol. 2009, 136, 270–277. [Google Scholar] [CrossRef]
- Levitan, E.B.; Shikany, J.M.; Ahmed, A.; Snetselaar, L.G.; Martin, L.W.; Curb, J.D.; Lewis, C.E. Calcium, magnesium and potassium intake and mortality in women with heart failure: The Women’s Health Initiative. Br. J. Nutr. 2013, 110, 179–185. [Google Scholar] [CrossRef]
- Misialek, J.R.; Lopez, F.L.; Lutsey, P.L.; Huxley, R.R.; Peacock, J.M.; Chen, L.Y.; Soliman, E.Z.; Agarwal, S.K.; Alonso, A. Serum and dietary magnesium and incidence of atrial fibrillation in whites and in African Americans—Atherosclerosis Risk in Communities (ARIC) study. Circ. J. 2013, 77, 323–329. [Google Scholar] [CrossRef]
- Sueta, C.A.; Patterson, J.H.; Adams, K.F., Jr. Antiarrhythmic action of pharmacological administration of magnesium in heart failure: A critical review of new data. Magnes. Res. 1995, 8, 389–401. [Google Scholar]
- The ASCEND Study Collaborative Group; Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; Haynes, R.; et al. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar] [CrossRef]
- Laville, M.; Segrestin, B.; Alligier, M.; Ruano-Rodriguez, C.; Serra-Majem, L.; Hiesmayr, M.; Schols, A.; La Vecchia, C.; Boirie, Y.; Rath, A.; et al. Evidence-based practice within nutrition: What are the barriers for improving the evidence and how can they be dealt with? Trials 2017, 18, 425. [Google Scholar] [CrossRef]
- Manson, J.E.; Bassuk, S.S.; Lee, I.M.; Cook, N.R.; Albert, M.A.; Gordon, D.; Zaharris, E.; Macfadyen, J.G.; Danielson, E.; Lin, J.; et al. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp. Clin. Trials 2012, 33, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Rodriguez, L.A.; Cantero, O.F.; Logan, R.; Hernan, M.A. Observational data for comparative effectiveness research: An emulation of randomised trials of statins and primary prevention of coronary heart disease. Stat. Methods Med. Res. 2013, 22, 70–96. [Google Scholar] [CrossRef]
- Matthews, A.A.; Danaei, G.; Islam, N.; Kurth, T. Target trial emulation: Applying principles of randomised trials to observational studies. BMJ 2022, 378, e071108. [Google Scholar] [CrossRef] [PubMed]
- Hernan, M.A.; Robins, J.M. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am. J. Epidemiol. 2016, 183, 758–764. [Google Scholar] [CrossRef]
- Hernan, M.A.; Wang, W.; Leaf, D.E. Target Trial Emulation: A Framework for Causal Inference From Observational Data. JAMA 2022, 328, 2446–2447. [Google Scholar] [CrossRef]
- Cheng, Y.; Zullo, A.R.; Yin, Y.; Shao, Y.; Liu, S.; Zeng-Treitler, Q.; Wu, W.C. Nonprescription Magnesium Supplement Use and Risk of Heart Failure in Patients with Diabetes: A Target Trial Emulation. J. Am. Heart Assoc. 2025, 14, e038870. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. EJI Frequently Asked Questions. Available online: https://www.atsdr.cdc.gov/place-health/php/eji/eji-frequently-asked-questions.html (accessed on 3 December 2024).
- Elze, M.C.; Gregson, J.; Baber, U.; Williamson, E.; Sartori, S.; Mehran, R.; Nichols, M.; Stone, G.W.; Pocock, S.J. Comparison of Propensity Score Methods and Covariate Adjustment: Evaluation in 4 Cardiovascular Studies. J. Am. Coll. Cardiol. 2017, 69, 345–357. [Google Scholar] [CrossRef]
- Ali, M.S.; Groenwold, R.H.; Klungel, O.H. Best (but oft-forgotten) practices: Propensity score methods in clinical nutrition research. Am. J. Clin. Nutr. 2016, 104, 247–258. [Google Scholar] [CrossRef]
- Negru, A.G.; Pastorcici, A.; Crisan, S.; Cismaru, G.; Popescu, F.G.; Luca, C.T. The Role of Hypomagnesemia in Cardiac Arrhythmias: A Clinical Perspective. Biomedicines 2022, 10, 2356. [Google Scholar] [CrossRef]
- Curran, J.; Ross-White, A.; Sibley, S. Magnesium prophylaxis of new-onset atrial fibrillation: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0292974. [Google Scholar] [CrossRef] [PubMed]
- Shiga, T.; Wajima, Z.; Inoue, T.; Ogawa, R. Magnesium prophylaxis for arrhythmias after cardiac surgery: A meta-analysis of randomized controlled trials. Am. J. Med. 2004, 117, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Na, L.; Chang, J.; Li, X.; Che, X.; Sun, Y.; Cui, W.; Zhang, W.; Xue, X. Association Between Intravenous Magnesium Sulfate and All-Cause Mortality in Patients with Acute Heart Failure: A Propensity Score-Matched Cohort Study in MIMIC-IV. Rev. Cardiovasc. Med. 2025, 26, 39206. [Google Scholar] [CrossRef] [PubMed]
- Stepura, O.B.; Martynow, A.I. Magnesium orotate in severe congestive heart failure (MACH). Int. J. Cardiol. 2009, 134, 145–147. [Google Scholar] [CrossRef]
- Guasch-Ferre, M.; Bullo, M.; Estruch, R.; Corella, D.; Martinez-Gonzalez, M.A.; Ros, E.; Covas, M.; Aros, F.; Gomez-Gracia, E.; Fiol, M.; et al. Dietary magnesium intake is inversely associated with mortality in adults at high cardiovascular disease risk. J. Nutr. 2014, 144, 55–60. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Gao, J.; Yang, Q.; Qu, H.; Shi, J. Association between dietary magnesium and 10-year risk of a first hard atherosclerotic cardiovascular disease event. Am. J. Med. Sci. 2024, 368, 355–360. [Google Scholar] [CrossRef]
- Song, L.; Ying, J.; Li, M.; Ying, L.; Zhao, C. Propensity score matched cohort study on magnesium supplementation and mortality in critically ill patients with HFpEF. Sci. Rep. 2025, 15, 1944. [Google Scholar] [CrossRef]
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Hollifield, J.W. Magnesium depletion, diuretics, and arrhythmias. Am. J. Med. 1987, 82, 30–37. [Google Scholar] [CrossRef]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Aging, Health and Diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopath. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef]
- Martin, B.J.; Milligan, K. Diuretic-associated hypomagnesemia in the elderly. Arch. Intern. Med. 1987, 147, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Kuller, L.; Farrier, N.; Caggiula, A.; Borhani, N.; Dunkle, S. Relationship of diuretic therapy and serum magnesium levels among participants in the Multiple Risk Factor Intervention Trial. Am. J. Epidemiol. 1985, 122, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Guerrera, M.P.; Volpe, S.L.; Mao, J.J. Therapeutic uses of magnesium. Am. Fam. Physician 2009, 80, 157–162. [Google Scholar] [PubMed]
- African American Policy Forum. Table 2 Common Magnesium Formulations and Dosages. Available online: https://www.aafp.org/pubs/afp/issues/2009/0715/p157/jcr:content/root/aafp-article-primary-content-container/aafp_article_main_par/aafp_tables_content1.print.html (accessed on 13 November 2025).
- National Institutes of Health. Magnesium: Fact Sheet for Health Professional. Available online: https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/ (accessed on 17 November 2025).



| Target Trial | Emulation in Observational Data | |
|---|---|---|
| Aim | Evaluate the association between magnesium supplement use and all-cause hospitalization or death in the U.S. military veterans with HF | |
| Eligibility | (1) Newly diagnosed with HF no more than 1 year (2) Never used magnesium supplement or replacement treatment before (3) No hospitalization in the past 30 days (4) No end-stage renal disease (ESRD) | (1) Had the initial HF diagnosis within 1 year before (2) Had at least two outpatient clinical notes within the previous 12 months (to confirm magnesium supplement use status) (3) Had no record of prescribed or supplemental magnesium use prior to this date (4) Had no hospitalization within 30 days before or at the eligible date (5) Had no ESRD any time before or at the eligible date |
| Treatment Strategies | (1) Magnesium supplement intervention: Initiation of magnesium supplement (2) Magnesium supplement non-intervention: No initiation of magnesium supplement | (1) Magnesium supplement users: new documentation of the use of magnesium supplements in an outpatient clinic encounter (2) Magnesium supplement non-users: No documentation of magnesium supplement use in an outpatient clinic encounter |
| Assignment | Randomly assigned 1:1 to each treatment strategy in a parallel design | Veterans were assigned to treatment arms based on EMR documentation. All magnesium supplement users were included. For the non-user group, randomization was emulated by randomly selecting a group of non-user patients with the same eligible date as a user. Inverse probability weighting was used to balance observed confounders between treatment groups. |
| Follow-up | From date of randomization up until end of 5 years, outcome, or censoring due to events such as violation of the assigned treatment strategy | Followed from the time when a patient became eligible for the trial (index date) until the outcome of interest occurred, or the censoring date due to magnesium supplement use status change (i.e., stop use in users or start use in non-users), prescribed magnesium replacement was started, or the end of 5th year from the index date, whichever occurred first. |
| Outcomes | All-cause mortality or all-cause hospitalization (time to events) | Same |
| Causal Contrast | The difference in risk of all-cause mortality or all-cause hospitalization had everyone followed strategy (1) vs. strategy (2) | Same |
| Statistical Analysis | Cox’s regression | Per-protocol analysis using Cox’s regression weighted by inverse probability of treatment |
| Baseline Characteristics | Overall (N = 19,800) | Magnesium Users (N = 9900) | Magnesium Non-Users (N = 9900) | ASD Before IPTW (%) | |||
|---|---|---|---|---|---|---|---|
| Mean/N | SD/% | Mean/N | SD/% | Mean/N | SD/% | ||
| Demographics (8 Variables) | |||||||
| Age, Years | 72.6 | 11.1 | 73.3 | 10.7 | 72.0 | 11.4 | 12 |
| Male | 19,133 | 96.6% | 9544 | 96.4% | 9589 | 96.9% | 3 |
| Race | |||||||
| White | 15,220 | 76.9% | 7872 | 79.5% | 7348 | 74.2% | 13 |
| Black | 2426 | 12.3% | 869 | 8.8% | 1557 | 15.7% | 21 |
| Others | 2154 | 10.9% | 1159 | 11.7% | 995 | 10.1% | 5 |
| Ethnicity | |||||||
| Non-Hispanics | 17,965 | 90.7% | 8991 | 90.8% | 8974 | 90.6% | 1 |
| Hispanics | 640 | 3.2% | 244 | 2.5% | 396 | 4.0% | 8 |
| Unknown | 1195 | 6.0% | 665 | 6.7% | 530 | 5.4% | 5 |
| Married | 11,583 | 58.5% | 6262 | 63.3% | 5321 | 53.7% | 20 |
| Median Income (lowest to highest) of Zip Code | |||||||
| 1st Quartile | 4128 | 20.8% | 1914 | 19.3% | 2214 | 22.4% | 8 |
| 2nd Quartile | 4811 | 24.3% | 2478 | 25.0% | 2333 | 23.6% | 3 |
| 3rd Quartile | 4871 | 24.6% | 2472 | 25.0% | 2399 | 24.2% | 2 |
| 4th Quartile | 5530 | 27.9% | 2865 | 28.9% | 2665 | 26.9% | 4 |
| Unknown | 460 | 2.3% | 171 | 1.7% | 289 | 2.9% | 8 |
| Environmental Justice Index Socio-Environmental Percentile Ranking of Zip code | 48.0 | 22.6 | 46.3 | 22.2 | 49.6 | 22.9 | 15 |
| Duration between HF Diagnosis and Index Date, Days | 129.6 | 117.7 | 105.6 | 118.1 | 153.7 | 112.2 | 42 |
| Comorbid Conditions (31 Variables) | |||||||
| Alcohol Disorder | 2818 | 14.2% | 1188 | 12.0% | 1630 | 16.5% | 13 |
| Smoking | 6083 | 30.7% | 2483 | 25.1% | 3600 | 36.4% | 25 |
| Hypertension | 17,218 | 87.0% | 8340 | 84.2% | 8878 | 89.7% | 16 |
| Hyperlipidemia | 16,079 | 81.2% | 7849 | 79.3% | 8230 | 83.1% | 10 |
| Myocardial Infarction | 3566 | 18.0% | 1536 | 15.5% | 2030 | 20.5% | 13 |
| Atherosclerosis | 4770 | 24.1% | 2208 | 22.3% | 2562 | 25.9% | 8 |
| Angina | 3394 | 17.1% | 1346 | 13.6% | 2048 | 20.7% | 19 |
| Atrial Fibrillation | 6705 | 33.9% | 3646 | 36.8% | 3059 | 30.9% | 12 |
| Cardiac Valve Disease | 3430 | 17.3% | 1574 | 15.9% | 1856 | 18.7% | 7 |
| Ischemic Stroke | 2460 | 12.4% | 1083 | 10.9% | 1377 | 13.9% | 9 |
| Hemorrhage Stroke | 172 | 0.9% | 62 | 0.6% | 110 | 1.1% | 5 |
| Transient Ischemic Attack | 1061 | 5.4% | 429 | 4.3% | 632 | 6.4% | 9 |
| Chronic Obstructive Pulmonary Disease | 7125 | 36.0% | 3324 | 33.6% | 3801 | 38.4% | 10 |
| Asthma | 1820 | 9.2% | 856 | 8.6% | 964 | 9.7% | 4 |
| Chronic Kidney Disease | 5612 | 28.3% | 2672 | 27.0% | 2940 | 29.7% | 6 |
| Anemia | 6541 | 33.0% | 3008 | 30.4% | 3533 | 35.7% | 11 |
| Arthritis | 9347 | 47.2% | 4398 | 44.4% | 4949 | 50.0% | 11 |
| Cancer | 6753 | 34.1% | 3129 | 31.6% | 3624 | 36.6% | 11 |
| Neurological Disorders | 7556 | 38.2% | 3572 | 36.1% | 3984 | 40.2% | 8 |
| Parkinson | 417 | 2.1% | 181 | 1.8% | 236 | 2.4% | 4 |
| Hypothyroidism | 2887 | 14.6% | 1511 | 15.3% | 1376 | 13.9% | 4 |
| Osteoporosis | 866 | 4.4% | 420 | 4.2% | 446 | 4.5% | 1 |
| Osteomyelitis | 500 | 2.5% | 183 | 1.8% | 317 | 3.2% | 9 |
| Urinary Tract Infection | 1238 | 6.3% | 487 | 4.9% | 751 | 7.6% | 11 |
| Liver Disease | 1875 | 9.5% | 828 | 8.4% | 1047 | 10.6% | 8 |
| Pneumonia | 2481 | 12.5% | 917 | 9.3% | 1564 | 15.8% | 20 |
| Respiratory Failure | 1087 | 5.5% | 373 | 3.8% | 714 | 7.2% | 15 |
| Sepsis | 662 | 3.3% | 210 | 2.1% | 452 | 4.6% | 14 |
| Opioid Use Disorder | 420 | 2.1% | 150 | 1.5% | 270 | 2.7% | 8 |
| Depression | 6883 | 34.8% | 2998 | 30.3% | 3885 | 39.2% | 19 |
| Fracture | 2223 | 11.2% | 915 | 9.2% | 1308 | 13.2% | 13 |
| Concurrent Medications (22 Variables) | |||||||
| Insulin | 5041 | 25.5% | 2214 | 22.4% | 2827 | 28.6% | 14 |
| Metformin | 3949 | 19.9% | 1855 | 18.7% | 2094 | 21.2% | 6 |
| Glucagon-Like Peptide-1 | 78 | 0.4% | 34 | 0.3% | 44 | 0.4% | 2 |
| Sodium–Glucose Cotrasporter-2 Inhibitors | 195 | 1.0% | 100 | 1.0% | 95 | 1.0% | 0 |
| Other Diabetes Medication | 3221 | 16.3% | 1470 | 14.8% | 1751 | 17.7% | 8 |
| Vitamin D Prescription | 3618 | 18.3% | 1433 | 14.5% | 2185 | 22.1% | 20 |
| Multivitamin (Rx) | 972 | 4.9% | 390 | 3.9% | 582 | 5.9% | 9 |
| Angiotensin-Converting Enzyme Inhibitor | 8021 | 40.5% | 3229 | 32.6% | 4792 | 48.4% | 33 |
| Angiotensin Receptor Blocker | 3137 | 15.8% | 1446 | 14.6% | 1691 | 17.1% | 7 |
| Calcium Channel Blocker | 5442 | 27.5% | 2211 | 22.3% | 3231 | 32.6% | 23 |
| Loop Diuretics | 8765 | 44.3% | 3750 | 37.9% | 5015 | 50.7% | 26 |
| Thiazide Diuretics | 3262 | 16.5% | 1372 | 13.9% | 1890 | 19.1% | 14 |
| Selective Beta Blocker | 7996 | 40.4% | 3287 | 33.2% | 4709 | 47.6% | 30 |
| Non-Selective Beta Blocker | 4425 | 22.3% | 1913 | 19.3% | 2512 | 25.4% | 15 |
| Other Antihypertensive | 5931 | 30.0% | 2598 | 26.2% | 3333 | 33.7% | 16 |
| Statins | 11,125 | 56.2% | 4679 | 47.3% | 6446 | 65.1% | 36 |
| Other Lipid-Lowering Medication | 1258 | 6.4% | 573 | 5.8% | 685 | 6.9% | 5 |
| Proton-Pump Inhibitors | 6662 | 33.6% | 2940 | 29.7% | 3722 | 37.6% | 17 |
| Aspirin | 5894 | 29.8% | 2067 | 20.9% | 3827 | 38.7% | 40 |
| Digoxin | 334 | 1.7% | 154 | 1.6% | 180 | 1.8% | 2 |
| Glucocorticoids | 2443 | 12.3% | 972 | 9.8% | 1471 | 14.9% | 16 |
| Platelet Inhibitor | 3058 | 15.4% | 1280 | 12.9% | 1778 | 18.0% | 14 |
| Health Care Utilization (11 Variables) | |||||||
| Hospitalization(s) in past year | |||||||
| 0 | 14,860 | 75.1% | 8364 | 84.5% | 6496 | 65.6% | 45 |
| 1 | 3035 | 15.3% | 976 | 9.9% | 2059 | 20.8% | 31 |
| 2+ | 1905 | 9.6% | 560 | 5.7% | 1345 | 13.6% | 27 |
| Number of visits in past year | 31.5 | 29.5 | 26.8 | 28.0 | 36.2 | 30.2 | 32 |
| Addiction Medicine | 483 | 2.4% | 186 | 1.9% | 297 | 3.0% | 7 |
| Cardiology | 8551 | 43.2% | 3170 | 32.0% | 5381 | 54.4% | 46 |
| Emergency Care | 5800 | 29.3% | 2025 | 20.5% | 3775 | 38.1% | 39 |
| Endocrinology | 990 | 5.0% | 425 | 4.3% | 565 | 5.7% | 6 |
| Hospice Medicine | 138 | 0.7% | 54 | 0.5% | 84 | 0.8% | 4 |
| Intensive Care Unit | 1255 | 6.3% | 379 | 3.8% | 876 | 8.8% | 21 |
| Internal Medicine | 6184 | 31.2% | 2628 | 26.5% | 3556 | 35.9% | 20 |
| Oncology | 980 | 4.9% | 404 | 4.1% | 576 | 5.8% | 8 |
| Palliative Care | 338 | 1.7% | 115 | 1.2% | 223 | 2.3% | 8 |
| Vital Signs and Labs (14 Variables) | |||||||
| Body Mass Index, kg/m2 | 30.0 | 6.9 | 30.2 | 6.9 | 29.8 | 6.9 | 6 |
| Systolic Blood Pressure, mmHg | 129.5 | 19.9 | 127.9 | 19.8 | 131.0 | 19.9 | 16 |
| Diastolic Blood Pressure, mmHg | 72.1 | 11.7 | 71.3 | 11.5 | 72.9 | 11.9 | 14 |
| Serum Magnesium, mg/dL | |||||||
| <1.7 mg/dL | 1003 | 5.1% | 757 | 7.6% | 246 | 2.5% | 23 |
| 1.7–2.5 mg/dL | 6454 | 32.6% | 2608 | 26.3% | 3846 | 38.8% | 27 |
| >2.5 mg/dL | 189 | 1.0% | 90 | 0.9% | 99 | 1.0% | 1 |
| Unknown | 12,154 | 61.4% | 6445 | 65.1% | 5709 | 57.7% | 15 |
| Serum 25-hydroxy vitamin D < 20 ng/dL | 927 | 4.7% | 382 | 3.9% | 545 | 5.5% | 8 |
| Low-Density Lipoprotein, mg/dL | 85.9 | 34.2 | 85.1 | 34.5 | 86.5 | 33.9 | 4 |
| Triglycerides, mg/dL | 146.3 | 111.5 | 147.1 | 110.3 | 145.6 | 112.6 | 1 |
| High-Density Lipoprotein, mg/dL | 43.2 | 14.2 | 43.3 | 14.4 | 43.0 | 14.0 | 2 |
| Cholesterol, mg/dL | 155.3 | 42.6 | 154.5 | 42.9 | 156.0 | 42.3 | 4 |
| Sodium, mmol/L | 138.9 | 3.2 | 138.7 | 3.3 | 139.0 | 3.1 | 9 |
| Potassium, mmol/L | 4.3 | 0.5 | 4.3 | 0.5 | 4.3 | 0.5 | 0 |
| Creatinine, mg/dL | 1.3 | 0.6 | 1.3 | 0.6 | 1.3 | 0.6 | 0 |
| Calcium, mg/dL | 9.2 | 0.5 | 9.2 | 0.5 | 9.2 | 0.5 | 0 |
| Hemoglobin A1C, % | 6.8 | 1.5 | 6.9 | 1.5 | 6.8 | 1.5 | 7 |
| Ejection Fraction | |||||||
| ≤40% | 5171 | 26.1% | 2600 | 26.9% | 2511 | 25.4% | 3 |
| >40% | 7091 | 35.8% | 2928 | 29.6% | 4163 | 42.1% | 26 |
| Unknown | 7538 | 38.1% | 4312 | 43.6% | 3226 | 32.6% | 23 |
| Baseline Characteristics | IPT-Weighted Overall (N = 19,925) | IPT-Weighted Magnesium Users (N = 10,096) | IPT-Weighted Magnesium Non-Users (N = 9829) | ASD After IPTW (%) | |||
|---|---|---|---|---|---|---|---|
| Mean/N | SD/% | Mean/N | SD/% | Mean/N | SD/% | ||
| Demographics (8 Variables) | |||||||
| Age, Years | 72.6 | 11.1 | 72.5 | 10.8 | 72.6 | 11.4 | 1 |
| Male | 19,240 | 96.6% | 9748 | 96.6% | 9492 | 96.6% | 0 |
| Race | |||||||
| White | 15,260 | 76.6% | 7711 | 76.4% | 7550 | 76.8% | 1 |
| Black | 2518 | 12.6% | 1300 | 12.9% | 1219 | 12.4% | 2 |
| Others | 2147 | 10.8% | 1086 | 10.8% | 1061 | 10.8% | 0 |
| Ethnicity | |||||||
| Non-Hispanics | 18,105 | 90.9% | 9171 | 90.8% | 8935 | 90.9% | 0 |
| Hispanics | 654 | 3.3% | 336 | 3.3% | 318 | 3.2% | 1 |
| Unknown | 1166 | 5.9% | 589 | 5.8% | 577 | 5.9% | 0 |
| Married | 11,565 | 58.0% | 5828 | 57.7% | 5737 | 58.4% | 1 |
| Median Income (lowest to highest) of Zip Code | |||||||
| 1st Quartile | 4197 | 21.1% | 2139 | 21.2% | 2058 | 20.9% | 1 |
| 2nd Quartile | 4797 | 24.1% | 2424 | 24.0% | 2373 | 24.1% | 0 |
| 3rd Quartile | 4825 | 24.2% | 2438 | 24.1% | 2387 | 24.3% | 0 |
| 4th Quartile | 5624 | 28.2% | 2842 | 28.1% | 2782 | 28.3% | 0 |
| Unknown | 482 | 2.4% | 253 | 2.5% | 229 | 2.3% | 1 |
| Environmental Justice Index Socio-Environmental Percentile Ranking of Zip code | 47.9 | 22.7 | 48.0 | 22.9 | 47.8 | 22.6 | 1 |
| Duration between Diabetes Diagnosis and Index Date, Days | 135.0 | 118.4 | 136.0 | 125.8 | 134.0 | 110.5 | 2 |
| Comorbid Conditions (31 Variables) | |||||||
| Alcohol Disorder | 2888 | 14.5% | 1471 | 14.6% | 1418 | 14.4% | 1 |
| Smoking | 6267 | 31.5% | 3210 | 31.8% | 3058 | 31.1% | 2 |
| Hypertension | 17,382 | 87.2% | 8804 | 87.2% | 8578 | 87.3% | 0 |
| Myocardial Infarction | 3670 | 18.4% | 1866 | 18.5% | 1804 | 18.4% | 0 |
| Atherosclerosis | 4884 | 24.5% | 2478 | 24.5% | 2405 | 24.5% | 0 |
| Angina | 3548 | 17.8% | 1817 | 18.0% | 1731 | 17.6% | 1 |
| Atrial Fibrillation | 6756 | 33.9% | 3424 | 33.9% | 3331 | 33.9% | 0 |
| Cardiac Valve Disease | 3565 | 17.9% | 1824 | 18.1% | 1741 | 17.7% | 1 |
| Ischemic Stroke | 2530 | 12.7% | 1284 | 12.7% | 1246 | 12.7% | 0 |
| Hemorrhage Stroke | 185 | 0.9% | 97 | 1.0% | 88 | 0.9% | 1 |
| Transient Ischemic Attack | 1092 | 5.5% | 552 | 5.5% | 539 | 5.5% | 0 |
| Chronic Obstructive Pulmonary Disease | 7263 | 36.5% | 3703 | 36.7% | 3560 | 36.2% | 1 |
| Asthma | 1922 | 9.6% | 989 | 9.8% | 934 | 9.5% | 1 |
| Chronic Kidney Disease | 5769 | 29.0% | 2919 | 28.9% | 2850 | 29.0% | 0 |
| Anemia | 6767 | 34.0% | 3452 | 34.2% | 3314 | 33.7% | 1 |
| Arthritis | 9532 | 47.8% | 4820 | 47.7% | 4712 | 47.9% | 0 |
| Cancer | 6905 | 34.7% | 3488 | 34.6% | 3417 | 34.8% | 0 |
| Neurological Disorders | 7785 | 39.1% | 3953 | 39.1% | 3832 | 39.0% | 0 |
| Parkinson | 421 | 2.1% | 218 | 2.2% | 204 | 2.1% | 1 |
| Hypothyroidism | 2944 | 14.8% | 1498 | 14.8% | 1446 | 14.7% | 0 |
| Osteoporosis | 888 | 4.5% | 459 | 4.6% | 428 | 4.4% | 1 |
| Osteomyelitis | 521 | 2.6% | 268 | 2.7% | 254 | 2.6% | 1 |
| Hyperlipidemia | 16,306 | 81.8% | 8244 | 81.7% | 8062 | 82.0% | 1 |
| Urinary Tract Infection | 1344 | 6.7% | 711 | 7.0% | 633 | 6.4% | 2 |
| Liver Disease | 1971 | 9.9% | 1006 | 10.0% | 965 | 9.8% | 1 |
| Pneumonia | 2592 | 13.0% | 1341 | 13.3% | 1251 | 12.7% | 2 |
| Respiratory Failure | 1175 | 5.9% | 622 | 6.2% | 553 | 5.6% | 3 |
| Sepsis | 699 | 3.5% | 362 | 3.6% | 338 | 3.4% | 1 |
| Opioid Use Disorder | 437 | 2.2% | 229 | 2.3% | 208 | 2.1% | 1 |
| Depression | 7050 | 35.4% | 3595 | 35.6% | 3454 | 35.1% | 1 |
| Fracture | 2378 | 11.9% | 1235 | 12.2% | 1144 | 11.6% | 2 |
| Concurrent Medications (22 Variables) | |||||||
| Insulin | 5219 | 26.2% | 2656 | 26.3% | 2564 | 26.1% | 0 |
| Metformin | 4099 | 20.6% | 2049 | 20.3% | 2051 | 20.9% | 1 |
| Glucagon-Like Peptide-1 | 84 | 0.4% | 42 | 0.4% | 42 | 0.4% | 0 |
| Sodium–Glucose Cotrasporter-2 Inhibitors | 208 | 1.0% | 106 | 1.0% | 103 | 1.0% | 0 |
| Other Diabetes Medication | 3315 | 16.6% | 1663 | 16.5% | 1652 | 16.8% | 1 |
| Vitamin D Prescription | 3789 | 19.0% | 1941 | 19.2% | 1848 | 18.8% | 1 |
| Multivitamin (Rx) | 1044 | 5.2% | 536 | 5.3% | 507 | 5.2% | 0 |
| Angiotensin-Converting Enzyme Inhibitor | 8280 | 41.6% | 4227 | 41.9% | 4053 | 41.2% | 1 |
| Angiotensin Receptor Blocker | 3253 | 16.3% | 1633 | 16.2% | 1620 | 16.5% | 1 |
| Calcium Channel Blocker | 5663 | 28.4% | 2892 | 28.6% | 2771 | 28.2% | 1 |
| Loop Diuretics | 9176 | 46.1% | 4666 | 46.2% | 4510 | 45.9% | 1 |
| Thiazide Diuretics | 3339 | 16.8% | 1683 | 16.7% | 1657 | 16.9% | 1 |
| Selective Beta Blocker | 8278 | 41.5% | 4202 | 41.6% | 4077 | 41.5% | 0 |
| Non-Selective Beta Blocker | 4636 | 23.3% | 2362 | 23.4% | 2274 | 23.1% | 1 |
| Other Antihypertensive | 6195 | 31.1% | 3143 | 31.1% | 3052 | 31.1% | 0 |
| Statins | 11,391 | 57.2% | 5788 | 57.3% | 5603 | 57.0% | 1 |
| Other Lipid-Lowering Medication | 1276 | 6.4% | 630 | 6.2% | 646 | 6.6% | 2 |
| Proton-Pump Inhibitors | 6903 | 34.6% | 3487 | 34.5% | 3415 | 34.7% | 0 |
| Aspirin | 6180 | 31.0% | 3181 | 31.5% | 2999 | 30.5% | 2 |
| Digoxin | 332 | 1.7% | 164 | 1.6% | 168 | 1.7% | 1 |
| Glucocorticoids | 2584 | 13.0% | 1320 | 13.1% | 1264 | 12.9% | 1 |
| Platelet Inhibitor | 3171 | 15.9% | 1605 | 15.9% | 1567 | 15.9% | 0 |
| Health Care Utilization (11 Variables) | |||||||
| Hospitalization(s) in past year | |||||||
| 0 | 14,672 | 73.6% | 7372 | 73.0% | 7300 | 74.3% | 3 |
| 1 | 3217 | 16.1% | 1657 | 16.4% | 1561 | 15.9% | 1 |
| 2+ | 2036 | 10.2% | 1068 | 10.6% | 968 | 9.9% | 2 |
| Number of visits in past year | 32.8 | 30.3 | 33.1 | 31.6 | 32.6 | 29.0 | 2 |
| Addiction Medicine | 491 | 2.5% | 251 | 2.5% | 240 | 2.4% | 1 |
| Cardiology | 8874 | 44.5% | 4550 | 45.1% | 4324 | 44.0% | 2 |
| Emergency Care | 6134 | 30.8% | 3146 | 31.2% | 2988 | 30.4% | 2 |
| Endocrinology | 1058 | 5.3% | 529 | 5.2% | 529 | 5.4% | 1 |
| Hospice Medicine | 164 | 0.8% | 92 | 0.9% | 71 | 0.7% | 2 |
| Intensive Care Unit | 1322 | 6.6% | 684 | 6.8% | 638 | 6.5% | 1 |
| Internal Medicine | 6416 | 32.2% | 3293 | 32.6% | 3123 | 31.8% | 2 |
| Oncology | 1052 | 5.3% | 548 | 5.4% | 504 | 5.1% | 1 |
| Palliative Care | 389 | 2.0% | 213 | 2.1% | 175 | 1.8% | 2 |
| Vital Signs and Labs (14 Variables) | |||||||
| Body Mass Index, kg/m2 | 30.0 | 6.9 | 29.9 | 7.0 | 30.0 | 6.8 | 1 |
| Systolic Blood Pressure, mmHg | 129.7 | 20.2 | 129.8 | 20.9 | 129.6 | 19.6 | 1 |
| Diastolic Blood Pressure, mmHg | 72.3 | 12.0 | 72.4 | 12.2 | 72.2 | 11.7 | 2 |
| Serum Magnesium, mg/dL | |||||||
| <1.7 mg/dL | 1070 | 5.4% | 511 | 5.1% | 559 | 5.7% | 3 |
| 1.7–2.5 mg/dL | 6711 | 33.7% | 3448 | 34.2% | 3263 | 33.2% | 2 |
| >2.5 mg/dL | 190 | 1.0% | 95 | 0.9% | 95 | 1.0% | 1 |
| Unknown | 11,954 | 60.0% | 6042 | 59.8% | 5912 | 60.2% | 1 |
| Serum 25-hydroxy vitamin D < 20 ng/dL | 956 | 4.8% | 492 | 4.9% | 464 | 4.7% | 1 |
| Low-Density Lipoprotein, mg/dL | 85.8 | 34.5 | 85.8 | 36.2 | 85.8 | 32.9 | 0 |
| Triglycerides, mg/dL | 145.5 | 111.2 | 145.5 | 109.7 | 145.6 | 112.5 | 0 |
| High-Density Lipoprotein, mg/dL | 43.2 | 14.2 | 43.3 | 14.5 | 43.2 | 13.9 | 1 |
| Cholesterol, mg/dL | 155.1 | 43.2 | 155.1 | 45.4 | 155.1 | 41.0 | 0 |
| Sodium, mmol/L | 138.9 | 3.3 | 138.9 | 3.4 | 138.9 | 3.1 | 0 |
| Potassium, mmol/L | 4.3 | 0.5 | 4.3 | 0.5 | 4.3 | 0.5 | 0 |
| Creatinine, mg/dL | 1.3 | 0.6 | 1.3 | 0.6 | 1.3 | 0.6 | 0 |
| Calcium, mg/dL | 9.2 | 0.5 | 9.2 | 0.6 | 9.2 | 0.5 | 0 |
| Hemoglobin A1C, % | 6.8 | 1.5 | 6.8 | 1.6 | 6.8 | 1.5 | 0 |
| Ejection Fraction | |||||||
| ≤40% | 5307 | 26.6% | 2683 | 26.6% | 2624 | 26.7% | 0 |
| >40% | 7269 | 36.5% | 3708 | 36.7% | 3561 | 36.2% | 1 |
| Unknown | 7349 | 36.9% | 3704 | 36.7% | 3644 | 37.1% | 1 |
| # Events | # Patients at Risk | Event/Patients at Risk (%) | Mean (STD) Follow-Up Years | Median (IQR) Follow-Up Years | Total Person Years | Incidence Rate (per 1000 Person-Years) | |
|---|---|---|---|---|---|---|---|
| Magnesium non-users | 3013 | 9829 | 30.7% | 0.85 (0.96) | 0.70 (0.10–1.00) | 8365.5 | 360.2 |
| Magnesium users | 2452 | 10096 | 24.3% | 0.82 (0.95) | 0.60 (0.10–1.00) | 8234.1 | 297.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Zullo, A.R.; Yin, Y.; Shao, Y.; Tekle, S.; Liu, S.; Zeng-Treitler, Q.; Wu, W.-C. Association Between Over-the-Counter Magnesium Supplement Use and Health Outcomes in Veterans with Newly Diagnosed Heart Failure. Nutrients 2025, 17, 3687. https://doi.org/10.3390/nu17233687
Cheng Y, Zullo AR, Yin Y, Shao Y, Tekle S, Liu S, Zeng-Treitler Q, Wu W-C. Association Between Over-the-Counter Magnesium Supplement Use and Health Outcomes in Veterans with Newly Diagnosed Heart Failure. Nutrients. 2025; 17(23):3687. https://doi.org/10.3390/nu17233687
Chicago/Turabian StyleCheng, Yan, Andrew R. Zullo, Ying Yin, Yijun Shao, Senait Tekle, Simin Liu, Qing Zeng-Treitler, and Wen-Chih Wu. 2025. "Association Between Over-the-Counter Magnesium Supplement Use and Health Outcomes in Veterans with Newly Diagnosed Heart Failure" Nutrients 17, no. 23: 3687. https://doi.org/10.3390/nu17233687
APA StyleCheng, Y., Zullo, A. R., Yin, Y., Shao, Y., Tekle, S., Liu, S., Zeng-Treitler, Q., & Wu, W.-C. (2025). Association Between Over-the-Counter Magnesium Supplement Use and Health Outcomes in Veterans with Newly Diagnosed Heart Failure. Nutrients, 17(23), 3687. https://doi.org/10.3390/nu17233687





