Therapeutic Modulation of Mitophagy by Cafestol in Pressure Overload-Induced Cardiac Hypertrophy and Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Experimental Model
2.3. Echocardiographic and Hemodynamic Assessment
2.4. Histological Evaluation
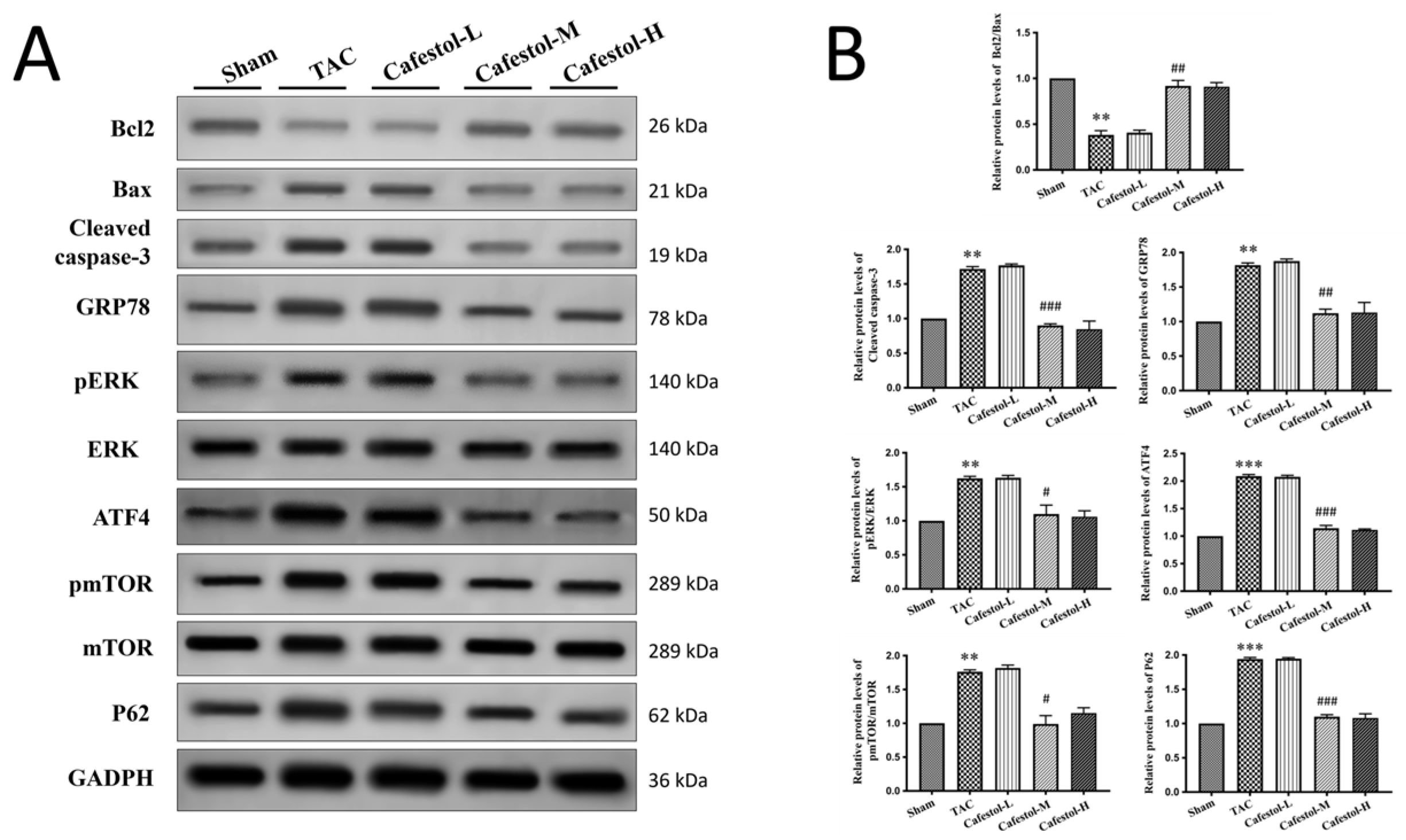
2.5. Western Blot Analysis
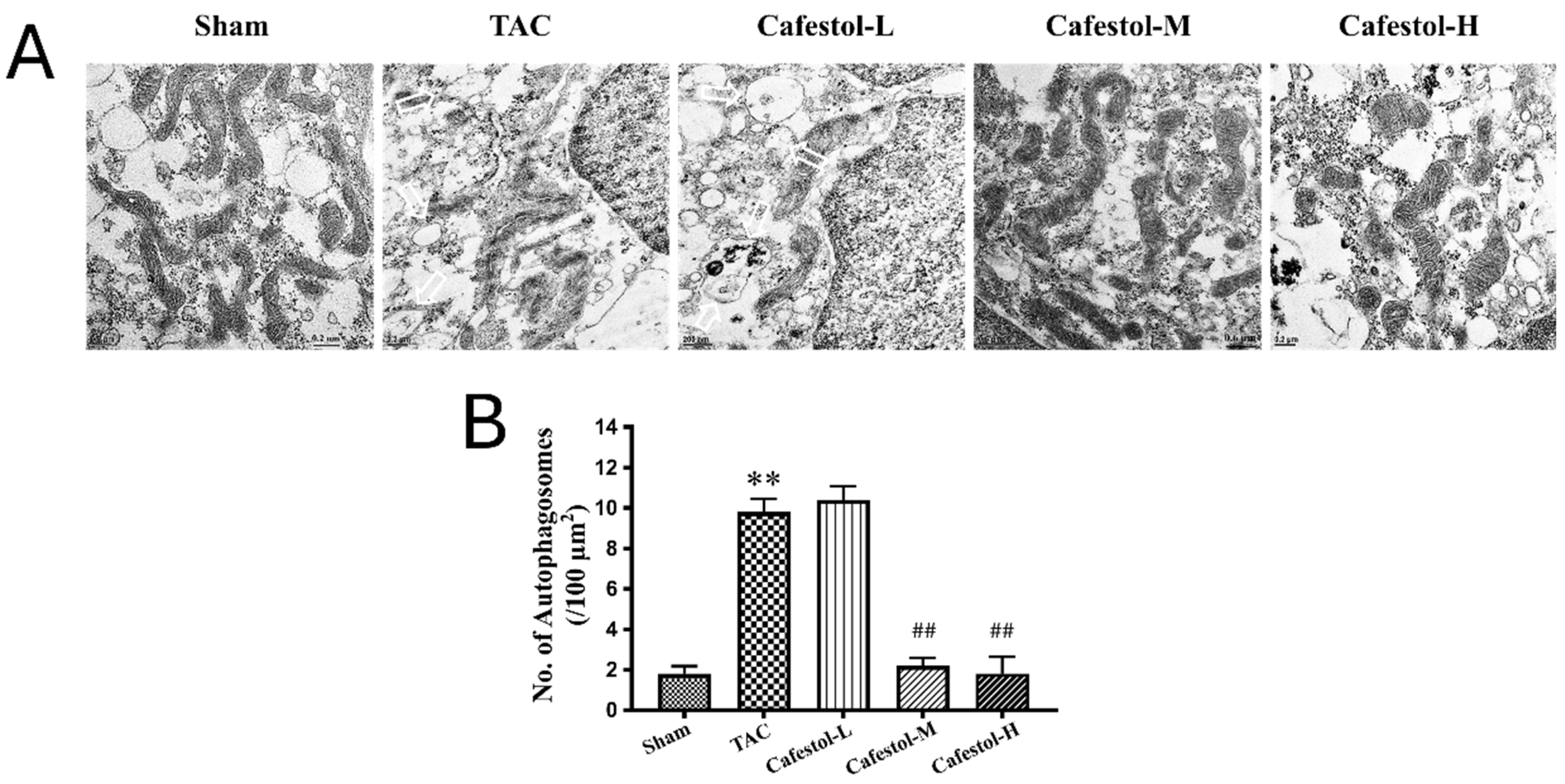
2.6. Transmission Electron Microscopy (TEM)
2.7. Statistical Analysis
3. Results
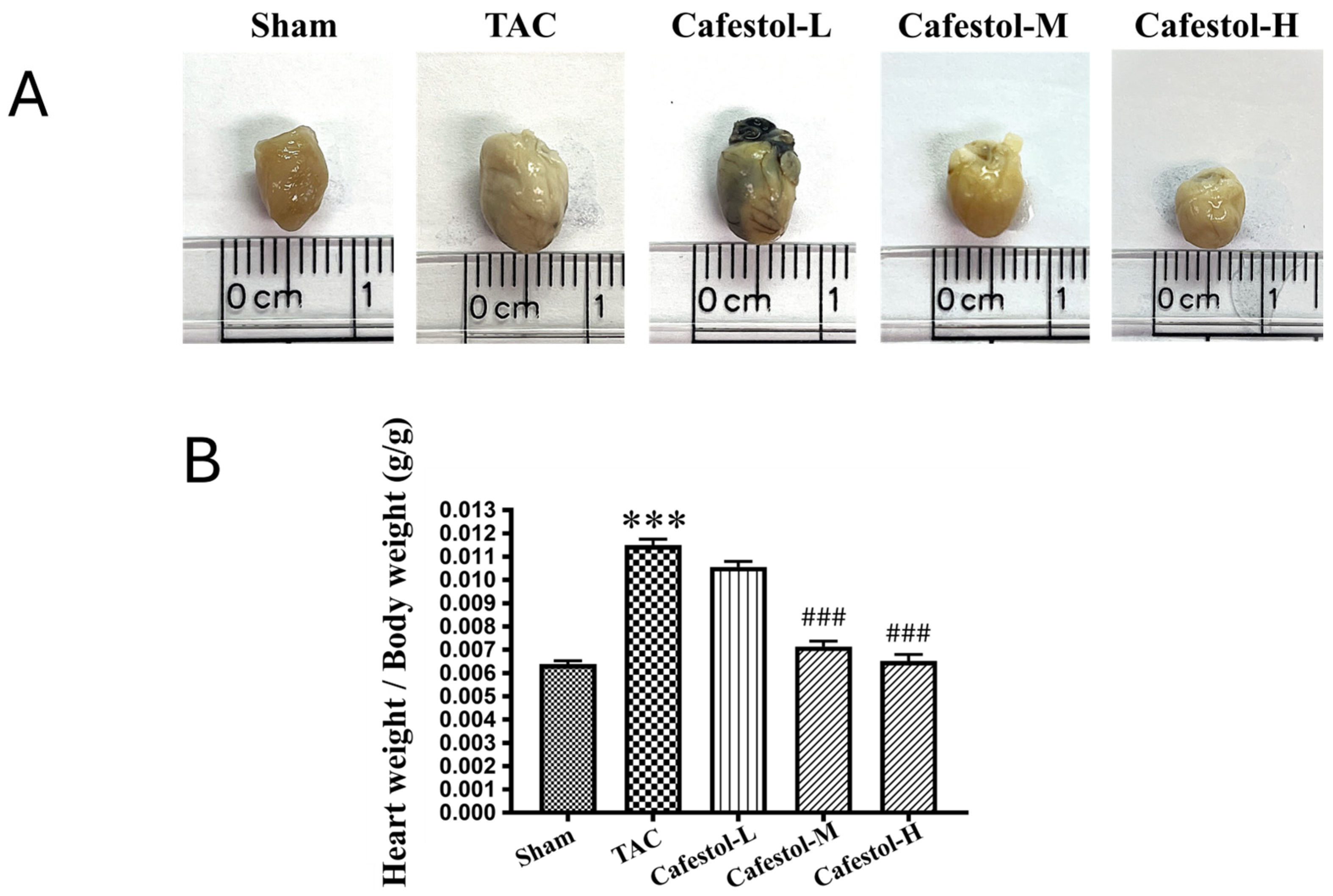
3.1. Cafestol Attenuates Cardiac and Ventricular Enlargement After Transverse Aortic Constriction-Induced Hypertrophy
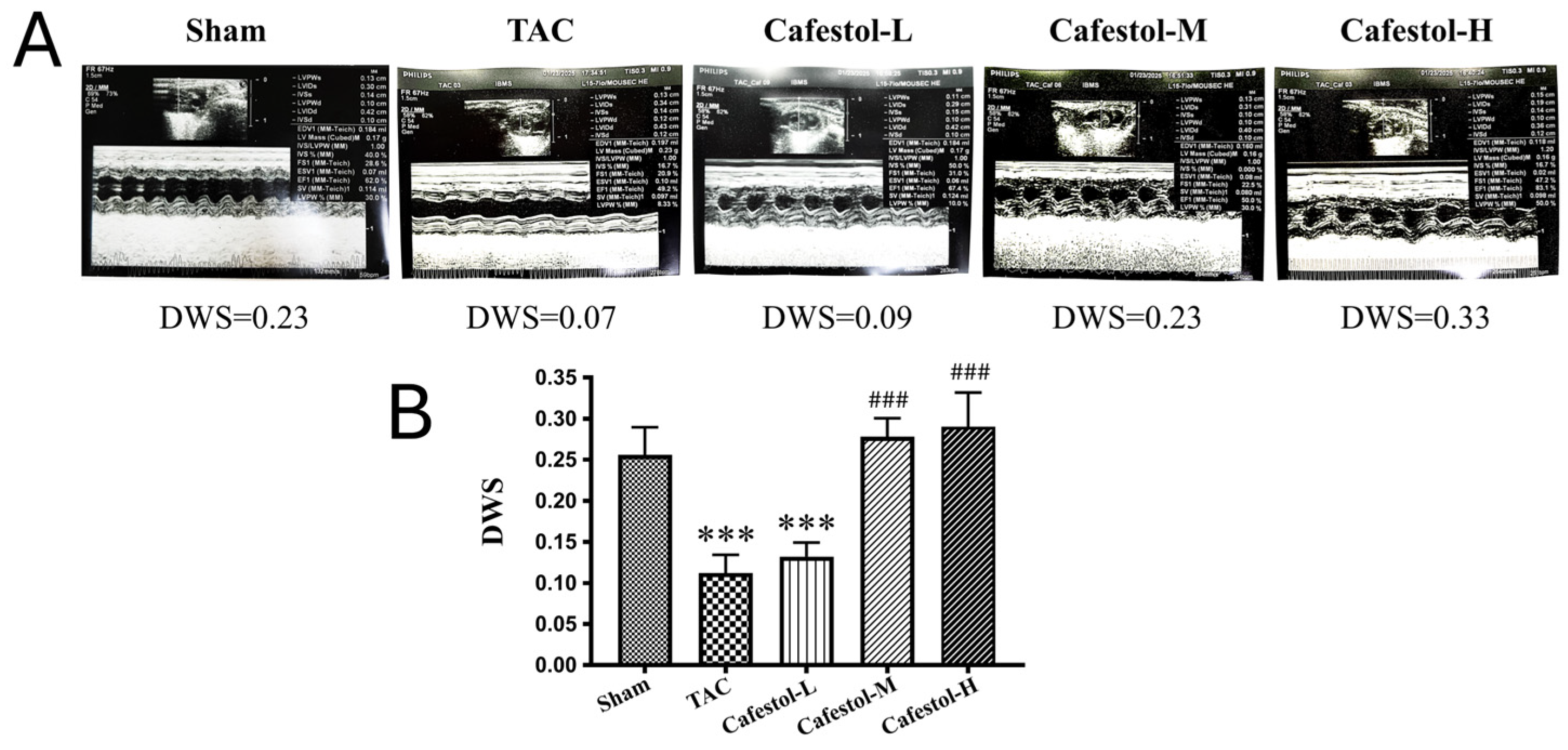
3.2. Cafestol Improves Cardiac Performance in TAC-Induced Hypertrophy
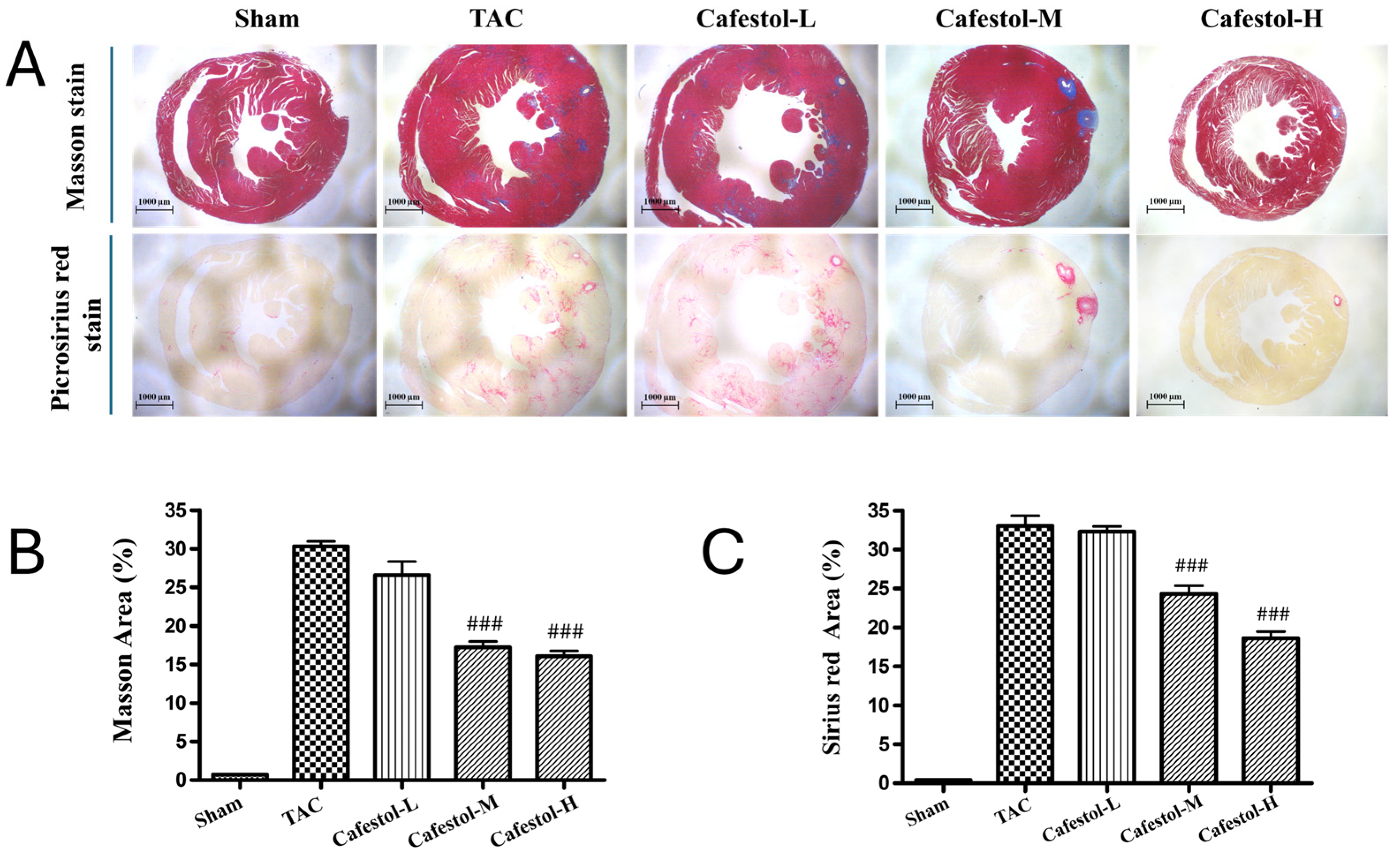
3.3. Cafestol Mitigates Histopathological Alterations in TAC-Induced Cardiac Hypertrophy
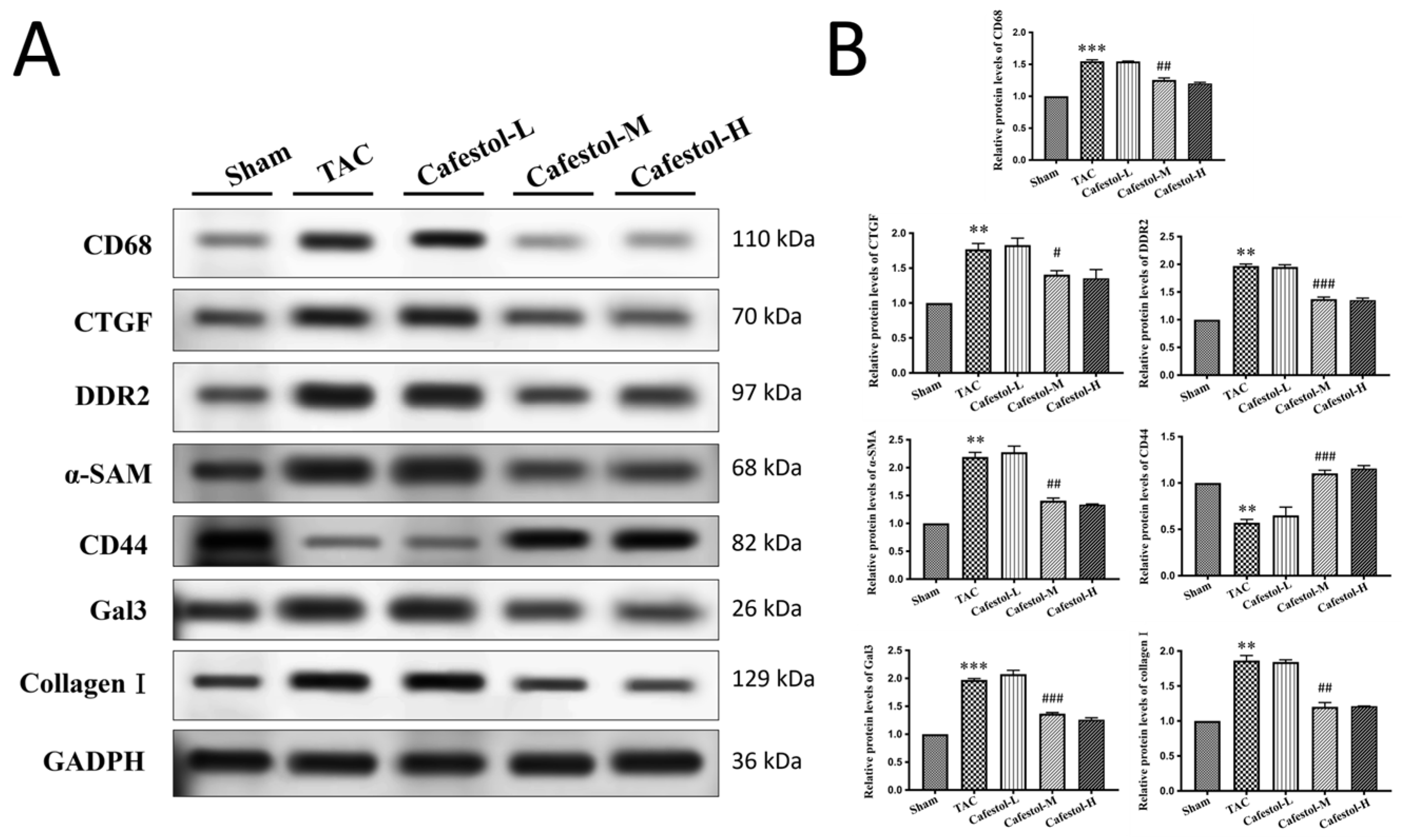
3.4. Cafestol Regulates Fibrotic and Inflammatory Signaling in TAC-Induced Cardiac Remodeling
3.5. Transmission Electron Microscopy Reveals Cafestol-Mediated Mitochondrial Protection in Hypertrophic Hearts
4. Discussion
4.1. Mitophagy as a Therapeutic Target
4.2. Antifibrotic and Anti-Inflammatory Effects of Cafestol
4.3. Apoptosis and Endoplasmic Reticulum Stress Modulation
4.4. Ultrastructural Insights from TEM
4.5. Dose-Dependent Effects and Translational Relevance
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TAC | Transverse Aortic Constriction |
| TEM | Transmission Electron Microscopy |
| HF | Heart Failure |
| ERK | Extracellular Signal-Regulated Kinase |
| mTOR | mammalian target of Rapamycin |
| BCA | Bicinchoninic Acid |
| PVDF | Polyvinylidene Fluoride |
| HRP | Horseradish Peroxidase |
| ECL | Enhanced Chemiluminescence |
| SD | Standard Deviation |
| ANOVA | Analysis of Variance |
| NIH | National Institutes of Health |
| IACUC | Institutional Animal Care and Use Committee |
| Gal-3 | Galectin-3 |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| SIRT1 | Sirtuin 1 |
| FUNDC1 | FUN14 Domain Containing 1 |
| LVEF | Left Ventricular Ejection Fraction |
| LVEDV | Left Ventricular End-Diastolic Volume |
| LVESV | Left Ventricular End-Systolic Volume |
| SV | Stroke Volume |
| LVIDs | Left Ventricular Internal Dimension at Systole |
| LVIDd | Left Ventricular Internal Dimension at Diastole |
| LVPWd | Left Ventricular Posterior Wall Thickness at Diastole |
| IVSs | Interventricular Septal Thickness at Systole |
| IVSd | Interventricular Septal Thickness at Diastole |
References
- Mavrogeni, S.; Piaditis, G.; Bacopoulou, F.; Chrousos, G.P. Cardiac Remodeling in Hypertension: Clinical Impact on Brain, Heart, and Kidney Function. Horm. Metab. Res. 2022, 54, 273–279. [Google Scholar] [CrossRef]
- Gaydarski, L.; Petrova, K.; Stanchev, S.; Pelinkov, D.; Iliev, A.; Dimitrova, I.N.; Kirkov, V.; Landzhov, B.; Stamenov, N. Morphometric and Molecular Interplay in Hypertension-Induced Cardiac Remodeling with an Emphasis on the Potential Therapeutic Implications. Int. J. Mol. Sci. 2025, 26, 4022. [Google Scholar] [CrossRef]
- Nishida, M.; Mi, X.; Ishii, Y.; Kato, Y.; Nishimura, A. Cardiac remodeling: Novel pathophysiological mechanisms and therapeutic strategies. J. Biochem. 2024, 176, 255–262. [Google Scholar] [CrossRef]
- Gomes, D.S.; Romanelli, M.A.; Gomes, S.P.S.; Brand, A.L.M.; da Silva, R.M.V.; Oliveira, S.S.C.; Santos, A.L.S.; Rezende, C.M.; Lara, L.S. Pharmacological Potential of Cafestol, a Bioactive Substance in Coffee, in Preventing Ischemia-Reperfusion-Induced Acute Kidney Injury. ACS Omega 2025, 10, 22825–22836. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.; Silva, A.; Andriolo, C.; Mellinger, C.; Uekane, T.; Garrett, R.; Rezende, C. Bioaccessibility of Cafestol from Coffee Brew: A Metabolic Study Employing an In Vitro Digestion Model and LC-HRMS. J. Agric. Food Chem. 2024, 72, 27876–27883. [Google Scholar] [CrossRef]
- Al-Kenany, S.A.; Al-Shawi, N.N. Protective effect of cafestol against doxorubicin-induced cardiotoxicity in rats by activating the Nrf2 pathway. Front. Pharmacol. 2023, 14, 1206782. [Google Scholar] [CrossRef]
- Belka, M.; Gostyńska-Stawna, A.; Stawny, M.; Krajka-Kuźniak, V. Activation of Nrf2 and FXR via Natural Compounds in Liver Inflammatory Disease. Int. J. Mol. Sci. 2024, 25, 11213. [Google Scholar] [CrossRef]
- Liu, J.C.; Chen, P.Y.; Hao, W.R.; Liu, Y.C.; Lyu, P.C.; Hong, H.J. Cafestol Inhibits High-Glucose-Induced Cardiac Fibrosis in Cardiac Fibroblasts and Type 1-Like Diabetic Rats. Evid. Based Complement. Alternat. Med. 2020, 2020, 4503747. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.R.; Sung, L.C.; Chen, C.C.; Chen, P.Y.; Cheng, T.H.; Chao, H.H.; Liu, J.C.; Chen, J.J. Cafestol Inhibits Cyclic-Strain-Induced Interleukin-8, Intercellular Adhesion Molecule-1, and Monocyte Chemoattractant Protein-1 Production in Vascular Endothelial Cells. Oxid. Med. Cell Longev. 2018, 2018, 7861518. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wei, J.; Liu, X.; Zhang, Z.; Huang, R.; Jiang, Z. Semaglutide ameliorates pressure overload-induced cardiac hypertrophy by improving cardiac mitophagy to suppress the activation of NLRP3 inflammasome. Sci. Rep. 2024, 14, 11824. [Google Scholar] [CrossRef]
- Yu, H.; Gan, D.; Luo, Z.; Yang, Q.; An, D.; Zhang, H.; Hu, Y.; Ma, Z.; Zeng, Q.; Xu, D.; et al. α-Ketoglutarate improves cardiac insufficiency through NAD(+)-SIRT1 signaling-mediated mitophagy and ferroptosis in pressure overload-induced mice. Mol. Med. 2024, 30, 15. [Google Scholar] [CrossRef]
- Abudureyimu, M.; Yu, W.; Cao, R.Y.; Zhang, Y.; Liu, H.; Zheng, H. Berberine Promotes Cardiac Function by Upregulating PINK1/Parkin-Mediated Mitophagy in Heart Failure. Front. Physiol. 2020, 11, 565751. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yin, L.; Sun, X.; Wu, J.; Dong, Z.; Hu, K.; Sun, A.; Ge, J. Alpha-lipoic acid protects against pressure overload-induced heart failure via ALDH2-dependent Nrf1-FUNDC1 signaling. Cell Death Dis. 2020, 11, 599. [Google Scholar] [CrossRef]
- Castiglioni, L.; Gelosa, P.; Muluhie, M.; Mercuriali, B.; Rzemieniec, J.; Gotti, M.; Fiordaliso, F.; Busca, G.; Sironi, L. Fenofibrate reduces cardiac remodeling by mitochondrial dynamics preservation in a renovascular model of cardiac hypertrophy. Eur. J. Pharmacol. 2024, 978, 176767. [Google Scholar] [CrossRef]
- Dong, X.; Zhuang, H.W.; Wen, R.J.; Huang, Y.S.; Liang, B.X.; Li, H.; Xian, S.X.; Li, C.; Wang, L.J.; Wang, J.Y. Xinyang tablet alleviated cardiac dysfunction in a cardiac pressure overload model by regulating the receptor-interacting serum/three-protein kinase 3/FUN14 domain containing 1-mediated mitochondrial unfolded protein response and mitophagy. J. Ethnopharmacol. 2024, 330, 118152. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, Z.; Li, X.; Zhang, H.; Han, D.; Xiong, W.; Zhou, H.; Yang, X.; Zeng, Q.; Ren, H.; et al. Metformin Collaborates with PINK1/Mfn2 Overexpression to Prevent Cardiac Injury by Improving Mitochondrial Function. Biology 2023, 12, 582. [Google Scholar] [CrossRef]
- Tan, Y.; Li, M.; Wu, G.; Lou, J.; Feng, M.; Xu, J.; Zhou, J.; Zhang, P.; Yang, H.; Dong, L.; et al. Short-term but not long-term high fat diet feeding protects against pressure overload-induced heart failure through activation of mitophagy. Life Sci. 2021, 272, 119242. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.M.; Hou, Y.C.; Tsai, K.W.; Hu, W.C.; Yang, H.C.; Liao, M.T.; Lu, K.C. Resveratrol Mitigates Uremic Toxin-Induced Intestinal Barrier Dysfunction in Chronic Kidney Disease by Promoting Mitophagy and Inhibiting Apoptosis Pathways. Int. J. Med. Sci. 2024, 21, 2437–2449. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, J.; Wang, Y.; Wang, X.; Ma, Q.; Li, Y.; Zhang, X.; Wang, S.; Zhang, Q.; Mi, F.; et al. Cafestol inhibits colon cancer cell proliferation and tumor growth in xenograft mice by activating LKB1/AMPK/ULK1-dependent autophagy. J. Nutr. Biochem. 2024, 129, 109623. [Google Scholar] [CrossRef]
- Heise, N.V.; Kozubek, M.; Hoenke, S.; Ludwig, S.; Deigner, H.P.; Al-Harrasi, A.; Csuk, R. Towards Cytotoxic Derivatives of Cafestol. Molecules 2025, 30, 2291. [Google Scholar] [CrossRef]
- Hao, W.R.; Cheng, C.H.; Chen, H.Y.; Cheng, T.H.; Liu, J.C.; Chen, J.J. Fucoidan Attenuates Cardiac Remodeling by Inhibiting Galectin-3 Secretion, Fibrosis, and Inflammation in a Mouse Model of Pressure Overload. Biomedicines 2024, 12, 2847. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X.; Xu, T.; Gan, D.; Ma, Z.; Zhang, H.; Zhang, J.; Zeng, Q.; Xu, D. PINK1-mediated mitophagy attenuates pathological cardiac hypertrophy by suppressing the mtDNA release-activated cGAS-STING pathway. Cardiovasc. Res. 2025, 121, 128–142. [Google Scholar] [CrossRef]
- Ji, J.; Wu, L.; Feng, J.; Mo, W.; Wu, J.; Yu, Q.; Li, S.; Zhang, J.; Dai, W.; Xu, X.; et al. Cafestol preconditioning attenuates apoptosis and autophagy during hepatic ischemia-reperfusion injury by inhibiting ERK/PPARγ pathway. Int. Immunopharmacol. 2020, 84, 106529. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, Z.; Li, J.; Li, Y.; Huang, H.; Yuan, F.; Liu, M.; Fang, Z. Qian Yang Yu Yin Granule prevents hypertensive cardiac remodeling by inhibiting NLRP3 inflammasome activation via Nrf2. J. Ethnopharmacol. 2025, 337, 118820. [Google Scholar] [CrossRef]
- Hao, W.R.; Sung, L.C.; Chen, C.C.; Hong, H.J.; Liu, J.C.; Chen, J.J. Cafestol Activates Nuclear Factor Erythroid-2 Related Factor 2 and Inhibits Urotensin II-Induced Cardiomyocyte Hypertrophy. Am. J. Chin. Med. 2019, 47, 337–350. [Google Scholar] [CrossRef]
- Park, J.B. Kahweol Found in Coffee Inhibits IL-2 Production Via Suppressing the Phosphorylations of ERK and c-Fos in Lymphocytic Jurkat Cells. J. Diet. Suppl. 2021, 18, 433–443. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Sung, L.C.; Haw, W.R.; Chen, C.C.; Huang, S.F.; Liu, J.C.; Cheng, T.H.; Chen, P.Y.; Loh, S.H.; Tsai, C.S. Cafestol, a coffee diterpene, inhibits urotensin II-induced interleukin-8 expression in human umbilical vein endothelial cells. Eur. J. Pharmacol. 2018, 820, 106–112. [Google Scholar] [CrossRef]
- Chaanine, A.H. Morphological Stages of Mitochondrial Vacuolar Degeneration in Phenylephrine-Stressed Cardiac Myocytes and in Animal Models and Human Heart Failure. Medicina 2019, 55, 239. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, W.C.; Zhang, Y.L.; Lin, Q.Y.; Liao, J.W.; Song, G.R.; Ma, X.L.; Li, H.H.; Zhang, B. SOCS3 Negatively Regulates Cardiac Hypertrophy via Targeting GRP78-Mediated ER Stress During Pressure Overload. Front. Cell Dev. Biol. 2021, 9, 629932. [Google Scholar] [CrossRef] [PubMed]

| Sham | TAC | Cafestol-L | Cafestol-M | Cafestol-H | |
|---|---|---|---|---|---|
| LVEDV (mL) | 0.131 ± 0.036 | 0.208 ± 0.01 ** | 0.177 ± 0.021 # | 0.195 ± 0.025 | 0.143 ± 0.025 ### |
| LVESV (mL) | 0.038 ± 0.018 | 0.094 ± 0.021 ** | 0.058 ± 0.013 ## | 0.068 ± 0.012 # | 0.034 ± 0.012 ### |
| SV (mL) | 0.093 ± 0.019 | 0.114 ± 0.018 | 0.119 ± 0.011 | 0.127 ± 0.028 | 0.109 ± 0.02 |
| LVd Mass | 0.112 ± 0.031 | 0.234 ± 0.034 *** | 0.168 ± 0.013 ## | 0.158 ± 0.023 ## | 0.158 ± 0.007 ## |
| LVIDs | 0.230 ± 0.043 | 0.332 ± 0.023 ** | 0.282 ± 0.02 ## | 0.298 ± 0.017 # | 0.232 ± 0.029 ### |
| LVIDd | 0.370 ± 0.036 | 0.438 ± 0.007 ** | 0.414 ± 0.017 # | 0.428 ± 0.019 | 0.384 ± 0.023 ## |
| LVPWd | 0.084 ± 0.008 | 0.116 ± 0.008 *** | 0.100 ± 0.000 ## | 0.088 ± 0.01 ## | 0.100 ± 0.000 ## |
| IVSs | 0.112 ± 0.015 | 0.138 ± 0.012 * | 0.138 ± 0.012 | 0.124 ± 0.017 | 0.144 ± 0.005 |
| IVSd | 0.084 ± 0.008 | 0.12 ± 0.0155 * | 0.100 ± 0.000 # | 0.096 ± 0.01 # | 0.106 ± 0.008 |
| EF | 72.48 ± 6.049 | 54.82 ± 8.803 ** | 67.62 ± 4.214 # | 64.46 ± 8.395 | 76.5 ± 7.052 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, W.-R.; Chen, C.-C.; Huang, G.-C.; Lin, J.-H.; Chen, H.-Y.; Liu, J.-C.; Cheng, T.-H.; Chen, J.-J. Therapeutic Modulation of Mitophagy by Cafestol in Pressure Overload-Induced Cardiac Hypertrophy and Fibrosis. Nutrients 2025, 17, 3680. https://doi.org/10.3390/nu17233680
Hao W-R, Chen C-C, Huang G-C, Lin J-H, Chen H-Y, Liu J-C, Cheng T-H, Chen J-J. Therapeutic Modulation of Mitophagy by Cafestol in Pressure Overload-Induced Cardiac Hypertrophy and Fibrosis. Nutrients. 2025; 17(23):3680. https://doi.org/10.3390/nu17233680
Chicago/Turabian StyleHao, Wen-Rui, Chun-Chao Chen, Guan-Ci Huang, Jia-Hong Lin, Huan-Yuan Chen, Ju-Chi Liu, Tzu-Hurng Cheng, and Jin-Jer Chen. 2025. "Therapeutic Modulation of Mitophagy by Cafestol in Pressure Overload-Induced Cardiac Hypertrophy and Fibrosis" Nutrients 17, no. 23: 3680. https://doi.org/10.3390/nu17233680
APA StyleHao, W.-R., Chen, C.-C., Huang, G.-C., Lin, J.-H., Chen, H.-Y., Liu, J.-C., Cheng, T.-H., & Chen, J.-J. (2025). Therapeutic Modulation of Mitophagy by Cafestol in Pressure Overload-Induced Cardiac Hypertrophy and Fibrosis. Nutrients, 17(23), 3680. https://doi.org/10.3390/nu17233680










