Gut-Protective and Multifunctional Exopolysaccharide from Enterococcus faecium HDRsEf1: Structural Characterization and Protective Effects Against Enteropathogenic E. coli-Induced Intestinal Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strains and Cells
2.3. Extraction and Purification of Crude EPS
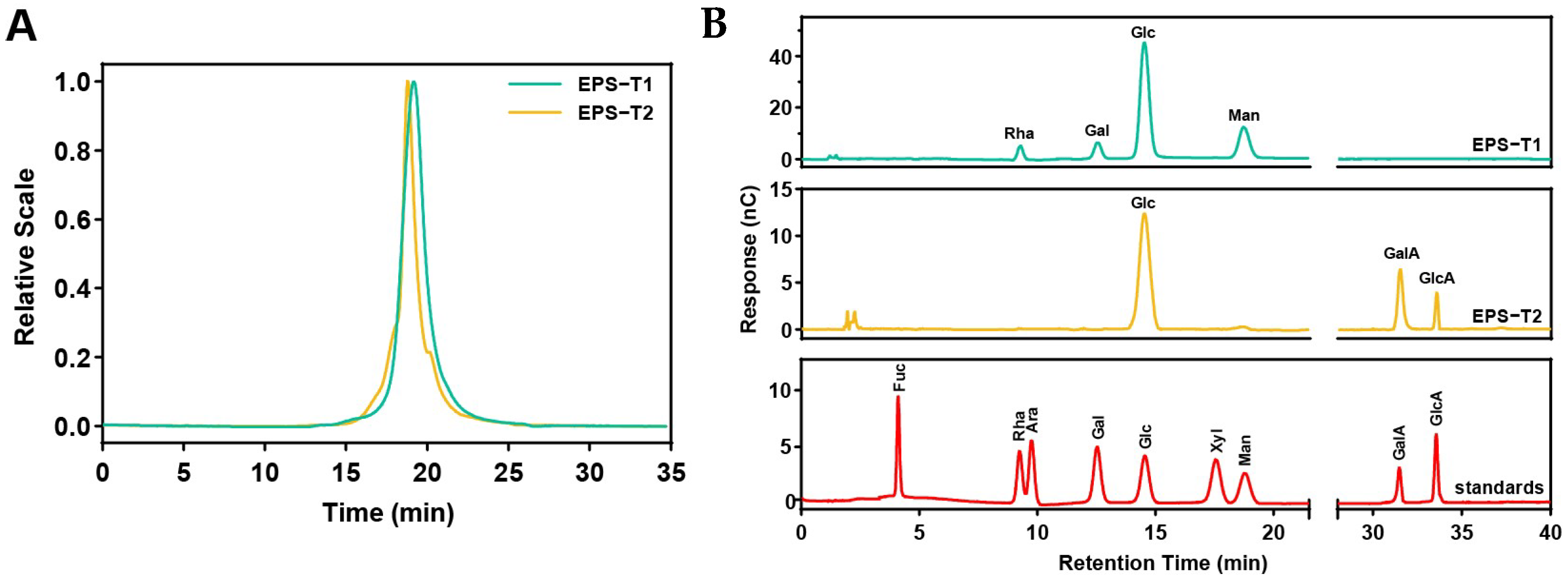
2.4. Determination of Molecular Weight and Monosaccharide Composition
2.5. Fourier-Transform Infrared Spectrometer (FT-IR) Analyses
2.6. Scanning Electron Microscopy (SEM)
2.7. Thermogravimetric (TG) and Differential Scanning Calorimetry (DSC) Analyses
2.8. Antibacterial Activity Assay
2.8.1. Minimum Inhibitory Concentration (MIC) Determination
2.8.2. Oxford Cup Agar Diffusion Assay
2.8.3. Transmission Electron Microscopy (TEM)
2.9. Antibiofilm Activity Assay
2.10. Biosafety Evaluation
2.11. Bacterial Adhesion Inhibition Assay
2.12. Antioxidant Activity Assay
2.12.1. ABTS Radical Scavenging Activity
2.12.2. DPPH Radical Scavenging Activity
2.12.3. Superoxide Anion Radical Scavenging Activity
2.12.4. Hydroxyl Radical Scavenging Activity
2.13. Animal Experiments
2.13.1. Animal Treatment and Administration
2.13.2. In Vivo and Ex Vivo Imaging of the Intestine
2.13.3. Fecal DNA Extraction and Quantification
2.13.4. Histological Examination
2.13.5. Cytokine Detection and Gene Expression Analysis
2.13.6. Antioxidant Analysis
2.14. Statistical Analysis
3. Results
3.1. Isolation and Purification of EPS
3.2. Molecular Weight and Monosaccharide Composition
3.3. FT-IR Spectroscopy and SEM
3.4. Thermal Analysis (TG and DSC)
3.5. Antibacterial Activity
3.6. Biofilm Inhibitory and Disruption Activities
3.7. Cell Viability
3.8. Inhibitory Effects on Adhesion
3.9. Antioxidant Activity
3.10. Animal Results
3.10.1. Body Weight Changes
3.10.2. In Vivo Colonization and Intestinal Bacterial Load of EPEC66-Lux
3.10.3. Histopathological Analyses
3.10.4. Inflammatory Cytokine Levels
3.10.5. Antioxidant Activity in Serum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EPS | Exopolysaccharides |
| LAB | Lactic acid bacteria |
| Ara | Arabinose |
| Fuc | Fucose |
| Gal | Galactose |
| GalA | Galacturonic acid |
| Glc | Glucose |
| Man | Mannose |
| Rha | Rhamnose |
| Xyl | xylose |
| CFU | Colony-forming units |
| MIC | Minimum inhibitory concentration |
| MOI | Multiplicity of infection |
| H&E | Staining hematoxylin-eosin staining |
| GSH-PX | Glutathione peroxidase |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| T-AOC | Total antioxidant capacity |
| NO | Nitric oxide |
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-1β | Interleukin-1β |
| TCA | Trichloroacetic acid |
References
- Farré, R.; Fiorani, M.; Abdu Rahiman, S.; Matteoli, G. Intestinal Permeability, Inflammation and the Role of Nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef]
- Brodin, P. Immune-microbe interactions early in life: A determinant of health and disease long term. Science 2022, 376, 945–950. [Google Scholar] [CrossRef]
- Beraldo, L.G.; Borges, C.A.; Maluta, R.P.; Cardozo, M.V.; de Ávila, F.A. Molecular analysis of enteropathogenic Escherichia coli (EPEC) isolates from healthy food-producing animals and humans with diarrhoea. Zoonoses Public Health 2023, 70, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Arais, L.R.; Barbosa, A.V.; Andrade, J.R.C.; Gomes, T.A.T.; Asensi, M.D.; Aires, C.A.M.; Cerqueira, A.M.F. Zoonotic potential of atypical enteropathogenic Escherichia coli (aEPEC) isolated from puppies with diarrhoea in Brazil. Vet. Microbiol. 2018, 227, 45–51. [Google Scholar] [CrossRef]
- Lee, J.B.; Kim, S.K.; Yoon, J.W. Pathophysiology of enteropathogenic Escherichia coli during a host infection. J. Vet. Sci. 2022, 23, e28. [Google Scholar] [CrossRef]
- Clarke, S.C.; Haigh, R.D.; Freestone, P.P.; Williams, P.H. Virulence of enteropathogenic Escherichia coli, a global pathogen. Clin. Microbiol. Rev. 2003, 16, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, Q.; Zhang, J.; Zhu, X. Occurrence and Characterization of Enteropathogenic Escherichia coli (EPEC) in Retail Ready-to-Eat Foods in China. Foodborne Pathog. Dis. 2016, 13, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ju, Y.; Liu, N.; Shi, S.; Hao, L. Structural characteristics of microbial exopolysaccharides in association with their biological activities: A review. Chem. Biol. Technol. Agric. 2023, 10, 137. [Google Scholar] [CrossRef]
- Ayyash, M.; Stathopoulos, C.; Abu-Jdayil, B.; Esposito, G.; Baig, M.; Turner, M.S.; Baba, A.S.; Apostolopoulos, V.; Al-Nabulsi, A.; Osaili, T. Exopolysaccharide produced by potential probiotic Enterococcus faecium MS79: Characterization, bioactivities and rheological properties influenced by salt and pH. LWT 2020, 131, 109741. [Google Scholar] [CrossRef]
- Tan, X.; Ma, B.; Wang, X.; Cui, F.; Li, X.; Li, J. Characterization of Exopolysaccharides from Lactiplantibacillus plantarum PC715 and Their Antibiofilm Activity Against Hafnia alvei. Microorganisms 2024, 12, 2229. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, A.; Lee, H.-J. Antioxidant potential of exopolysaccharides from lactic acid bacteria: A comprehensive review. Int. J. Biol. Macromol. 2024, 281, 135536. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, M.-M.; Li, X.; Ding, Y.-R.; Wei, X.-Y.; Zhou, T. Antioxidant and anti-inflammatory activities and action mechanisms of exopolysaccharides from Lactiplantibacillus plantarum Z-1. Food Biosci. 2024, 62, 105247. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Xiao, L.; Zhao, X.; Ma, K.; Ji, F.; Azarpazhooh, E.; Ajami, M.; Rui, X.; Li, W. Digestive characteristics of extracellular polysaccharide from Lactiplantibacillus plantarum T1 and its regulation of intestinal microbiota. Int. J. Biol. Macromol. 2024, 259, 129112. [Google Scholar] [CrossRef]
- Tarique, M.; Ali, A.H.; Kizhakkayil, J.; Liu, S.; Oz, F.; Dertli, E.; Kamal-Eldin, A.; Ayyash, M. Exopolysaccharides from Enterococcus faecium and Streptococcus thermophilus: Bioactivities, gut microbiome effects, and fermented milk rheology. Food Chem. X 2024, 21, 101073. [Google Scholar] [CrossRef]
- Kavitake, D.; Devi, P.B.; Delattre, C.; Reddy, G.B.; Shetty, P.H. Exopolysaccharides produced by Enterococcus genus—An overview. Int. J. Biol. Macromol. 2023, 226, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liang, W.; Lu, Y.; Xiong, J.; Liu, D.; Jia, X. Identification, Antioxidant and Immunomodulatory Activities of a Neutral Exopolysaccharide from Lactiplantibacillus plantarum DMDL 9010. Nutrients 2025, 17, 2265. [Google Scholar] [CrossRef]
- Du, R.; Yu, L.; Yu, N.; Ping, W.; Song, G.; Ge, J. Characterization of exopolysaccharide produced by Levilactobacillus brevis HDE-9 and evaluation of its potential use in dairy products. Int. J. Biol. Macromol. 2022, 217, 303–311. [Google Scholar] [CrossRef]
- Ding, C.; Wu, H.; Cao, X.; Gao, Z.; Tang, Z.; Fan, W.; Yan, L.; Liu, B.; Lin, H.; Song, S. Lactobacillus crispatus-derived exopolysaccharides with antibacterial activity limit Salmonella typhimurium invasion by inhibiting inflammasome-mediated pyroptosis. Food Funct. 2022, 13, 10501–10515. [Google Scholar] [CrossRef]
- Abedin, M.M.; Chourasia, R.; Phukon, L.C.; Sarkar, P.; Ray, R.C.; Singh, S.P.; Rai, A.K. Lactic acid bacteria in the functional food industry: Biotechnological properties and potential applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 10730–10748. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Xiao, Y.; Bi, D.; Xiong, Y.; Wang, X.; Gao, X.; Li, Z.; Zhou, Z.; Liu, M.; Xu, Q. A Beneficial Enterococcus faecium Strain’s Screening and Application. CN102747003B, 13 November 2013. [Google Scholar]
- Zhuo, W.X.; Zhao, Y.; Zhao, X.L.; Yao, Z.M.; Qiu, X.X.; Huang, Y.X.; Li, H.X.; Shen, J.; Zhu, Z.H.; Li, T.T.; et al. Enteropathogenic Escherichia coli is a predominant pathotype in healthy pigs in Hubei Province of China. J. Appl. Microbiol. 2023, 134, lxad260. [Google Scholar] [CrossRef]
- Lv, H.; Teng, Q.; Chen, J.; Peng, L.; Ren, Z.; Ma, L.; Yang, W.; Yu, B.; Wu, Z.; Wan, C. Probiotic potential of a novel exopolysaccharide produced by Bifidobacterium animalis subsp. Lactis SF. LWT 2024, 193, 115764. [Google Scholar] [CrossRef]
- Akkerman, R.; Oerlemans, M.M.P.; Ferrari, M.; Fernández-Lainez, C.; Walvoort, M.T.C.; de Vos, P. Exopolysaccharides from Bifidobacterium longum subsp. infantis and Bifidobacterium adolescentis modulate Toll-like receptor signaling. Carbohydr. Polym. 2025, 349, 123017. [Google Scholar] [CrossRef] [PubMed]
- Pramudito, T.E.; Desai, K.; Voigt, C.; Smid, E.J.; Schols, H.A. Dextran and levan exopolysaccharides from tempeh-associated lactic acid bacteria with bioactivity against enterotoxigenic Escherichia coli (ETEC). Carbohydr. Polym. 2024, 328, 121700. [Google Scholar] [CrossRef]
- Tilwani, Y.M.; Lakra, A.K.; Domdi, L.; Yadav, S.; Jha, N.; Arul, V. Optimization and physicochemical characterization of low molecular levan from Enterococcus faecium MC-5 having potential biological activities. Process Biochem. 2021, 110, 282–291. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, J.; Zhou, H.; Wu, Z.; Qiu, Y.; Ye, C. Dihydromyricetin alleviates ETEC K88-induced intestinal inflammatory injury by inhibiting quorum sensing-related virulence factors. BMC Microbiol. 2025, 25, 201. [Google Scholar] [CrossRef]
- Zhao, H.; Abbas, S.; Ren, J.; Huang, H.; Song, Y.; Su, X.; Wu, Q.; Ma, Y.; Tang, H.; Gao, Y.Z.; et al. Dextran from human feces-derived Weissella cibaria facilitates intestinal mucosal barrier function by modulating gut bacteria and propionate levels. Carbohydr. Polym. 2025, 354, 123300. [Google Scholar] [CrossRef] [PubMed]
- Tilwani, Y.M.; Lakra, A.K.; Domdi, L.; Jha, N.; Arul, V. Characterization of potential probiotic bacteria Enterococcus faecium MC-5 isolated from the gut content of Cyprinus carpio specularis. Microb. Pathog. 2022, 172, 105783. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhou, H.; Zhao, H.; Pei, Y.; Su, F.; Li, Y.; Luo, T. Isolation, In Vitro Antioxidant Capacity, Hypoglycemic Activity and Immunoactivity Evaluation of Polysaccharides from Coriandrum sativum L. Antioxidants 2025, 14, 149. [Google Scholar] [CrossRef]
- Ledwaba, S.E.; Costa, D.V.S.; Bolick, D.T.; Giallourou, N.; Medeiros, P.; Swann, J.R.; Traore, A.N.; Potgieter, N.; Nataro, J.P.; Guerrant, R.L. Enteropathogenic Escherichia coli Infection Induces Diarrhea, Intestinal Damage, Metabolic Alterations, and Increased Intestinal Permeability in a Murine Model. Front. Cell. Infect. Microbiol. 2020, 10, 595266. [Google Scholar] [CrossRef]
- MacArthur Clark, J.A.; Sun, D. Guidelines for the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892-2018 [Issued 6 February 2018 Effective from 1 September 2018]. Animal Model Exp Med. 2020, 3, 103–113. [Google Scholar] [CrossRef]
- Wong, D.V.; Lima-Júnior, R.C.; Carvalho, C.B.; Borges, V.F.; Wanderley, C.W.; Bem, A.X.; Leite, C.A.; Teixeira, M.A.; Batista, G.L.; Silva, R.L.; et al. The Adaptor Protein Myd88 Is a Key Signaling Molecule in the Pathogenesis of Irinotecan-Induced Intestinal Mucositis. PLoS ONE 2015, 10, e0139985. [Google Scholar] [CrossRef]
- Unkovič, A.; Boštjančič, E.; Belič, A.; Perše, M. Selection and Evaluation of mRNA and miRNA Reference Genes for Expression Studies (qPCR) in Archived Formalin-Fixed and Paraffin-Embedded (FFPE) Colon Samples of DSS-Induced Colitis Mouse Model. Biology 2023, 12, 190. [Google Scholar] [CrossRef]
- Ren, Y.; Pei, F.; Cao, X.; Zhang, W.; Du, R.; Ge, J.; Ping, W. Purification of exopolysaccharides from Lactobacillus rhamnosus and changes in their characteristics by regulating quorum sensing genes via polyphenols. Int. J. Biol. Macromol. 2023, 240, 124414. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, K.; Zhang, N.; Chitrakar, B.; Huang, P.; Wang, X.; Yang, B.; Sang, Y. Structural characterization and immunomodulatory effects of extracellular polysaccharide from Lactobacillus paracasei VL8 obtained by gradient ethanol precipitation. J. Food Sci. 2022, 87, 2034–2047. [Google Scholar] [CrossRef]
- Yuksekdag, Z.; Ahlatcı, N.S.; Hajikhani, R.; Darilmaz, D.O.; Beyatli, Y. Safety and metabolic characteristics of 17 Enterococcus faecium isolates. Arch. Microbiol. 2021, 203, 5683–5694. [Google Scholar] [CrossRef]
- Bhat, B.; Bajaj, B.K. Hypocholesterolemic and bioactive potential of exopolysaccharide from a probiotic Enterococcus faecium K1 isolated from kalarei. Bioresour. Technol. 2018, 254, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, P.; Balraj, M.; Ayyanna, R.; Ankaiah, D.; Arul, V. Physicochemical and biosorption properties of novel exopolysaccharide produced by Enterococcus faecalis. LWT 2016, 68, 606–614. [Google Scholar] [CrossRef]
- Jia, K.; Tao, X.; Liu, Z.; Zhan, H.; He, W.; Zhang, Z.; Zeng, Z.; Wei, H. Characterization of novel exopolysaccharide of Enterococcus faecium WEFA23 from infant and demonstration of its in vitro biological properties. Int. J. Biol. Macromol. 2019, 128, 710–717. [Google Scholar] [CrossRef]
- Ali, A.H.; Bamigbade, G.; Tarique, M.; Esposito, G.; Obaid, R.; Abu-Jdayil, B.; Ayyash, M. Physicochemical, rheological, and bioactive properties of exopolysaccharide produced by a potential probiotic Enterococcus faecalis 84B. Int. J. Biol. Macromol. 2023, 240, 124425. [Google Scholar] [CrossRef] [PubMed]
- Kansandee, W.; Moonmangmee, D.; Moonmangmee, S.; Itsaranuwat, P. Characterization and Bifidobacterium sp. growth stimulation of exopolysaccharide produced by Enterococcus faecalis EJRM152 isolated from human breast milk. Carbohydr. Polym. 2019, 206, 102–109. [Google Scholar] [CrossRef]
- Dong, J.; Chi, Z.; Lu, S.; Xie, X.; Gong, P.; Li, H.; Liu, W. Bacterial exopolysaccharides: Characteristics and antioxidant mechanism. Int. J. Biol. Macromol. 2025, 289, 138849. [Google Scholar] [CrossRef]
- Salimi, F.; Farrokh, P. Recent advances in the biological activities of microbial exopolysaccharides. World J. Microbiol. Biotechnol. 2023, 39, 213. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Xu, D.; Ma, K.; Zhang, C.; Wang, G.; Dong, M.; Li, W. Structural and prebiotic activity analysis of the polysaccharide produced by Lactobacillus helveticus SNA12. Carbohydr. Polym. 2022, 296, 119971. [Google Scholar] [CrossRef]
- Abid, Y.; Casillo, A.; Gharsallah, H.; Joulak, I.; Lanzetta, R.; Corsaro, M.M.; Attia, H.; Azabou, S. Production and structural characterization of exopolysaccharides from newly isolated probiotic lactic acid bacteria. Int. J. Biol. Macromol. 2018, 108, 719–728. [Google Scholar] [CrossRef]
- Vazquez-Rodriguez, A.; Vasto-Anzaldo, X.G.; Barboza Perez, D.; Vázquez-Garza, E.; Chapoy-Villanueva, H.; García-Rivas, G.; Garza-Cervantes, J.A.; Gómez-Lugo, J.J.; Gomez-Loredo, A.E.; Garza Gonzalez, M.T.; et al. Microbial Competition of Rhodotorula mucilaginosa UANL-001L and E. coli increase biosynthesis of Non-Toxic Exopolysaccharide with Applications as a Wide-Spectrum Antimicrobial. Sci. Rep. 2018, 8, 798. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Suganya, K.; Kumar, R.S.; Yuvaraj, N.; Pattukumar, V.; Paari, K.A.; Arul, V. Synthesis and functional characterization of antibiofilm exopolysaccharide produced by Enterococcus faecium MC13 isolated from the gut of fish. Appl. Biochem. Biotechnol. 2013, 169, 1001–1015. [Google Scholar] [CrossRef]
- Ying, M.; Yu, Q.; Zheng, B.; Wang, H.; Wang, J.; Chen, S.; Nie, S.; Xie, M. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr. Polym. 2020, 235, 115957. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, X.; Tang, C.; Wang, X.; Zhang, X.; Xiao, L.; Li, W. Protective effect of Paecilomyces cicadae TJJ11213 exopolysaccharide on intestinal mucosa and regulation of gut microbiota in immunosuppressed mice. Food Res. Int. 2023, 165, 112477. [Google Scholar] [CrossRef]
- Kšonžeková, P.; Bystrický, P.; Vlčková, S.; Pätoprstý, V.; Pulzová, L.; Mudroňová, D.; Kubašková, T.; Csank, T.; Tkáčiková, Ľ. Exopolysaccharides of Lactobacillus reuteri: Their influence on adherence of E. coli to epithelial cells and inflammatory response. Carbohydr. Polym. 2016, 141, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Pramudito, T.E.; Klostermann, C.; Smid, E.J.; Schols, H.A. Modulation of soy flour bioactivity against enterotoxigenic Escherichia coli by fermentation with exopolysaccharides-producing lactic acid bacteria. Carbohydr. Polym. 2025, 348, 122922. [Google Scholar] [CrossRef] [PubMed]
- Nehal, F.; Sahnoun, M.; Smaoui, S.; Jaouadi, B.; Bejar, S.; Mohammed, S. Characterization, high production and antimicrobial activity of exopolysaccharides from Lactococcus lactis F-mou. Microb. Pathog. 2019, 132, 10–19. [Google Scholar] [CrossRef]
- Rahbar Saadat, Y.; Yari Khosroushahi, A.; Pourghassem Gargari, B. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef]
- Bhat, B.; Bajaj, B.K. Hypocholesterolemic potential and bioactivity spectrum of an exopolysaccharide from a probiotic isolate Lactobacillus paracasei M7. Bioact. Carbohydr. Diet. Fibre 2019, 19, 100191. [Google Scholar] [CrossRef]
- Sharma, V.; Ghosh, M. Characterization of immunomodulatory, anticancer and antioxidant properties of an extracellular polymer produced by Enterococcus sp. in vegetable waste medium. Environ. Sustain. 2021, 4, 419–428. [Google Scholar] [CrossRef]
- Choudhuri, I.; Khanra, K.; Pariya, P.; Maity, G.N.; Mondal, S.; Pati, B.R.; Bhattacharyya, N. Structural Characterization of an Exopolysaccharide Isolated from Enterococcus faecalis, and Study on its Antioxidant Activity, and Cytotoxicity Against HeLa Cells. Curr. Microbiol. 2020, 77, 3125–3135. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.; Jayaraman, G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res. 2020, 487, 107881. [Google Scholar] [CrossRef] [PubMed]
- Ledwaba, S.E.; Bolick, D.T.; de Medeiros, P.; Kolling, G.L.; Traore, A.N.; Potgieter, N.; Nataro, J.P.; Guerrant, R.L. Enteropathogenic Escherichia coli (EPEC) expressing a non-functional bundle-forming pili (BFP) also leads to increased growth failure and intestinal inflammation in C57BL/6 mice. Braz. J. Microbiol. 2022, 53, 1781–1787. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Peng, X.; Zhu, Y.; Duan, W.; Ji, R.; Xiao, H.; Li, X.; Liu, G.; Yu, Y.; et al. Anti-inflammation mechanisms of a homogeneous polysaccharide from Phyllanthus emblica L. on DSS induced colitis mice via the gut microbiota and metabolites alteration. Food Chem. 2024, 459, 140346. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, J.; Liu, W.; Pi, X.; Zhou, Q.; Li, P.; Zhou, T.; Gu, Q. Effects of exopolysaccharide from Lactobacillus rhamnosus on human gut microbiota in in vitro fermentation model. LWT 2021, 139, 110524. [Google Scholar] [CrossRef]
- Peng, P.; Feng, T.; Yang, X.; Ding, R.; Wang, J.; Chen, P.; Guo, Y.; Li, P. Bioorthogonal conjugation and responsive nanocoating of probiotics for inflammatory bowel disease. J. Control. Release 2024, 374, 538–549. [Google Scholar] [CrossRef]
- Xie, A.; Ji, H.; Liu, Z.; Wan, Y.; Zhang, X.; Xiong, H.; Nie, S.P.; Wan, H. Modified Prebiotic-Based “Shield” Armed Probiotics with Enhanced Resistance of Gastrointestinal Stresses and Prolonged Intestinal Retention for Synergistic Alleviation of Colitis. ACS Nano 2023, 17, 14775–14791. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, M.; Yao, M.; Naseeb, J.; Sarwar, A.; Yang, Z.; Aziz, T.; Alhomrani, M.; Alsanie, W.F.; Alamri, A.S. Lactiplantibacillus plantarum NMGL2 exopolysaccharide ameliorates DSS-induced IBD in mice mainly by regulation of intestinal tight junction and NF-κB p65 protein expression. Front. Microbiol. 2024, 15, 1491727. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, M.; Ren, F. A Role of Exopolysaccharide Produced by Streptococcus thermophilus in the Intestinal Inflammation and Mucosal Barrier in Caco-2 Monolayer and Dextran Sulphate Sodium-Induced Experimental Murine Colitis. Molecules 2019, 24, 513. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, Q.; Liu, Y.; Yang, B.; Ahmed Sadiqb, F.; Li, X.; Mi, S.; Sang, Y. Immunoregulatory effect of Lactobacillus paracasei VL8 exopolysaccharide on RAW264.7 cells by NF-κB and MAPK pathways. J. Funct. Foods 2022, 95, 105166. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Wu, Q.; Gao, N.; Wang, Z.; Yang, Y.; Shan, A. Exopolysaccharides of Lactobacillus rhamnosus GG ameliorate Salmonella typhimurium-induced intestinal inflammation via the TLR4/NF-κB/MAPK pathway. J. Anim. Sci. Biotechnol. 2023, 14, 23. [Google Scholar] [CrossRef]
- Wang, K.; Niu, M.; Yao, D.; Zhao, J.; Wu, Y.; Lu, B.; Zheng, X. Physicochemical characteristics and in vitro and in vivo antioxidant activity of a cell-bound exopolysaccharide produced by Lactobacillus fermentum S1. Int. J. Biol. Macromol. 2019, 139, 252–261. [Google Scholar] [CrossRef]
- Huang, Q.; Liang, J.; Yang, C.; Li, K.; Niu, M.; Fan, J.; Zhang, X. Stimulation-responsive mucoadhesive probiotics for inflammatory bowel disease treatment by scavenging reactive oxygen species and regulating gut microbiota. Biomaterials 2023, 301, 122274. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Li, X.; Wu, Y.; Wang, Z.; Cui, W.; Hu, S.; Shi, D.; Huang, Q.; Xiao, Y.; Zhou, H.; et al. Gut-Protective and Multifunctional Exopolysaccharide from Enterococcus faecium HDRsEf1: Structural Characterization and Protective Effects Against Enteropathogenic E. coli-Induced Intestinal Inflammation. Nutrients 2025, 17, 3667. https://doi.org/10.3390/nu17233667
Dong Z, Li X, Wu Y, Wang Z, Cui W, Hu S, Shi D, Huang Q, Xiao Y, Zhou H, et al. Gut-Protective and Multifunctional Exopolysaccharide from Enterococcus faecium HDRsEf1: Structural Characterization and Protective Effects Against Enteropathogenic E. coli-Induced Intestinal Inflammation. Nutrients. 2025; 17(23):3667. https://doi.org/10.3390/nu17233667
Chicago/Turabian StyleDong, Zeyuan, Xinyang Li, Yaxin Wu, Zhaoyang Wang, Weitao Cui, Sishun Hu, Deshi Shi, Qi Huang, Yuncai Xiao, Hongbo Zhou, and et al. 2025. "Gut-Protective and Multifunctional Exopolysaccharide from Enterococcus faecium HDRsEf1: Structural Characterization and Protective Effects Against Enteropathogenic E. coli-Induced Intestinal Inflammation" Nutrients 17, no. 23: 3667. https://doi.org/10.3390/nu17233667
APA StyleDong, Z., Li, X., Wu, Y., Wang, Z., Cui, W., Hu, S., Shi, D., Huang, Q., Xiao, Y., Zhou, H., Li, Z., & Zhou, Z. (2025). Gut-Protective and Multifunctional Exopolysaccharide from Enterococcus faecium HDRsEf1: Structural Characterization and Protective Effects Against Enteropathogenic E. coli-Induced Intestinal Inflammation. Nutrients, 17(23), 3667. https://doi.org/10.3390/nu17233667







