Dietary and Lifestyle Patterns and Their Associations with Cardiovascular and Inflammatory Biomarkers in Vegans, Vegetarians, Pescatarians, and Omnivores: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
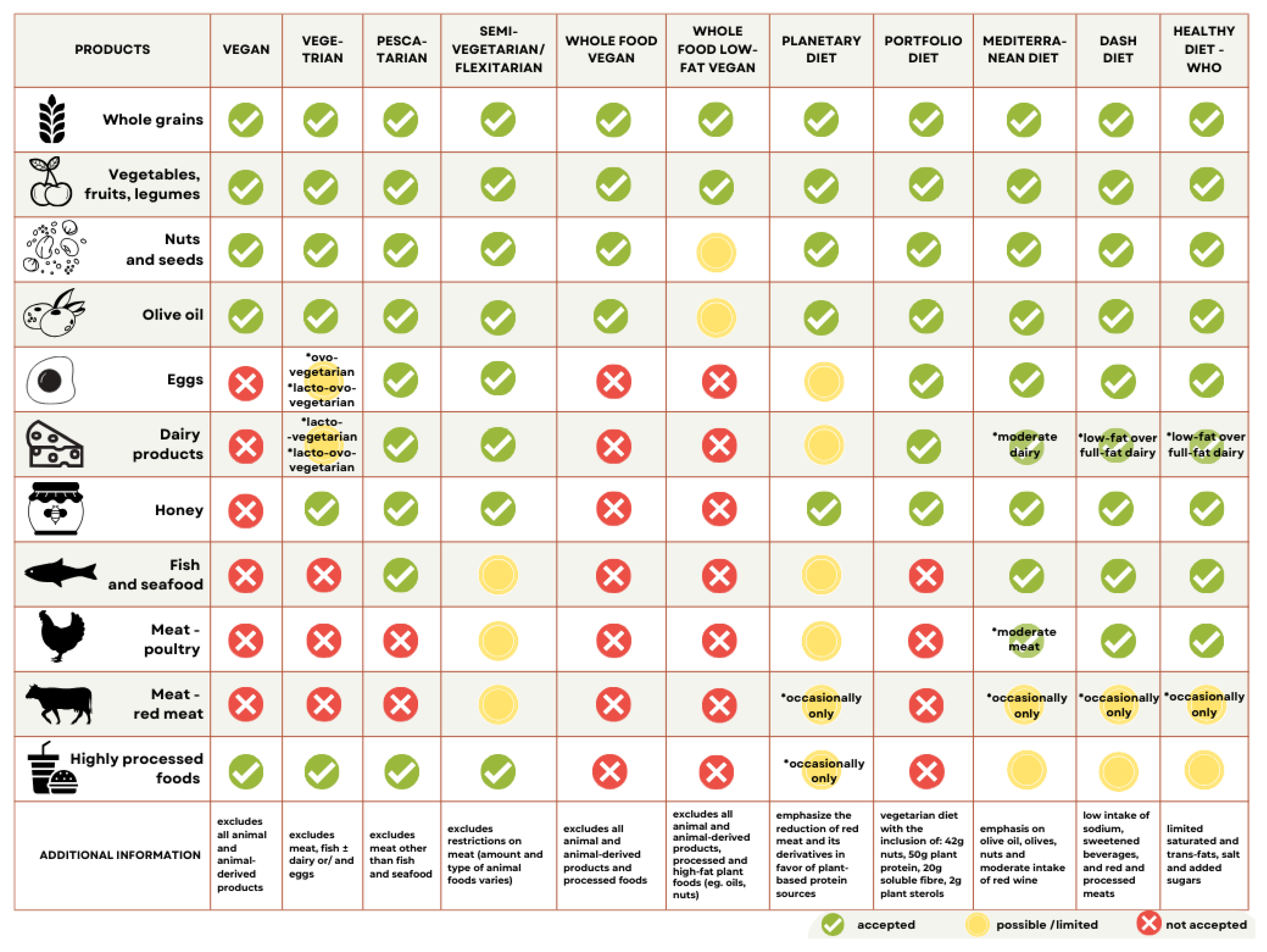
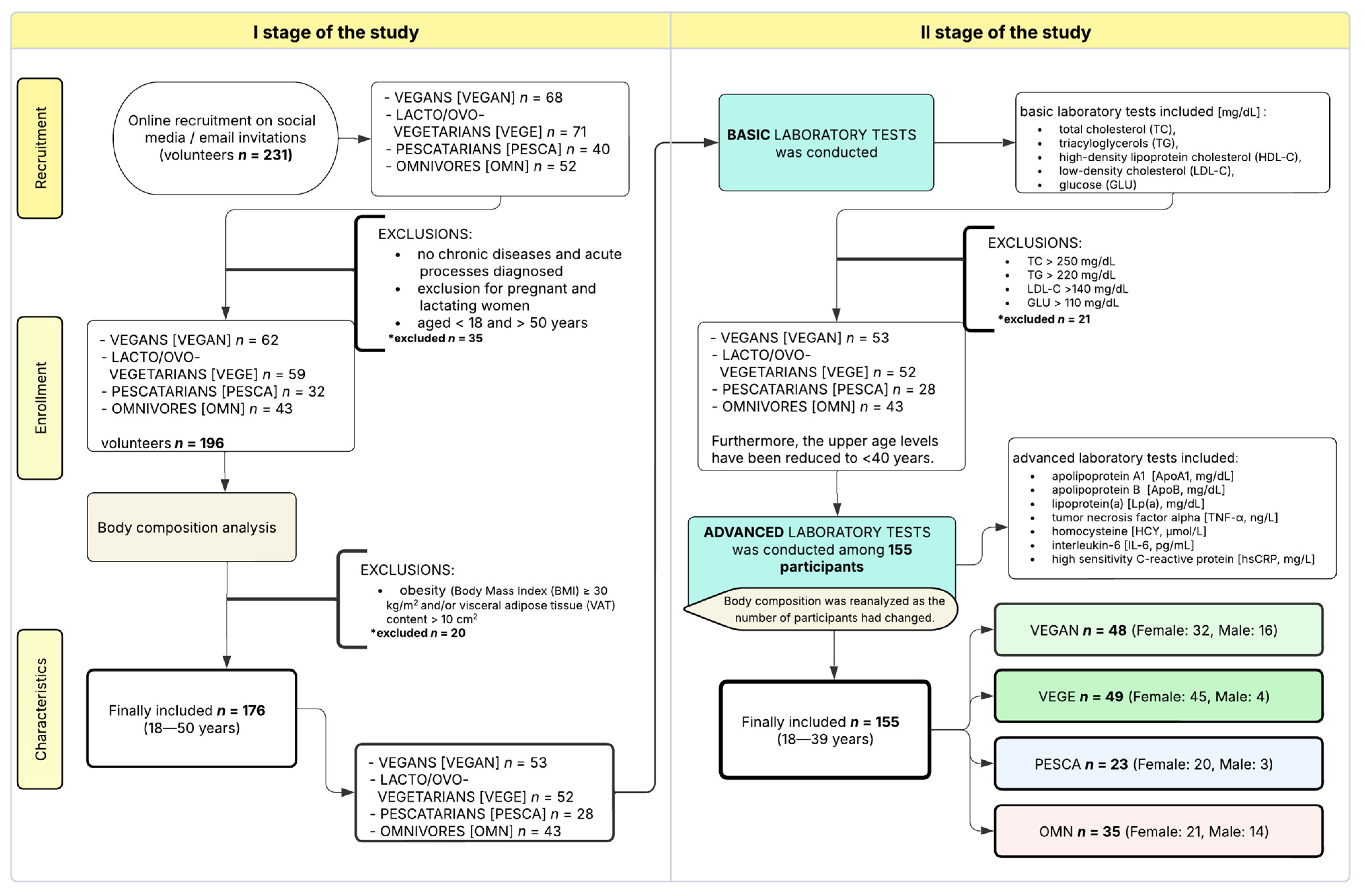
2.2. Subjects
- -
- Vegan diet (VEGAN): excludes all animal-derived products;
- -
- Lacto-/ovo-vegetarian diet (VEGE): includes dairy and/or eggs, excludes meat and fish;
- -
- Pescatarian diet (PESCA): includes fish, eggs, and dairy products;
- -
- Omnivore/mixed/traditional diet (OMN): regular consumption of meat.
2.3. Body Composition and Behavioral Factors Questionnaire Analysis
2.4. Biochemical Tests
2.5. Processing of Data—Subgroups
- -
- Appropriate Behavioral Factors (ABF): score > 3 points
- -
- Inappropriate Behavioral Factors (IBF): score ≤ 3 points
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristic and Behavioral Factors Outcomes
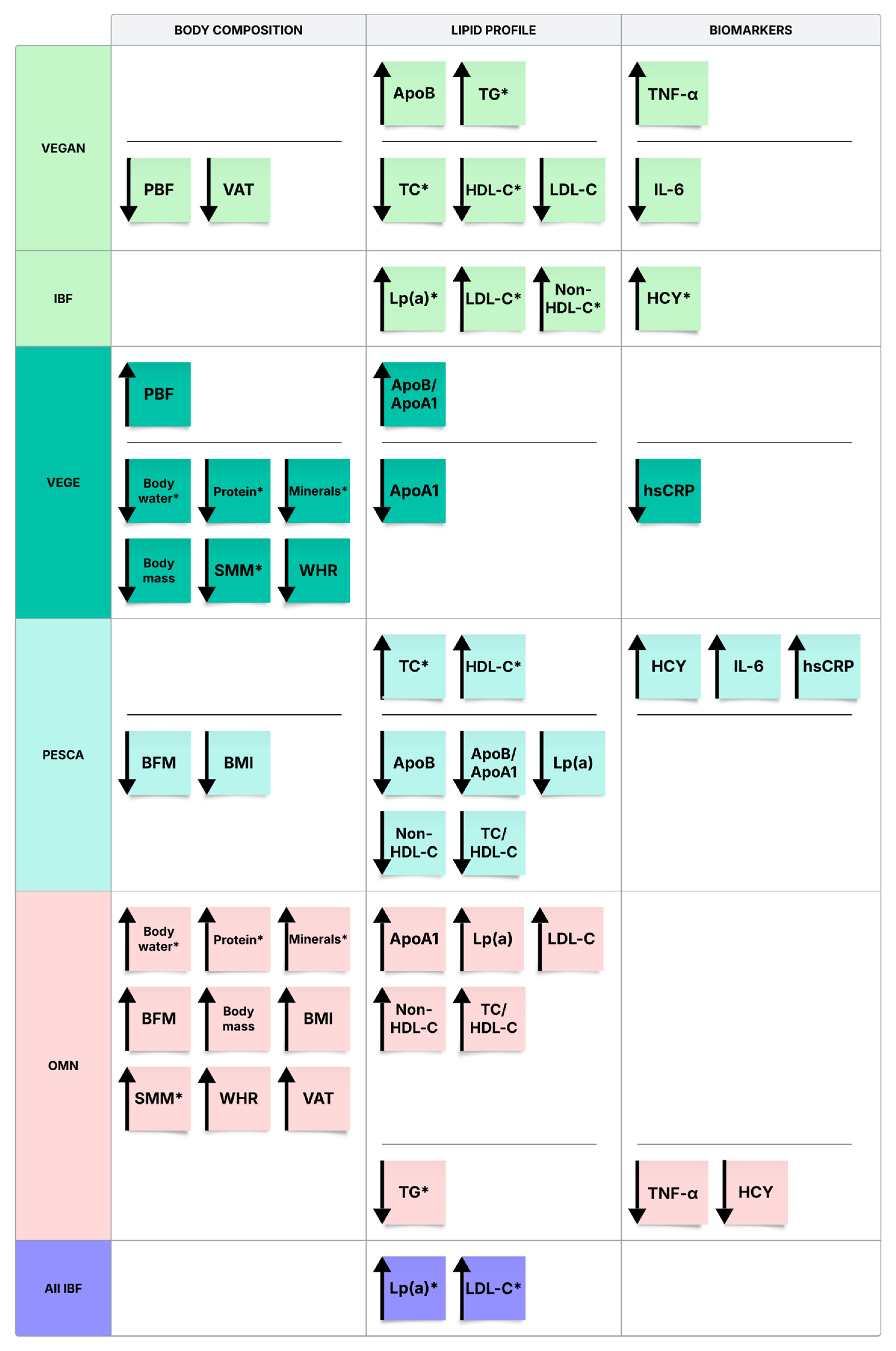
3.2. Body Composition Analysis
3.3. Biochemical Blood Tests
3.4. Correlations Between the Biochemical Analyses and Body Composition Parameters in Subgroups
4. Discussion
4.1. Body Composition and Lifestyle Characteristics Across Dietary Groups
4.2. Connection Between Lifestyle and Lipid Panel Values
4.2.1. Lipid Profile
4.2.2. Apolipoproteins and Lipoprotein(a)
4.3. Connection Between Lifestyle, Body Composition, and Metabolic and Inflammatory Markers
4.3.1. Homocysteine (HCY)
4.3.2. TNF-α, IL-6, hsCRP
4.4. The Relation of the Behavioral Factors of VEGAN to Biochemical Blood Tests
4.4.1. ApoA1
4.4.2. ApoB
4.4.3. Non-HDL-C
4.4.4. IL-6
4.5. Integrative Perspective and Broader Implications
5. Strengths and Limitations of the Study
5.1. Strengths
5.2. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABF | Appropriate Behavioral Factors |
| ApoA1 | Apolipoprotein A1 |
| ApoB | Apolipoprotein B |
| ApoB/ApoA1 | ApoB to ApoA1 ratio |
| BFM | Body Fat Mass |
| BIA | Bioelectric Impedance |
| BMI | Body Mass Index |
| CI | Confidence Intervals |
| CRP | C-reactive protein |
| DASH | Dietary Approaches to Stop Hypertension |
| DII | Dietary Inflammatory Index |
| DSM-BIA | Direct Segmental Multi-frequency Bioelectrical Impedance Analysis |
| DXA | Dual-Energy X-ray Absorptiometry |
| EAS | European Atherosclerosis Society |
| ELISA | Immunoenzymatic Enzyme-Linked Immunosorbent Assay |
| ESC | European Society of Cardiology |
| F | Female |
| FFQ | Food Frequency Questionnaire |
| GLU | Glucose Levels |
| HCY | Homocysteine |
| HDL-C | High-Density Lipoprotein Cholesterol |
| hsCRP | High-Sensitivity C-Reactive Protein |
| IBF | Inappropriate Behavioral Factors |
| IL-6 | Interleukin 6 |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| Lp(a) | Lipoprotein(a) |
| M | Male |
| MUFAs | Monounsaturated Fatty Acids |
| NCDs | Non-Communicable Diseases |
| OMN | Omnivores |
| PBF | Percentage Body Fat |
| PESCA | Pescatarian Diet |
| PPBD | Predominantly Plant-Based Diets |
| PUFAs | Polyunsaturated Fatty Acids |
| r | Pearson Correlation |
| SMM | Skeletal Muscle Mass |
| TC | Total Cholesterol |
| TG | Triacylglycerols |
| TNF-α | Tumor Necrosis Factor-alpha |
| tS | Student’s t-test |
| tW | Welch’s t-test |
| U | Mann–Whitney U test |
| VAT | Visceral Adipose Tissue |
| VEGAN | Vegan Diet |
| VEGE | Lacto-/Ovo-Vegetarian Diet |
| WHO | Health Organization |
| WHR | Waist-Hip Ratio |
| ρ | Spearman Correlation |
References
- Lifestyle Medicine. Available online: https://lifestylemedicine.org (accessed on 20 February 2025).
- GBD 2015 Risk Factors Collaborators. Global, Regional, and National Comparative Risk Assessment of 79 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Regional Office for Europe European Health Report 2018: More than Numbers—Evidence for All: Highlights; World Health Organization: Copenhagen, Denmark, 2018; ISBN 978-92-890-5344-0. [Google Scholar]
- Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 24 February 2025).
- Ricker, M.A.; Haas, W.C. Anti-Inflammatory Diet in Clinical Practice: A Review. Nutr. Clin. Pract. 2017, 32, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, Metaflammation and Immunometabolic Disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory Mechanisms Linking Obesity and Metabolic Disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Lee, H.; Lee, I.S.; Choue, R. Obesity, Inflammation and Diet. Pediatr. Gastroenterol. Hepatol. Nutr. 2013, 16, 143–152. [Google Scholar] [CrossRef]
- Wirth, M.D.; Hébert, J.R.; Shivappa, N.; Hand, G.A.; Hurley, T.G.; Drenowatz, C.; McMahon, D.; Shook, R.P.; Blair, S.N. Anti-Inflammatory Dietary Inflammatory Index Scores Are Associated with Healthier Scores on Other Dietary Indices. Nutr. Res. 2016, 36, 214–219. [Google Scholar] [CrossRef]
- Wirth, M.D.; Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hébert, J.R. The Dietary Inflammatory Index Is Associated with Colorectal Cancer in the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Br. J. Nutr. 2015, 113, 1819–1827. [Google Scholar] [CrossRef]
- Zwickey, H.; Horgan, A.; Hanes, D.; Schiffke, H.; Moore, A.; Wahbeh, H.; Jordan, J.; Ojeda, L.; McMurry, M.; Elmer, P.; et al. Effect of the Anti-Inflammatory Diet in People with Diabetes and Pre-Diabetes: A Randomized Controlled Feeding Study. J. Restor. Med. 2019, 8, e20190107. [Google Scholar] [CrossRef]
- Tolkien, K.; Bradburn, S.; Murgatroyd, C. An Anti-Inflammatory Diet as a Potential Intervention for Depressive Disorders: A Systematic Review and Meta-Analysis. Clin. Nutr. 2019, 38, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Osmanlıoğlu, Ş.; Sanlier, N. The Relationship between Endometriosis and Diet. Hum. Fertil. 2021, 26, 649–664. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- Wang, P.; Wang, D.; Sui, J.; Liu, S.; Kong, Y.; Lei, H.; Zhang, M. The Comprehensive Relationship between Combined Anti-Inflammatory and Healthy Diets and All-Cause Mortality in Rheumatoid Arthritis: Results from NHANES 2003–2018. Arthritis Res. Ther. 2024, 26, 226. [Google Scholar] [CrossRef]
- Zhou, R.Y.; Wang, J.J.; Sun, J.C.; You, Y.; Ying, J.N.; Han, X.M. Attention Deficit Hyperactivity Disorder May Be a Highly Inflammation and Immune-Associated Disease. Mol. Med. Rep. 2017, 16, 5071–5077. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Hebert, J.R.; Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The Dietary Inflammatory Index (DII)—Lessons Learned, Improvements Made, and Future Directions. Adv. Nutr. 2019, 10, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Pu, H.; Voss, M. Overview of Anti-Inflammatory Diets and Their Promising Effects on Non-Communicable Diseases. Br. J. Nutr. 2024, 132, 898–918. [Google Scholar] [CrossRef] [PubMed]
- Ilari, S.; Proietti, S.; Milani, F.; Vitiello, L.; Muscoli, C.; Russo, P.; Bonassi, S. Dietary Patterns, Oxidative Stress, and Early Inflammation: A Systematic Review and Meta-Analysis Comparing Mediterranean, Vegan, and Vegetarian Diets. Nutrients 2025, 17, 548. [Google Scholar] [CrossRef]
- Romanello, M.; McGushin, A.; Di Napoli, C.; Drummond, P.; Hughes, N.; Jamart, L.; Kennard, H.; Lampard, P.; Rodriguez, B.S.; Arnell, N.; et al. The 2021 Report of the Lancet Countdown on Health and Climate Change: Code Red for a Healthy Future. Lancet 2021, 398, 1619–1662. [Google Scholar] [CrossRef]
- Ko, B.J.; Park, K.H.; Shin, S.; Zaichenko, L.; Davis, C.R.; Crowell, J.A.; Joung, H.; Mantzoros, C.S. Diet Quality and Diet Patterns in Relation to Circulating Cardiometabolic Biomarkers. Clin. Nutr. 2016, 35, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.L.; Harris, T.B.; Tylavsky, F.A.; Perry, S.E.; Houston, D.K.; Lee, J.S.; Kanaya, A.M.; Sahyoun, N.R. Dietary Patterns, Insulin Sensitivity and Inflammation in Older Adults. Eur. J. Clin. Nutr. 2012, 66, 18–24. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. UNESCO Making Every School a Health-Promoting School: Implementation Guidance; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-002507-3. [Google Scholar]
- World Health Organization. Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 17 May 2025).
- Gonzalez Fischer, C.; Garnett, T. Plates, Pyramids, Planet. Developments in National Healthy and Sustainable Dietary Guidelines: A State of Play Assessment; Food and Agriculture Organization and The University of Oxford: Oxford, UK, 2016. [Google Scholar]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed. Available online: https://odphp.health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf (accessed on 24 February 2025).
- Kent, G.; Kehoe, L.; Flynn, A.; Walton, J. Plant-Based Diets: A Review of the Definitions and Nutritional Role in the Adult Diet. Proc. Nutr. Soc. 2022, 81, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Nishi, S.K.; Khan, T.A.; Braunstein, C.R.; Glenn, A.J.; Mejia, S.B.; Rahelić, D.; Kahleova, H.; Salas-Salvado, J.; Jenkins, D.J.; et al. Portfolio Dietary Pattern and Cardiovascular Disease: A Systematic Review and Meta-Analysis of Controlled Trials. Prog. Cardiovasc. Dis. 2018, 61, 43–53. [Google Scholar] [CrossRef]
- Rogerson, D.; Maçãs, D.; Milner, M.; Liu, Y.; Klonizakis, M. Contrasting Effects of Short-Term Mediterranean and Vegan Diets on Microvascular Function and Cholesterol in Younger Adults: A Comparative Pilot Study. Nutrients 2018, 10, 1897. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Kendall, C.W.; Sievenpiper, J.L. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, Vegan Diets and Multiple Health Outcomes: A Systematic Review with Meta-Analysis of Observational Studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef]
- Rodriguez, N.R.; DiMarco, N.M.; Langley, S.; American Dietetic Association; Dietitians of Canada; American College of Sports Medicine. Nutrition and Athletic Performance. J. Am. Diet. Assoc. 2009, 109, 509–527. [Google Scholar] [CrossRef]
- British Dietetic Association. British Dietetic Association Confirms Well-Planned Vegan Diets Can Support Healthy Living in People of All Ages. Available online: https://www.vegansociety.com/whats-new/news/british-dietetic-association-confirms-vegan-diets-support-healthy-living (accessed on 17 May 2025).
- Tan, J.; Wang, C.; Tomiyama, A.J. Dietary Approaches to Stop Hypertension (DASH) Diet and Mental Well-Being: A Systematic Review. Nutr. Rev. 2023, 82, 60–75. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Crowe, F.L.; Appleby, P.N.; Schmidt, J.A.; Travis, R.C.; Key, T.J. Serum Concentrations of Cholesterol, Apolipoprotein A-I and Apolipoprotein B in a Total of 1694 Meat-Eaters, Fish-Eaters, Vegetarians and Vegans. Eur. J. Clin. Nutr. 2014, 68, 178–183. [Google Scholar] [CrossRef]
- Huang, T.; Yang, B.; Zheng, J.; Li, G.; Wahlqvist, M.L.; Li, D. Cardiovascular Disease Mortality and Cancer Incidence in Vegetarians: A Meta-Analysis and Systematic Review. Ann. Nutr. Metab. 2012, 60, 233–240. [Google Scholar] [CrossRef]
- Tonstad, S.; Stewart, K.; Oda, K.; Batech, M.; Herring, R.P.; Fraser, G.E. Vegetarian Diets and Incidence of Diabetes in the Adventist Health Study-2. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 292–299. [Google Scholar] [CrossRef]
- Orlich, M.J.; Singh, P.N.; Sabaté, J.; Jaceldo-Siegl, K.; Fan, J.; Knutsen, S.; Beeson, W.L.; Fraser, G.E. Vegetarian Dietary Patterns and Mortality in Adventist Health Study 2. JAMA Intern. Med. 2013, 173, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Nishimura, K.; Barnard, N.D.; Takegami, M.; Watanabe, M.; Sekikawa, A.; Okamura, T.; Miyamoto, Y. Vegetarian Diets and Blood Pressure: A Meta-Analysis. JAMA Intern. Med. 2014, 174, 577–587. [Google Scholar] [CrossRef]
- Rosi, A.; Mena, P.; Pellegrini, N.; Turroni, S.; Neviani, E.; Ferrocino, I.; Di Cagno, R.; Ruini, L.; Ciati, R.; Angelino, D.; et al. Environmental Impact of Omnivorous, Ovo-Lacto-Vegetarian, and Vegan Diet. Sci. Rep. 2017, 7, 6105. [Google Scholar] [CrossRef]
- Appleby, P.N.; Key, T.J. The Long-Term Health of Vegetarians and Vegans. Proc. Nutr. Soc. 2016, 75, 287–293. [Google Scholar] [CrossRef]
- Kwiatkowska, I.; Olszak, J.; Formanowicz, P.; Formanowicz, D. Nutritional Status and Habits among People on Vegan, Lacto/Ovo-Vegetarian, Pescatarian and Traditional Diets. Nutrients 2022, 14, 4591. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R.S.; Moore, C.E.; Montgomery, B.D. Consumption of a Defined, Plant-Based Diet Reduces Lipoprotein(a), Inflammation, and Other Atherogenic Lipoproteins and Particles within 4 Weeks. Clin. Cardiol. 2018, 41, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.W.; Vadiveloo, M.K. Diet Quality of Vegetarian Diets Compared with Nonvegetarian Diets: A Systematic Review. Nutr. Rev. 2019, 77, 144–160. [Google Scholar] [CrossRef]
- Clarys, P.; Deriemaeker, P.; Huybrechts, I.; Hebbelinck, M.; Mullie, P. Dietary Pattern Analysis: A Comparison between Matched Vegetarian and Omnivorous Subjects. Nutr. J. 2013, 12, 82. [Google Scholar] [CrossRef]
- Conrad, Z.; Karlsen, M.; Chui, K.; Jahns, L. Diet Quality on Meatless Days: National Health and Nutrition Examination Survey (NHANES), 2007–2012. Public Health Nutr. 2017, 20, 1564–1573. [Google Scholar] [CrossRef]
- de Souza, R.J.; Zulyniak, M.A.; Desai, D.; Shaikh, M.R.; Campbell, N.C.; Lefebvre, D.L.; Gupta, M.; Wilson, J.; Wahi, G.; Atkinson, S.A.; et al. Harmonization of Food-Frequency Questionnaires and Dietary Pattern Analysis in 4 Ethnically Diverse Birth Cohorts. J. Nutr. 2016, 146, 2343–2350. [Google Scholar] [CrossRef]
- Thiele, S.; Mensink, G.B.M.; Beitz, R. Determinants of Diet Quality. Public Health Nutr. 2004, 7, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.L.; Rollo, M.E.; Schumacher, T.; Collins, C.E. Diet Quality Scores of Australian Adults Who Have Completed the Healthy Eating Quiz. Nutrients 2017, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Turner-McGrievy, G.M.; Barnard, N.D.; Cohen, J.; Jenkins, D.J.A.; Gloede, L.; Green, A.A. Changes in Nutrient Intake and Dietary Quality among Participants with Type 2 Diabetes Following a Low-Fat Vegan Diet or a Conventional Diabetes Diet for 22 Weeks. J. Am. Diet. Assoc. 2008, 108, 1636–1645. [Google Scholar] [CrossRef]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of Nutritional Quality of the Vegan, Vegetarian, Semi-Vegetarian, Pesco-Vegetarian and Omnivorous Diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef]
- Kahleova, H.; Petersen, K.F.; Shulman, G.I.; Alwarith, J.; Rembert, E.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. Effect of a Low-Fat Vegan Diet on Body Weight, Insulin Sensitivity, Postprandial Metabolism, and Intramyocellular and Hepatocellular Lipid Levels in Overweight Adults: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2025454. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Laganà, A.S.; Rapisarda, A.M.C.; La Ferrera, G.M.G.; Buscema, M.; Rossetti, P.; Nigro, A.; Muscia, V.; Valenti, G.; Sapia, F.; et al. Vitamin B12 among Vegetarians: Status, Assessment and Supplementation. Nutrients 2016, 8, 767. [Google Scholar] [CrossRef]
- The Jamovi Project (2022). Jamovi. (Version 2.3) [Computer software]. Available online: https://www.jamovi.org (accessed on 22 October 2025).
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on Diabetes, Pre-Diabetes, and Cardiovascular Diseases Developed in Collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef]
- Understanding Your Cholesterol Test Results. Available online: https://www.heartuk.org.uk/cholesterol/understanding-your-cholesterol-test-results- (accessed on 22 October 2025).
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Desideri, G.; Castaldo, G.; Lombardi, A.; Mussap, M.; Testa, A.; Pontremoli, R.; Punzi, L.; Borghi, C. Is It Time to Revise the Normal Range of Serum Uric Acid Levels? Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1295–1306. [Google Scholar]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Vanacore, D.; Messina, G.; Lama, S.; Bitti, G.; Ambrosio, P.; Tenore, G.; Messina, A.; Monda, V.; Zappavigna, S.; Boccellino, M.; et al. Effect of Restriction Vegan Diets on Muscle Mass, Oxidative Status, and Myocytes Differentiation: A Pilot Study. J. Cell. Physiol. 2018, 233, 9345–9353. [Google Scholar] [CrossRef] [PubMed]
- Monteyne, A.J.; Coelho, M.O.; Murton, A.J.; Abdelrahman, D.R.; Blackwell, J.R.; Koscien, C.P.; Knapp, K.M.; Fulford, J.; Finnigan, T.J.; Dirks, M.L.; et al. Vegan and Omnivorous High Protein Diets Support Comparable Daily Myofibrillar Protein Synthesis Rates and Skeletal Muscle Hypertrophy in Young Adults. J. Nutr. 2023, 153, 1680–1695. [Google Scholar] [CrossRef]
- Dorard, G.; Mathieu, S. Vegetarian and Omnivorous Diets: A Cross-Sectional Study of Motivation, Eating Disorders, and Body Shape Perception. Appetite 2021, 156, 104972. [Google Scholar] [CrossRef]
- Tong, T.Y.; Key, T.J.; Sobiecki, J.G.; Bradbury, K.E. Anthropometric and Physiologic Characteristics in White and British Indian Vegetarians and Nonvegetarians in the UK Biobank. Am. J. Clin. Nutr. 2018, 107, 909–920. [Google Scholar] [CrossRef]
- Hsu, T.L.; Chou, Y.H.; Ho, C.C.; Tantoh, D.M.; Lu, W.Y.; Lung, C.C.; Jan, C.F.; Wang, L.; Liaw, Y.P. Spine, Hip, and Femoral Neck Bone Mineral Density in Relation to Vegetarian Type and Status among Taiwanese Adults. Arch. Osteoporos. 2023, 18, 134. [Google Scholar] [CrossRef]
- Iguacel, I.; Miguel-Berges, M.L.; Gómez-Bruton, A.; Moreno, L.A.; Julián, C. Veganism, Vegetarianism, Bone Mineral Density, and Fracture Risk: A Systematic Review and Meta-Analysis. Nutr. Rev. 2019, 77, 1–18. [Google Scholar] [CrossRef]
- Austin, G.; Ferguson, J.J.A.; Eslick, S.; Oldmeadow, C.; Wood, L.G.; Garg, M.L. Bone Mineral Density and Body Composition in Australians Following Plant-Based Diets vs. Regular Meat Diets. Front. Nutr. 2024, 11, 1411003. [Google Scholar] [CrossRef] [PubMed]
- Britton, K.A.; Massaro, J.M.; Murabito, J.M.; Kreger, B.E.; Hoffmann, U.; Fox, C.S. Body Fat Distribution, Incident Cardiovascular Disease, Cancer, and All-Cause Mortality. J. Am. Coll. Cardiol. 2013, 62, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B. Adipose Tissue, Inflammation and Atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 332–341. [Google Scholar] [CrossRef]
- Eyre, H.; Kahn, R.; Robertson, R.M.; Clark, N.G.; Doyle, C.; Hong, Y.; Gansler, T.; Glynn, T.; Smith, R.A.; ACS/ADA/AHA Collaborative Writing Committee; et al. Preventing Cancer, Cardiovascular Disease, and Diabetes: A Common Agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Stroke 2004, 35, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and Visceral Adipose Tissue: Structural and Functional Differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Vij, V.; Deshmukh, K.; Vijayageetha, M.; Goyal, C.; Gumashta, J.; Gandhi, A.P. Effect of Predominantly Plant-Based Diets on Visceral Fat: A Systematic Review and Meta-Analysis. J. Hum. Nutr. Diet. 2025, 38, e70055. [Google Scholar] [CrossRef]
- Serra-Prat, M.; Lorenzo, I.; Palomera, E.; Yébenes, J.C.; Campins, L.; Cabré, M. Intracellular Water Content in Lean Mass Is Associated with Muscle Strength, Functional Capacity, and Frailty in Community-Dwelling Elderly Individuals: A Cross-Sectional Study. Nutrients 2019, 11, 661. [Google Scholar] [CrossRef]
- Lorenzo, I.; Serra-Prat, M.; Yébenes, J.C. The Role of Water Homeostasis in Muscle Function and Frailty: A Review. Nutrients 2019, 11, 1857. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, N.; Lee, Y.; An, X.; Chung, Y.S.; Park, Y.K. Muscle Mass Changes after Daily Consumption of Protein Mix Supplemented with Vitamin D in Adults over 50 Years of Age: Subgroup Analysis According to the Serum 25(OH)D Levels of a Randomized Controlled Trial. Clin. Nutr. Res. 2023, 12, 184–198. [Google Scholar] [CrossRef]
- Sharma Ghimire, P.; Ding, X.; Eckart, A. Exploring the Role of Dietary Calcium Intake in Muscle and Cardiovascular Performance among Young Athletes. Sports 2024, 12, 288. [Google Scholar] [CrossRef]
- Alhassan, A.; Young, J.; Lean, M.E.J.; Lara, J. Consumption of Fish and Vascular Risk Factors: A Systematic Review and Meta-Analysis of Intervention Studies. Atherosclerosis 2017, 266, 87–94. [Google Scholar] [CrossRef]
- Moosavi, D.; Vuckovic, I.; Kunz, H.E.; Lanza, I.R. A Randomized Trial of ω-3 Fatty Acid Supplementation and Circulating Lipoprotein Subclasses in Healthy Older Adults. J. Nutr. 2022, 152, 1675–1689. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Levin, S.M.; Barnard, N.D. Association between Plant-Based Diets and Plasma Lipids: A Systematic Review and Meta-Analysis. Nutr. Rev. 2017, 75, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, A.; Lebiedzińska, A.; Fijałkowski, M.; Gałaska, R.; Kreft, E.; Totoń, M.; Czaja, K.; Kozłowska, A.; Ćwiklińska, A.; Kortas-Stempak, B.; et al. Impact of Plant-Based Diet on Lipid Risk Factors for Atherosclerosis. Cardiol. J. 2016, 23, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Saintila, J.; Lozano López, T.E.; Ruiz Mamani, P.G.; White, M.; Huancahuire-Vega, S. Health-Related Quality of Life, Blood Pressure, and Biochemical and Anthropometric Profile in Vegetarians and Nonvegetarians. J. Nutr. Metab. 2020, 2020, 3629742. [Google Scholar] [CrossRef]
- Jakše, B.; Jakše, B.; Godnov, U.; Pinter, S. Nutritional, Cardiovascular Health and Lifestyle Status of ‘Health Conscious’ Adult Vegans and Non-Vegans from Slovenia: A Cross-Sectional Self-Reported Survey. Int. J. Environ. Res. Public Health 2021, 18, 5968. [Google Scholar] [CrossRef]
- Dawczynski, C.; Weidauer, T.; Richert, C.; Schlattmann, P.; Dawczynski, K.; Kiehntopf, M. Nutrient Intake and Nutrition Status in Vegetarians and Vegans in Comparison to Omnivores—The Nutritional Evaluation (NuEva) Study. Front. Nutr. 2022, 9, 819106. [Google Scholar] [CrossRef]
- Reyes-Soffer, G.; Ginsberg, H.N.; Berglund, L.; Duell, P.B.; Heffron, S.P.; Kamstrup, P.R.; Lloyd-Jones, D.M.; Marcovina, S.M.; Yeang, C.; Koschinsky, M.L.; et al. Lipoprotein(a): A Genetically Determined, Causal, and Prevalent Risk Factor for Atherosclerotic Cardiovascular Disease: A Scientific Statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e48–e60. [Google Scholar] [CrossRef]
- Enkhmaa, B.; Petersen, K.S.; Kris-Etherton, P.M.; Berglund, L. Diet and Lp(a): Does Dietary Change Modify Residual Cardiovascular Risk Conferred by Lp(a)? Nutrients 2020, 12, 2024. [Google Scholar] [CrossRef]
- Stojko, M.; Spychał, A.; Nikel, K.; Kołodziej, R.; Zalejska-Fiolka, J. The Impact of Diet on Lipoprotein(a) Levels. Life 2024, 14, 1403. [Google Scholar] [CrossRef]
- Nuotio, P.; Lankinen, M.A.; Meuronen, T.; de Mello, V.D.; Sallinen, T.; Virtanen, K.A.; Pihlajamäki, J.; Laakso, M.; Schwab, U. Dietary n-3 Alpha-Linolenic and n-6 Linoleic Acids Modestly Lower Serum Lipoprotein(a) Concentration but Differentially Influence Other Atherogenic Lipoprotein Traits: A Randomized Trial. Atherosclerosis 2024, 395, 117562. [Google Scholar] [CrossRef]
- Riley, T.M.; Sapp, P.A.; Kris-Etherton, P.M.; Petersen, K.S. Effects of Saturated Fatty Acid Consumption on Lipoprotein(a): A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2024, 120, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Katsiki, N.; Ward, N.; Reiner, Ž. Flaxseed Supplementation Reduces Plasma Lipoprotein(a) Levels: A Meta-Analysis. Altern. Ther. Health Med. 2021, 27, 50–53. [Google Scholar]
- Obersby, D.; Chappell, D.C.; Dunnett, A.; Tsiami, A.A. Plasma Total Homocysteine Status of Vegetarians Compared with Omnivores: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2013, 109, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Alam, S.F. Role of Homocysteine in the Development of Cardiovascular Disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Zhang, A.; Zhong, F. Association between Homocysteine Levels and All-Cause Mortality: A Dose-Response Meta-Analysis of Prospective Studies. Sci. Rep. 2017, 7, 4769. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, D. Research Progress on the Effect of Exercise on Homocysteine. Curr. Med. Chem. 2025, in press. [Google Scholar] [CrossRef]
- Gu, Y.; Bai, J.; Li, Y.; Han, L.; Wang, D. The Effect of Tai Chi on Plasma Homocysteine in 1176 Adults: A Propensity Score Matching-Based Study. BMC Cardiovasc. Disord. 2025, 25, 61. [Google Scholar] [CrossRef]
- Krajcovicová-Kudlácková, M.; Blazícek, P.; Kopcová, J.; Béderová, A.; Babinská, K. Homocysteine Levels in Vegetarians versus Omnivores. Ann. Nutr. Metab. 2000, 44, 135–138. [Google Scholar] [CrossRef]
- Majchrzak, D.; Singer, I.; Männer, M.; Rust, P.; Genser, D.; Wagner, K.-H.; Elmadfa, I. B-Vitamin Status and Concentrations of Homocysteine in Austrian Omnivores, Vegetarians and Vegans. Ann. Nutr. Metab. 2006, 50, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Koebnick, C.; Garcia, A.L.; Dagnelie, P.C.; Strassner, C.; Lindemans, J.; Katz, N.; Leitzmann, C.; Hoffmann, I. Long-Term Consumption of a Raw Food Diet Is Associated with Favorable Serum LDL Cholesterol and Triglycerides but Also with Elevated Plasma Homocysteine and Low Serum HDL Cholesterol in Humans. J. Nutr. 2005, 135, 2372–2378. [Google Scholar] [CrossRef]
- Krivosíková, Z.; Krajcovicová-Kudlácková, M.; Spustová, V.; Stefíková, K.; Valachovicová, M.; Blazícek, P.; Nemcová, T. The Association between High Plasma Homocysteine Levels and Lower Bone Mineral Density in Slovak Women: The Impact of Vegetarian Diet. Eur. J. Nutr. 2010, 49, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Su, T.-C.; Jeng, J.-S.; Wang, J.-D.; Torng, P.-L.; Chang, S.-J.; Chen, C.-F.; Liau, C.-S. Homocysteine, Circulating Vascular Cell Adhesion Molecule and Carotid Atherosclerosis in Postmenopausal Vegetarian Women and Omnivores. Atherosclerosis 2006, 184, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.; Schorr, H.; Purschwitz, K.; Rassoul, F.; Richter, V. Total Homocysteine, Vitamin B(12), and Total Antioxidant Status in Vegetarians. Clin. Chem. 2001, 47, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, P.; Meleady, R.; Fitzgerald, T.; Graham, I.; European COMAC Group. Smoking and Plasma Homocysteine. Eur. Heart J. 2002, 23, 1580–1586. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Wang, N.; Zhu, M.; Liu, X.; Wang, R.; Jiang, F.; Chen, Y.; Zhao, Q.; Zhao, G. Central but Not General Obesity Is Positively Associated with the Risk of Hyperhomocysteinemia in Middle-Aged Women. Nutrients 2019, 11, 1614. [Google Scholar] [CrossRef]
- Ulloque-Badaracco, J.R.; Alarcon-Braga, E.A.; Hernandez-Bustamante, E.A.; von-Koeller-Jones, B.M.; Huayta-Cortez, M.; Saavedra-Custodio, E.; Herrera-Añazco, P.; Benites-Zapata, V.A. Vitamin B12, Folate, and Homocysteine Levels in Children and Adolescents with Obesity: A Systematic Review and Meta-Analysis. Front. Public Health 2025, 13, 1481002. [Google Scholar] [CrossRef]
- Klein, L.; Lenz, C.; Krüger, K.; Lorkowski, S.; Kipp, K.; Dawczynski, C. Comparative Analysis of Fatty Acid Profiles across Omnivorous, Flexitarians, Vegetarians, and Vegans: Insights from the NuEva Study. Lipids Health Dis. 2025, 24, 133. [Google Scholar] [CrossRef]
- Jenko Pražnikar, Z.; Šik Novak, K.; Bogataj Jontez, N.; Petelin, A.; Mohorko, N.; Kenig, S. Inflammatory and Intestinal Permeability Biomarkers in Healthy Participants on Long-Term Vegan, Vegetarian, Omnivore and Low-Carbohydrate High-Fat Diet. Sci. Rep. 2023, 13, 17286. [Google Scholar] [CrossRef]
- Montalcini, T.; De Bonis, D.; Ferro, Y.; Carè, I.; Mazza, E.; Accattato, F.; Greco, M.; Foti, D.; Romeo, S.; Gulletta, E.; et al. High Vegetable Fats Intake Is Associated with High Resting Energy Expenditure in Vegetarians. Nutrients 2015, 7, 5933–5947. [Google Scholar] [CrossRef]
- Franco-de-Moraes, A.C.; de Almeida-Pititto, B.; da Rocha Fernandes, G.; Gomes, E.P.; da Costa Pereira, A.; Ferreira, S.R.G. Worse Inflammatory Profile in Omnivores than in Vegetarians Associates with the Gut Microbiota Composition. Diabetol. Metab. Syndr. 2017, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Menzel, J.; Biemann, R.; Longree, A.; Isermann, B.; Mai, K.; Schulze, M.B.; Abraham, K.; Weikert, C. Associations of a Vegan Diet with Inflammatory Biomarkers. Sci. Rep. 2020, 10, 1933. [Google Scholar] [CrossRef]
- Wu, T.T.; Chang, C.Y.; Hsu, W.M.; Wang, I.K.; Hsu, C.H.; Cheng, S.H.; Liang, C.C.; Chang, C.T.; Huang, C.C. Nutritional Status of Vegetarians on Maintenance Haemodialysis. Nephrology 2011, 16, 582–587. [Google Scholar] [CrossRef]
- Gorczyca, D.; Prescha, A.; Szeremeta, K. Impact of Vegetarian Diet on Serum Immunoglobulin Levels in Children. Clin. Pediatr. 2013, 52, 241–246. [Google Scholar] [CrossRef]
- Fontana, L.; Shew, J.L.; Holloszy, J.O.; Villareal, D.T. Low Bone Mass in Subjects on a Long-Term Raw Vegetarian Diet. Arch. Intern. Med. 2005, 165, 684–689. [Google Scholar] [CrossRef]
- Krajcovicova-Kudlackova, M.; Blazicek, P. C-Reactive Protein and Nutrition. Bratisl. Lek. Listy 2005, 106, 345–347. [Google Scholar] [PubMed]
- Gorshkova, I.N.; Sviridov, D.O.; Bukrinsky, M.; Remaley, A.T. Human apoA-I [Lys107del] mutation affects lipid surface behavior of apoA-I and its ability to form large nascent HDL. J. Lipid Res. 2023, 64, 100319. [Google Scholar] [CrossRef]
- Walldius, G.; Jungner, I. The ApoB/ApoA-I Ratio: A Strong, New Risk Factor for Cardiovascular Disease and a Target for Lipid-Lowering Therapy—A Review of the Evidence. J. Intern. Med. 2006, 259, 493–519. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Gu, Y.; Xu, J.; Xiao, Y.; Wang, C.; Chen, Y.; Huang, H.; Chen, Q.; Chen, Y.; Chen, Q.; et al. Association between Total and Regional Body Fat Measured by Dual-Energy X-ray Absorptiometry and Apolipoprotein B. Sci. Rep. 2025, 15, 25734. [Google Scholar] [CrossRef]
- Vazirian, F.; Darroudi, S.; Rahimi, H.R.; Latifi, M.; Shakeri, B.; Abolbashari, S.; Mohammadpour, A.H.; Esmaily, H.; Mouhebati, M.; Samadi, S.; et al. Non-HDL Cholesterol and Long-Term Follow-Up Outcomes in Patients with Metabolic Syndrome. Lipids Health Dis. 2023, 22, 165. [Google Scholar] [CrossRef]
- Katsi, V.; Argyriou, N.; Fragoulis, C.; Tsioufis, K. The Role of Non-HDL Cholesterol and Apolipoprotein B in Cardiovascular Disease: A Comprehensive Review. J. Cardiovasc. Dev. Dis. 2025, 12, 256. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, P.S.; Handelsman, Y.; Rosenblit, P.D.; Bloomgarden, Z.T.; Fonseca, V.A.; Garber, A.J.; Grunberger, G.; Guerin, C.K.; Bell, D.S.H.; Mechanick, J.I.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocr. Pract. 2017, 23, 1–87. [Google Scholar] [CrossRef] [PubMed]
- Shantaram, D.; Hoyd, R.; Blaszczak, A.M.; Antwi, L.; Jalilvand, A.; Wright, V.P.; Liu, J.; Smith, A.J.; Bradley, D.; Lafuse, W.; et al. Obesity-Associated Microbiomes Instigate Visceral Adipose Tissue Inflammation by Recruitment of Distinct Neutrophils. Nat. Commun. 2024, 15, 5434. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Bickel, P.E.; Scherer, P.E. Targeting Adipose Tissue in the Treatment of Obesity-Associated Diabetes. Nat. Rev. Drug Discov. 2016, 15, 639–660. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream to Identify Novel Targets for Atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-Reactive Protein, Interleukin 6, and Risk of Developing Type 2 Diabetes Mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Bowker, N.; Shah, R.L.; Sharp, S.J.; Luan, J.A.; Stewart, I.D.; Wheeler, E.; Ferreira, M.A.; Baras, A.; Wareham, N.J.; Langenberg, C.; et al. Meta-Analysis Investigating the Role of Interleukin-6 Mediated Inflammation in Type 2 Diabetes. eBioMedicine 2020, 61, 103062. [Google Scholar] [CrossRef]
- Jafarnezhad, F.; Nazarzadeh, A.; Bazavar, H.; Keramat, S.; Ryszkiel, I.; Stanek, A. Vegan and Plant-Based Diets in the Management of Metabolic Syndrome: A Narrative Review from Anti-Inflammatory and Antithrombotic Perspectives. Nutrients 2025, 17, 2656. [Google Scholar] [CrossRef]
- Smidowicz, A.; Regula, J. Effect of Nutritional Status and Dietary Patterns on Human Serum C-Reactive Protein and Interleukin-6 Concentrations. Adv. Nutr. 2015, 6, 738–747. [Google Scholar] [CrossRef]
- Formanowicz, D.; Sackmann, A.; Kozak, A.; Błażewicz, J.; Formanowicz, P. Some aspects of the anemia of chronic disorders modeled and analyzed by petri net based approach. Bioprocess Biosyst. Eng. 2011, 34, 581–595. [Google Scholar] [CrossRef]
- Formanowicz, D. Pathomechanisms of Disturbances Underlying Chronic Disorders. Biomedicines 2024, 12, 131. [Google Scholar] [CrossRef]
- Kwiatkowska, I.; Olszak, J.; Formanowicz, P.; Formanowicz, D. Dietary Habits and Lifestyle, including Cardiovascular Risk among Vegetarians and Omnivores during the COVID-19 Pandemic in the Polish Population. Nutrients 2023, 15, 442. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Fan, S.; Zhi, X.; Wang, Y.; Wang, Y.; Zheng, Q.; Sun, G. Prevalence of hyperhomocysteinemia in China: A systematic review and meta-analysis. Nutrients 2014, 7, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, Q.; Zhang, L.; Hao, Y.; Fan, R.; Peng, X.; Liu, S.; Chen, Z.; Zhang, T.; Chen, S.; et al. Elevated total plasma homocysteine levels are associated with type 2 diabetes in women with hypertension. Asia Pacific J. Clin. Nutr. 2015, 24, 683–691. [Google Scholar]
- Feng, Y.; Kang, K.; Xue, Q.; Chen, Y.; Wang, W.; Cao, J. Value of Plasma Homocysteine to Predict Stroke, Cardiovascular Diseases, and New-Onset Hypertension: A Retrospective Cohort Study. Medicine 2020, 99, e21541. [Google Scholar] [CrossRef]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., III; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef] [PubMed]
| Studied Groups | Behavioral Factors Characteristics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level of Activity [min/week] | Sleep Duration [h/night] | Smoking | Vodka Consumption | |||||||||
| Low (<150) | Medium (150–300) | High (>300) | ≤6 | 7–8 | ≥9 | Never | Occasionally | Regularly | Never | Rare | Monthly/Weekly/Daily | |
| points * | 0 | 0.5 | 1 | 0 | 1 | 0.5 | 1 | 0.5 | 0 | 1 | 0.5 | 0 |
| n (%) | ||||||||||||
| VEGAN n = 48 F/M: 32/16 | 9 (18.8) | 31 (64.6) | 8 (16.7) | 3 (6.3) | 38 (79.2) | 7 (14.6) | 40 (83.3) | 3 (6.3) | 5 (10.5) | 35 (72.9) | 10 (20.8) | 3 (6.3) |
| VEGE n = 49 F/M: 45/4 | 7 (14.3) | 35 (71.4) | 7 (14.3) | 13 (26.5) | 32 (65.3) | 4 (8.2) | 38 (77.5) | 7 (14.3) | 4 (8.2) | 30 (61.2) | 14 (28.6) | 5 (10.2) |
| PESCA n = 23 F/M: 20/3 | 0 (0.0) | 19 (82.6) | 4 (17.4) | 3 (13.0) | 20 (87.0) | 0 (0.0) | 15 (65.2) | 4 (17.4) | 4 (17.4) | 14 (60.9) | 4 (17.4) | 5 (21.7) |
| OMN n = 35 F/M: 21/14 | 4 (11.4) | 21 (60) | 10 (28.6) | 7 (20) | 27 (77.1) | 1 (2.9) | 29 (82.9) | 1 (2.9) | 5 (14.3) | 17 (48.6) | 14 (40) | 4 (11.4) |
| Subgroups | VEGAN | VEGE | PESCA | OMN |
|---|---|---|---|---|
| n (%) | ||||
| ABF (>3 points) | 25 (52.08) | 16 (32.65) | 9 (39.13) | 11 (31.43) |
| IBF (≤3 points) | 23 (47.92) | 33 (67.35) | 14 (60.87) | 24 (68.57) |
| Studied Groups | n | Body Composition Parameters Mean ± SD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Body Water [l] | Protein [kg] | Minerals [kg] | BFM [kg] | Body Mass [kg] | BMI [kg/m2] | PBF [%] | SMM [kg] | WHR | VAT [cm2] | ||
| VEGAN | 48 | 36.0 ± 6.78 | 9.66 ± 1.85 | 3.41 ± 0.576 | 13.3 ± 4.77 | 62.4 ± 9.48 | 21.0 ± 2.24 | 21.4 ± 7.22 | 27.2 ± 5.59 | 0.831 ± 0.041 | 5.27 ± 2.39 |
| VEGE | 49 | 32.4 ± 4.57 | 8.68 ± 1.25 | 3.14 ± 0.432 | 14.4 ± 3.48 | 58.7 ± 7.62 | 20.9 ± 1.96 | 24.6 ± 7.22 | 24.2 ± 3.77 | 0.824 ± 0.038 | 5.57 ± 1.65 |
| PESCA | 23 | 33.1 ± 5.47 | 8.85 ± 1.51 | 3.19 ± 0.512 | 14.1 ± 4.91 | 59.2 ± 10.5 | 20.8 ± 2.24 | 23.4 ± 5.94 | 24.7 ± 4.57 | 0.832 ± 0.042 | 5.52 ± 2.21 |
| OMN | 35 | 37.4 ± 8.71 | 10.1 ± 2.38 | 3.53 ± 0.742 | 14.7 ± 5.05 | 65.7 ± 14.4 | 21.9 ± 3.10 | 22.4 ± 6.00 | 28.4 ± 7.19 | 0.846 ± 0.044 | 5.66 ± 2.41 |
| p-value * | 0.016 | 0.015 | 0.033 | 0.404 | 0.088 | 0.694 | 0.065 | 0.014 | 0.281 | 0.713 | |
| Studied Groups and Subgroups | n | Lipid Profile Mean ± SD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ApoA1 [mg/mL] | ApoB [mg/mL] | ApoB/ ApoA1 | Lp(a) [mg/dL] | TC [mg/dL] | HDL-C [mg/dL] | LDL-C [mg/dL] | TG [mg/dL] | Non-HDL-C [mg/dL] | TC/ HDL-C | |||
| VEGAN | All | 48 | 1.80 ± 1.87 | 1.15 ± 0.983 | 0.900 ± 1.06 | 22.5 ± 17.6 | 142 ± 29.2 | 51.7 ± 10.1 | 70.0 ± 21.0 | 95.1 ± 26.4 | 90.1 ± 25.9 | 2.79 ± 0.596 |
| VEGE | All | 49 | 1.30 ± 0.948 | 1.04 ± 0.700 | 0.944 ± 0.684 | 23.6 ± 17.4 | 157 ± 22.8 | 59.3 ± 13.4 | 74.1 ± 18.2 | 94.0 ± 27.2 | 98.1 ± 19.9 | 2.76 ± 0.613 |
| PESCA | All | 23 | 1.56 ± 1.09 | 0.957 ± 0.495 | 0.871 ± 0.811 | 19.1 ± 16.1 | 160 ± 34.7 | 60.6 ± 13.0 | 75.1 ± 24.8 | 92.4 ± 45.9 | 99.6 ± 35.0 | 2.73 ± 0.698 |
| OMN | All | 35 | 2.14 ± 1.77 | 1.05 ± 0.744 | 0.914 ± 1.28 | 23.8 ± 18.0 | 158 ± 30.3 | 55.8 ± 14.3 | 80.1 ± 20.9 | 77.9 ± 34.7 | 102 ± 27.9 | 2.94 ± 0.778 |
| p-value (1)* | 0.380 | 0.983 | 0.107 | 0.404 | 0.032 | 0.006 | 0.133 | 0.006 | 0.297 | 0.746 | ||
| All participants | ABF | 94 | 1.61 ± 1.454 | 1.01 ± 0.663 | 1.00 ± 1.149 | 19.46 ± 12.956 | 149 ± 30.898 | 56.08 ± 13.817 | 71.32 ± 20.285 | 91.21 ± 36.109 | 93.90 ± 27.260 | 2.78 ± 0.702 |
| IBF | 61 | 1.799 ± 1.625 | 1.139 ± 0.935 | 0.773 ± 0.593 | 27.493 ± 21.659 | 157 ± 26.080 | 56.770 ± 11.694 | 79.046 ± 21.085 | 89.098 ± 26.414 | 100.967 ± 24.647 | 2.858 ± 0.587 | |
| p-value (2)* | 0.311 (U) | 0.965 (U) | 0.335 (U) | 0.042 (U) | 0.107 (tS) | 0.554 (U) | 0.024 (tS) | 0.812 (U) | 0.104 (tS) | 0.188 (U) | ||
| Female and Male Division | n | Lipid Profile Mean ± SD | |||
|---|---|---|---|---|---|
| ApoA1 [mg/mL] | ApoB/ ApoA1 | HDL-C [mg/dL] | |||
| VEGAN | F | 32 | 1.92 ± 2.01 | 0.805 ± 0.669 | 53.4 ± 10.6 |
| M | 16 | 1.55 ± 1.59 | 1.09 ± 1.60 | 48.3 ± 8.43 | |
| VEGE | F | 45 | 1.33 ± 0.980 | 0.975 ± 0.702 | 60.7 ± 12.9 |
| M | 4 | 0.946 ± 0.317 | 0.600 ± 0.290 | 43.8 ± 6.99 | |
| PESC | F | 20 | 1.64 ± 1.15 | 0.873 ± 0.859 | 61.9 ± 13.0 |
| M | 3 | 1.05 ± 0.151 | 0.852 ± 0.461 | 52.0 ± 11.0 | |
| OMN | F | 21 | 2.17 ± 1.78 | 0.763 ± 0.680 | 60.8 ± 15.6 |
| M | 14 | 2.09 ± 1.83 | 1.14 ± 1.87 | 48.4 ± 8.04 | |
| p-value for F | 0.561 | 0.172 | 0.055 | ||
| p-value for M | 0.608 | 0.820 | 0.607 | ||
| VEGAN Subgroups | n | Lipids Profile Mean ± SD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ApoA1 [mg/mL] | ApoB [mg/mL] | ApoB/ ApoA1 | Lp(a) [mg/dL] | TC [mg/dL] | HDL-C [mg/dL] | LDL-C [mg/dL] | TG [mg/dL] | Non-HDL-C [mg/dL] | TC/ HDL-C | ||
| ABF | 23 | 1.308 ± 1.361 | 0.777 ± 0.484 | 0.956 ± 1.347 | 17.061 ± 10.064 | 133.435 ± 32.020 | 51.287 ± 10.160 | 61.322 ± 20.617 | 99.652 ± 32.730 | 82.148 ± 27.410 | 2.659 ± 0.575 |
| IBF | 25 | 2.250 ± 2.173 | 1.499 ± 1.191 | 0.846 ± 0.7333 | 27.457 ± 21.449 | 149.560 ± 24.607 | 52.080 ± 10.243 | 78.048 ± 18.308 | 90.480 ± 18.948 | 97.480 ± 22.600 | 2.942 ± 0.585 |
| p-value * | 0.110 (U) | 0.050 (U) | 0.902 (U) | 0.029 (U) | 0.055 (tS) | 0.789 (tS) | 0.005 (tS) | 0.248 (tW) | 0.039 (tS) | 0.110 (U) | |
| Studied Groups and Subgroups | n | Metabolic and Inflammatory Markers Mean ± SD | ||||
|---|---|---|---|---|---|---|
| TNF-α [ng/L] | HCY [µmol/L] | IL-6 [pg/mL] | hsCRP [mg/L] | |||
| VEGAN | All | 48 | 128 ± 90.6 | 14.3 ± 10.9 | 2.79 ± 2.27 | 4.76 ± 3.07 |
| VEGE | All | 49 | 121 ± 83.8 | 12.8 ± 8.17 | 2.87 ± 2.31 | 3.83 ± 2.50 |
| PESCA | All | 23 | 111 ± 49.1 | 15.3 ± 8.78 | 3.16 ± 2.40 | 5.47 ± 3.45 |
| OMN | All | 35 | 89.1 ± 39.2 | 10.0 ± 5.53 | 3.05 ± 1.87 | 5.19 ± 3.01 |
| p-value (1)* | 0.149 | 0.096 | 0.833 | 0.100 | ||
| All participants | ABF | 94 | 111.18 ± 64.12 | 12.29 ± 7.84 | 3.15 ± 2.16 | 4.75 ± 3.08 |
| IBF | 61 | 119.282 ± 89.12 | 14.166 ± 10.13 | 2.601 ± 2.25 | 4.540 ± 2.85 | |
| p-value (2)* | 0.281 | 0.714 | 0.076 | 0.815 | ||
| VEGAN | ABF | 23 | 93.403 ± 37.8 | 9.914 ± 6.41 | 3.171 ± 2.28 | 5.305 ± 3.15 |
| IBF | 25 | 159.909 ± 112.0 | 18.371 ± 12.57 | 2.444 ± 2.26 | 4.260 ± 2.97 | |
| p-value (3)* | 0.110 | 0.007 | 0.122 | 0.187 | ||
| Blood Lipids and Lipoproteins | Body Composition Parameter | ||
|---|---|---|---|
| ApoA1 [mg/dL] | BFM [kg] | PBF [%] | VAT [cm2] |
| 0.452 (ρ) p < 0.05 | 0.476 (ρ) p < 0.05 | 0.433 (ρ) p < 0.05 | |
| ApoB [mg/dL] | Body mass [kg] | BMI [kg/m2] | WHR |
| 0.461 (ρ) p < 0.05 | 0.517 (ρ) p < 0.05 | 0.489 (ρ) p < 0.05 | |
| Non-HDL-C [mg/dL] | BFM [kg] | PBF [%] | VAT [cm2] |
| 0.432 (r) p < 0.05 | 0.42 (r) p < 0.05 | 0.461 (r) p < 0.05 | |
| Metabolic and inflammatory markers | |||
| IL-6 [pg/mL] | Body mass [kg] | WHR | |
| 0.526 (ρ) p < 0.01 | 0.499 (ρ) p < 0.05 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowska, I.; Olszak, J.; Formanowicz, D. Dietary and Lifestyle Patterns and Their Associations with Cardiovascular and Inflammatory Biomarkers in Vegans, Vegetarians, Pescatarians, and Omnivores: A Cross-Sectional Study. Nutrients 2025, 17, 3634. https://doi.org/10.3390/nu17233634
Kwiatkowska I, Olszak J, Formanowicz D. Dietary and Lifestyle Patterns and Their Associations with Cardiovascular and Inflammatory Biomarkers in Vegans, Vegetarians, Pescatarians, and Omnivores: A Cross-Sectional Study. Nutrients. 2025; 17(23):3634. https://doi.org/10.3390/nu17233634
Chicago/Turabian StyleKwiatkowska, Izabela, Jakub Olszak, and Dorota Formanowicz. 2025. "Dietary and Lifestyle Patterns and Their Associations with Cardiovascular and Inflammatory Biomarkers in Vegans, Vegetarians, Pescatarians, and Omnivores: A Cross-Sectional Study" Nutrients 17, no. 23: 3634. https://doi.org/10.3390/nu17233634
APA StyleKwiatkowska, I., Olszak, J., & Formanowicz, D. (2025). Dietary and Lifestyle Patterns and Their Associations with Cardiovascular and Inflammatory Biomarkers in Vegans, Vegetarians, Pescatarians, and Omnivores: A Cross-Sectional Study. Nutrients, 17(23), 3634. https://doi.org/10.3390/nu17233634







