Influence of Diets Differing in Macronutrient Composition on Metabolic Regulation During Exercise in Adults with Type 1 Diabetes
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Design and Ethical Approval
2.2. Screening Procedures and Eligibility Criteria
2.3. Composition of the Diets
2.4. Experimental Trial Day Procedures
2.5. Blood Sampling Procedures
2.6. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Metabolic and Hormonal Responses During Laboratory Trial Days
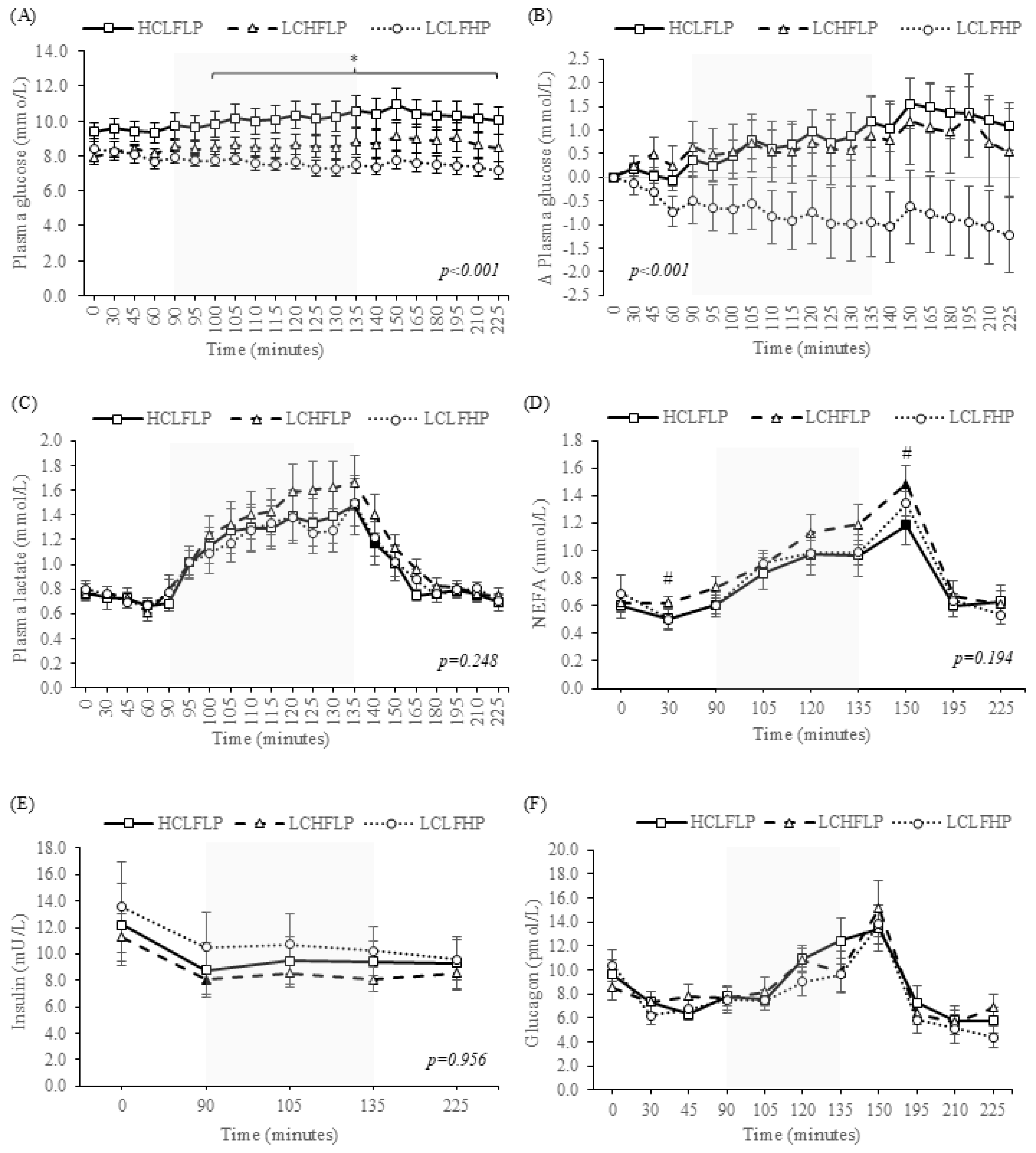
3.2.1. Plasma Glucose Responses
3.2.2. Plasma Lactate Responses
3.2.3. Non-Esterified Fatty Acid Responses
3.2.4. Insulin Responses
3.2.5. Glucagon Responses
3.3. Cardiopulmonary and Calorimetric Data
4. Discussion
4.1. Substrate Availability and Fuel Oxidation
4.2. Glucose Responses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065. [Google Scholar] [CrossRef]
- American Diabetes Association. 5. Lifestyle Management: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42 (Suppl. S1), S46–S60. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.P.; McCarthy, O.M.; Hoeg-Jensen, T.; Wellman, B.M.; Bracken, R.M. Factors Influencing Insulin Absorption Around Exercise in Type 1 Diabetes. Front. Endocrinol. 2020, 11, 793. [Google Scholar] [CrossRef]
- McCarthy, O.M.; Christensen, M.B.; Kristensen, K.B.; Schmidt, S.; Ranjan, A.G.; Bain, S.C.; Bracken, R.M.; Nørgaard, K. Automated Insulin Delivery Around Exercise in Adults with Type 1 Diabetes: A Pilot Randomized Controlled Study. Diabetes Technol. Ther. 2023, 25, 476–484. [Google Scholar] [CrossRef]
- McCarthy, O.; Deere, R.; Churm, R.; Dunseath, G.J.; Jones, C.; Eckstein, M.L.; Williams, D.M.; Hayes, J.; Pitt, J.; Bain, S.C.; et al. Extent and prevalence of post-exercise and nocturnal hypoglycemia following peri-exercise bolus insulin adjustments in individuals with type 1 diabetes. Nutr. Metab. Cardiovasc. Dis. 2020, 31, 227–236. [Google Scholar] [CrossRef]
- Riddell, M.C.; Gallen, I.W.; Smart, C.E.; Taplin, C.E.; Adolfsson, P.; Lumb, A.N.; Kowalski, A.; Rabasa-Lhoret, R.; McCrimmon, R.J.; Hume, C.; et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017, 5, 377–390. [Google Scholar] [CrossRef]
- Adolfsson, P.; Riddell, M.C.; Taplin, C.E.; Davis, E.A.; Fournier, P.A.; Annan, F.; Scaramuzza, A.E.; Hasnani, D.; Hofer, S.E. ISPAD Clinical Practice Consensus Guidelines 2018: Exercise in children and adolescents with diabetes. Pediatr. Diabetes 2018, 19, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, M.J.; Bach, C.W.; Baur, D.A. Pre-Exercise Nutrition: The Role of Macronutrients, Modified Starches and Supplements on Metabolism and Endurance Performance. Nutrients 2014, 6, 1782. [Google Scholar] [CrossRef] [PubMed]
- van Hall, G. The Physiological Regulation of Skeletal Muscle Fatty Acid Supply and Oxidation During Moderate-Intensity Exercise. Sports Med. 2015, 45 (Suppl. S1), 23. [Google Scholar] [CrossRef]
- Hawley, J.A.; Burke, L.M.; Angus, D.J.; Fallon, K.E.; Martin, D.T.; Febbraio, M.A. Effect of altering substrate availability on metabolism and performance during intense exercise. Br. J. Nutr. 2000, 84, 829–838. [Google Scholar] [CrossRef]
- Helge, J.W.; Watt, P.W.; Richter, E.A.; Rennie, M.J.; Kiens, B. Fat utilization during exercise: Adaptation to a fat-rich diet increases utilization of plasma fatty acids and very low density lipoprotein-triacylglycerol in humans. J. Physiol. 2001, 537, 1009–1020. [Google Scholar] [CrossRef]
- Kiens, B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol. Rev. 2006, 86, 205–243. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Angus, D.J.; Cox, G.R.; Cummings, N.K.; Febbraio, M.A.; Gawthorn, K.; Hawley, J.A.; Minehan, M.; Martin, D.T.; Hargreaves, M. Effect of fat adaptation and carbohydrate restoration on metabolism and performance during prolonged cycling. J. Appl. Physiol. 2000, 89, 2413–2421. [Google Scholar] [CrossRef]
- Stellingwerff, T.; Spriet, L.L.; Watt, M.J.; Kimber, N.E.; Hargreaves, M.; Hawley, J.A.; Burke, L.M. Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E380–E388. [Google Scholar] [CrossRef]
- Burke, L.M.; Hawley, J.A.; Angus, D.J.; Cox, G.R.; Clark, S.A.; Cummings, N.K.; Desbrow, B.; Hargreaves, M. Adaptations to short-term high-fat diet persist during exercise despite high carbohydrate availability. Med. Sci. Sports Exerc. 2002, 34, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M. Re-Examining High-Fat Diets for Sports Performance: Did We Call the ‘Nail in the Coffin’ Too Soon? Sports Med. 2015, 45 (Suppl. S1), 33. [Google Scholar] [CrossRef] [PubMed]
- Goedecke, J.H.; Christie, C.; Wilson, G.; Dennis, S.C.; Noakes, T.D.; Hopkins, W.G.; Lambert, E.V. Metabolic adaptations to a high-fat diet in endurance cyclists. Metabolism 1999, 48, 1509–1517. [Google Scholar] [CrossRef]
- De Bock, K.; Richter, E.A.; Russell, A.; Eijnde, B.O.; Derave, W.; Ramaekers, M.; Koninckx, E.; Léger, B.; Verhaeghe, J.; Hespel, P. Exercise in the fasted state facilitates fibre type-specific intramyocellular lipid breakdown and stimulates glycogen resynthesis in humans. J. Physiol. 2005, 564 Pt 2, 649. [Google Scholar] [CrossRef]
- Fel, S.; Rochette, E.; Walther, G.; Echaubard, S.; Pereira, B.; Merlin, E.; Terral, D.; Duché, P. Maximal Fat Oxidation During Exercise Is Already Impaired in Pre-pubescent Children with Type 1 Diabetes Mellitus. Front. Physiol. 2021, 12, 664211. [Google Scholar] [CrossRef]
- Dumke, C.L.; Keck, N.A.; Mcarthur, M.C.; Corcoran, M.H. Patients with Type 1 Diabetes Oxidize Fat at a Greater Rate than Age– and Sex–Matched Controls. Phys. Sportsmed. 2013, 41, 78–85. [Google Scholar] [CrossRef]
- Robitaille, M.; Dubé, M.-C.; Weisnagel, S.J.; Prud'HOmme, D.; Massicotte, D.; Péronnet, F.; Lavoie, C. Substrate source utilization during moderate intensity exercise with glucose ingestion in Type 1 diabetic patients. J. Appl. Physiol. 2007, 103, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Raguso, C.A.; Coggan, A.R.; Gastaldelli, A.; Sidossis, L.S.; Bastyr, E.J.; Wolfe, R.R. Lipid and Carbohydrate Metabolism in IDDM During Moderate and Intense Exercise. Diabetes 1995, 44, 1066–1074. [Google Scholar] [CrossRef]
- Riddell, M.C.; Bar-Or, O.; Hollidge-Horvat, M.; Schwarcz, H.P.; Heigenhauser, G.J.F. Glucose ingestion and substrate utilization during exercise in boys with IDDM. J. Appl. Physiol. 2000, 88, 1239–1246. [Google Scholar] [CrossRef]
- Krzentowski, G.; Pirnay, F.; Pallikarakis, N.; Luyckx, A.S.; Lacroix, M.; Mosora, F.; Lefèbvre, P.J. Glucose utilization during exercise in normal and diabetic subjects. The role of insulin. Diabetes 1981, 30, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.B.; Ranjan, A.G.; McCarthy, O.M.; Holst, J.J.; Bracken, R.M.; Nørgaard, K.; Schmidt, S. Effects of a Low-Carbohydrate-High-Protein Pre-Exercise Meal in Type 1 Diabetes-a Randomized Crossover Trial. J. Clin. Endocrinol. Metab. 2023, 109, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.B.; Ranjan, A.G.; McCarthy, O.M.; Bracken, R.M.; Nørgaard, K.; Schmidt, S. Sensor-Based Glucose Metrics during Different Diet Compositions in Type 1 Diabetes—A Randomized One-Week Crossover Trial. Nutrients 2024, 16, 199. [Google Scholar] [CrossRef]
- The Official Dietary Guidelines—Good for Health and Climate. Available online: https://en.foedevarestyrelsen.dk/food/nutrition-and-health/the-official-dietary-guidelines (accessed on 20 September 2025).
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef]
- Albrechtsen, N.J.W.; Hartmann, B.; Veedfald, S.; Windeløv, J.A.; Plamboeck, A.; Bojsen-Møller, K.N.; Idorn, T.; Feldt-Rasmussen, B.; Knop, F.K.; Vilsbøll, T.; et al. Hyperglucagonaemia analysed by glucagon sandwich ELISA: Nonspecific interference or truly elevated levels? Diabetologia 2014, 57, 1919–1926. [Google Scholar] [CrossRef]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol.-Endocrinol. Metab. 1993, 265, E380–E391. [Google Scholar] [CrossRef]
- Lambert, E.V.; Speechly, D.P.; Dennis, S.C.; Noakes, T.D. Enhanced endurance in trained cyclists during moderate intensity exercise following 2 weeks adaptation to a high fat diet. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 287–293. [Google Scholar] [CrossRef]
- Cameron-Smith, D.; Burke, L.M.; Angus, D.J.; Tunstall, R.J.; Cox, G.R.; Bonen, A.; Hawley, J.A.; Hargreaves, M. A short-term, high-fat diet up-regulates lipid metabolism and gene expression in human skeletal muscle. Am. J. Clin. Nutr. 2003, 77, 313–318. [Google Scholar] [CrossRef]
- McCarthy, O.M.; Christensen, M.B.; Tawfik, S.; Kristensen, K.B.; Hartmann, B.; Holst, J.J.; Schmidt, S.; Nørgaard, K.; Bracken, R.M. Metabolic and Hormonal Responses to Isomaltulose Ingestion Before or During Sustained Submaximal Exercise in Adults with Type 1 Diabetes Using Automated Insulin Delivery Systems. Nutrients 2024, 16, 4098. [Google Scholar] [CrossRef] [PubMed]
- Zderic, T.W.; Davidson, C.J.; Schenk, S.; Byerley, L.O.; Coyle, E.F. High-fat diet elevates resting intramuscular triglyceride concentration and whole body lipolysis during exercise. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E217–E225. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.; Gleeson, M.; Greenhaff, L.P. Biochemistry of Exercise & Training; Oxford University Press Inc.: New York, NY, USA, 2005. [Google Scholar]
- McMahon, S.K.; Ferreira, L.D.; Ratnam, N.; Davey, R.J.; Youngs, L.M.; Davis, E.A.; Fournier, P.A.; Jones, T.W. Glucose requirements to maintain euglycemia after moderate-intensity afternoon exercise in adolescents with type 1 diabetes are increased in a biphasic manner. J. Clin. Endocrinol. Metab. 2007, 92, 963–968. [Google Scholar] [CrossRef]
- Campbell, M.D.; Walker, M.; Bracken, R.M.; Turner, D.; Stevenson, E.J.; Gonzalez, J.T.; Shaw, J.A.; West, D.J. Insulin therapy and dietary adjustments to normalize glycemia and prevent nocturnal hypoglycemia after evening exercise in type 1 diabetes: A randomized controlled trial. BMJ Open Diabetes Res. Care 2015, 3, e000085. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, O.M.; Christensen, M.B.; Kristensen, K.B.; Schmidt, S.; Ranjan, A.G.; Bain, S.C.; Bracken, R.M.; Nørgaard, K. Glycaemia around exercise in adults with type 1 diabetes using automated and non-automated insulin delivery pumps: A switch pilot trial. Diabetes Technol. Ther. 2023, 25, 287–292. [Google Scholar] [CrossRef]
- Brazeau, A.S.; Rabasa-Lhoret, R.; Strychar, I.; Mircescu, H. Barriers to Physical Activity Among Patients with Type 1 Diabetes. Diabetes Care 2008, 31, 2108. [Google Scholar] [CrossRef]
| Main Macronutrient Composition of the Diet | Carbohydrates | Fat | Protein |
|---|---|---|---|
| High carbohydrate (HCLFLP) | 250 g (48%) | 78 g (33%) | 98 g (19%) |
| High fat (LCHFLP) | 100 g (19%) | 145 g (62%) | 98 g (19%) |
| High protein (LCLFHP) | 100 g (19%) | 132 g (57%) | 126 g (24%) |
| Characteristic | Mean ± SD | Range (Min–Max) |
|---|---|---|
| Age (years) | 46 ± 15 | 48 (22–70) |
| BMI (kg·m−2) | 27.0 ± 4.3 | 14.6 (21.3–35.9) |
| HbA1c (%) | 7.3 ± 0.7 | 2.8 (5.5–8.3) |
| HbA1c (mmol·mol−1) | 55.9 ± 7.8 | 30.0 (37.0–67.9) |
| Diabetes duration (years) | 29 ± 15 | 41 (11–52) |
| Age of diabetes onset (years) | 17 ± 11 | 39 (4–43) |
| Total daily insulin dose (U) | 45.4 ± 20.0 | 108 (18.4–127.0) |
| Total daily insulin dose (U·kg−1) | 0.5 ± 0.2 | 0.7 (0.3–1.0) |
| Total daily basal insulin dose (percent of total) | 48.4 ± 9.0 | 29.0 (34.9–63.9) |
| Total daily bolus insulin dose (percent of total) | 51.6 ± 9.0 | 29.3 (36.1–65.4) |
| Average daily CHO intake (g) | 160 ± 66 | 201 (62–263) |
| Average 14-day mean SG (mmol·L−1) | 8.9 ± 0.9 | 3.0 (7.0–10.0) |
| Average 14-day SG CV (%) | 37.9 ± 4.5 | 17.0 (29.0–46.0) |
| Average 14-day SG TBR (%) | 10.8 ± 16.6 | 46 (0.0–46.0) |
| Average 14-day SG TIR (%) | 61.1 ± 12.3 | 44.0 (39.0–83.0) |
| Average 14-day SG TAR (%) | 28.2 ± 18.3 | 60.0 (1.0–61.0) |
| Systolic blood pressure (mmHg) | 142 ± 9 | 24 (131–155) |
| Diastolic blood pressure (mmHg) | 86 ± 6 | 23 (71–94) |
| Resting heart rate (bpm) | 68 ± 12 | 43 (52–95) |
| O2peak (L·min−1) | 3.0 ± 1.1 | 3.1 (1.5–4.6) |
| O2peak (mL·min·kg−1) | 34.8 ± 10.9 | 36.0 (21.1–57.1) |
| Powerpeak (L·min−1) | 256 ± 84 | 225 (155–380) |
| Powerpeak (Watts·kg−1) | 3.1 ± 0.9 | 2.8 (1.9–4.7) |
| Glycaemic Parameter | HCLFLP | LCHFLP | LCLFHP | p-Value |
|---|---|---|---|---|
| Pre-exercise period(0min to 60min) | ||||
| Mean PG (mmol/L) | 9.5 ± 2.1 | 8.2 ± 0.9 | 8.1 ± 1.6 | 0.052 |
| SD PG (mmol/L) | 0.4 ± 0.2 | 0.6 ± 0.4 | 0.6 ± 0.3 | 0.226 |
| CV PG (%) | 4.6 ± 2.0 | 7.4 ± 5.0 | 7.9 ± 4.7 | 0.087 |
| TBR PG (%) | - | - | - | - |
| TIR PG (%) | 66.7 ± 46.2 | 95.0 ± 17.3 | 86.7 ± 26.1 | 0.078 |
| TAR PG (%) | 33.3 ± 46.2 | 5.0 ± 17.3 | 13.3 ± 26.1 | 0.078 |
| Exercise period(90min to 135min) | ||||
| Mean PG (mmol/L) | 10.1 ± 2.8 | 8.5 ± 1.9 | 7.6 ± 1.3 | 0.004 * |
| SD PG (mmol/L) | 0.5 ± 0.2 | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.947 |
| CV PG (%) | 4.7 ± 1.6 | 5.5 ± 2.2 | 6.3 ± 4.8 | 0.501 |
| TBR PG (%) | - | - | - | - |
| TIR PG (%) | 59.2 ± 47.2 | 88.3 ± 30.1 | 99.2 ± 2.9 | 0.006 * |
| TAR PG (%) | 40.8 ± 47.2 | 11.7 ± 30.1 | 0.8 ± 2.9 | 0.006 * |
| Post-exercise period(140min to 225min) | ||||
| Mean PG (mmol/L) | 10.7 ± 3.2 | 8.8 ± 2.8 | 7.5 ± 1.8 | 0.003 * |
| SD PG (mmol/L) | 0.4 ± 0.2 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.040 |
| CV PG (%) | 3.5 ± 1.8 | 3.4 ± 1.4 | 3.4 ± 1.1 | 0.973 |
| TBR PG (%) | - | - | - | - |
| TIR PG (%) | 44.8 ± 44.7 | 81.3 ± 38.3 | 96.9 ± 10.8 | 0.002 *# |
| TAR PG (%) | 55.2 ± 44.7 | 18.8 ± 38.3 | 3.1 ± 10.8 | 0.002 *# |
| Overall period(0min to 225min) | ||||
| Mean PG (mmol/L) | 10.2 ± 2.7 | 8.6 ± 1.9 | 7.7 ± 1.3 | 0.004 * |
| SD PG (mmol/L) | 0.7 ± 0.4 | 0.9 ± 0.5 | 0.9 ± 0.4 | 0.462 |
| CV PG (%) | 7.2 ± 2.7 | 10.5 ± 4.9 | 11.8 ± 7.0 | 0.044 *# |
| TBR PG (%) | - | - | - | - |
| TIR PG (%) | 55.6 ± 43.9 | 87.3 ± 28.7 | 95.2 ± 7.9 | 0.003 *# |
| TAR PG (%) | 44.4 ± 43.9 | 12.7 ± 28.7 | 4.8 ± 7.9 | 0.003 *# |
| Parameter | HCLFLP | LCLFHP | LCHFLP | p Value |
|---|---|---|---|---|
| Heart rate (bpm) | 60 ± 10 | 59 ± 7 | 61 ± 8 | 0.289 |
| O2 (L/min) | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.858 |
| CO2 (L/min) | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.747 |
| RER | 0.8 ± 0.1 | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.203 |
| O2 pulse (mL/min) | 5.1 ± 1.5 | 5.2 ± 1.4 | 5.0 ± 1.1 | 0.465 |
| Carbohydrate oxidation (g/min) | 0.13 ± 0.08 | 0.12 ± 0.04 | 0.10 ± 0.04 | 0.292 |
| Carbohydrate oxidation (% total energy) | 36.3 ± 19.9 | 32.1 ± 6.9 | 27.9 ± 7.1 | 0.205 |
| Lipid oxidation (g/min) | 0.11 ± 0.05 | 0.12 ± 0.03 | 0.12 ± 0.02 | 0.364 |
| Lipid oxidation (% total energy) | 64.7 ± 19.9 | 67.9 ± 6.9 | 72.1 ± 7.1 | 0.205 |
| Total energy (Kcals) | 22.6 ± 4.9 | 23.2 ± 5.7 | 22.1 ± 4.4 | 0.442 |
| Total energy (KJ) | 94.4 ± 20.6 | 97.0 ± 23.6 | 92.6 ± 18.6 | 0.441 |
| Parameter | HCLFLP | LCLFHP | LCHFLP | p Value |
|---|---|---|---|---|
| Exercise duration (mins) | 43.9 ± 3.4 | 44.9 ± 0.3 | 44.8 ± 0.9 | 0.427 |
| Load (watts) | 95.7 ± 38.9 | 96.3 ± 37.9 | 95.8 ± 39.0 | 0.300 |
| Heart rate (bpm) | 119 ± 10 | 119 ± 13 | 122 ± 12 | 0.343 |
| O2 (L/min) | 1.6 ± 0.5 | 1.6 ± 0.5 | 1.6 ± 0.5 | 0.260 |
| CO2 (L/min) | 1.3 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.4 | 0.257 |
| RER | 0.83 ± 0.03 | 0.81 ± 0.04 | 0.80 ± 0.04 | 0.048 # |
| O2 pulse (mL/min) | 12.9 ± 4.1 | 12.7 ± 3.5 | 12.8 ± 4.4 | 0.810 |
| Carbohydrate oxidation (g/min) | 0.87 ± 0.27 | 0.75 ± 0.37 | 0.70 ± 0.36 | 0.089 |
| Carbohydrate oxidation (% total energy) | 46.5 ± 9.2 | 39.6 ± 14.4 | 35.7 ± 14.6 | 0.039 # |
| Lipid oxidation (g/min) | 0.46 ± 0.18 | 0.50 ± 0.20 | 0.54 ± 0.21 | 0.113 |
| Lipid oxidation (% total energy) | 53.5 ± 9.2 | 60.4 ± 14.4 | 64.3 ± 14.6 | 0.039 # |
| Total energy (Kcals) | 335.8 ± 111.4 | 337.9 ± 107.1 | 343.0 ± 112.6 | 0.552 |
| Total energy (KJ) | 1403.5 ± 465.8 | 1412.2 ± 447.6 | 1433.9 ± 470.7 | 0.552 |
| Parameter | HCLFLP | LCLFHP | LCHFLP | p Value |
|---|---|---|---|---|
| Heart rate (bpm) | 65 ± 10 | 61 ± 7 | 64 ± 10 | 0.231 |
| O2 (L/min) | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.842 |
| CO2 (L/min) | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.702 |
| RER | 0.75 ± 0.05 | 0.72 ± 0.04 | 0.74 ± 0.05 | 0.115 |
| O2 pulse (mL/min) | 5.0 ± 1.4 | 5.2 ± 1.5 | 5.0 ± 1.5 | 0.692 |
| Carbohydrate oxidation (g/min) | 0.12 ± 0.05 | 0.10 ± 0.04 | 0.11 ± 0.05 | 0.214 |
| Carbohydrate oxidation (% total energy) | 30.0 ± 14.2 | 22.8 ± 8.3 | 26.8 ± 9.9 | 0.099 |
| Lipid oxidation (g/min) | 0.13 ± 0.05 | 0.15 ± 0.06 | 0.14 ± 0.05 | 0.377 |
| Lipid oxidation (% total energy) | 70.0 ± 14.2 | 77.2 ± 8.3 | 73.2 ± 9.9 | 0.099 |
| Total energy (Kcals) | 25.2 ± 6.8 | 25.8 ± 9.1 | 25.1 ± 7.5 | 0.890 |
| Total energy (KJ) | 105.3 ± 28.3 | 108.0 ± 38.2 | 104.9 ± 31.2 | 0.889 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCarthy, O.M.; Kristensen, K.B.; Ranjan, A.G.; Nicholas, C.; Holst, J.J.; Bracken, R.M.; Nørgaard, K.; Schmidt, S. Influence of Diets Differing in Macronutrient Composition on Metabolic Regulation During Exercise in Adults with Type 1 Diabetes. Nutrients 2025, 17, 3637. https://doi.org/10.3390/nu17233637
McCarthy OM, Kristensen KB, Ranjan AG, Nicholas C, Holst JJ, Bracken RM, Nørgaard K, Schmidt S. Influence of Diets Differing in Macronutrient Composition on Metabolic Regulation During Exercise in Adults with Type 1 Diabetes. Nutrients. 2025; 17(23):3637. https://doi.org/10.3390/nu17233637
Chicago/Turabian StyleMcCarthy, Olivia Mary, Kasper Birch Kristensen, Ajenthen Gayathri Ranjan, Chloe Nicholas, Jens Juul Holst, Richard Michael Bracken, Kirsten Nørgaard, and Signe Schmidt. 2025. "Influence of Diets Differing in Macronutrient Composition on Metabolic Regulation During Exercise in Adults with Type 1 Diabetes" Nutrients 17, no. 23: 3637. https://doi.org/10.3390/nu17233637
APA StyleMcCarthy, O. M., Kristensen, K. B., Ranjan, A. G., Nicholas, C., Holst, J. J., Bracken, R. M., Nørgaard, K., & Schmidt, S. (2025). Influence of Diets Differing in Macronutrient Composition on Metabolic Regulation During Exercise in Adults with Type 1 Diabetes. Nutrients, 17(23), 3637. https://doi.org/10.3390/nu17233637







