1. Introduction
EoE is a chronic, immune-mediated esophageal disorder with increasing prevalence of around 1 in 1000 [
1]. Patients typically present with difficulty swallowing and food impaction. The disease displays a wide clinical spectrum, ranging from inflammatory to fibrostenotic forms, and it affects individuals across various age groups and demographics. Diagnosis is confirmed by endoscopy with histologic findings of at least 15 eosinophils per high-power field, basal layer thickening, and widened intercellular spaces, which may progress to strictures and a narrow-caliber esophagus. EoE is primarily triggered by an allergic response to dietary antigens, where epithelial signals such as thymic stromal lymphopoietin and interleukin (IL)-33 stimulate cytokines like IL-5 and IL-13, attracting eosinophils and mast cells that lead to tissue remodeling, scarring, and fibrosis. Its development is multifactorial, involving genetic susceptibility and environmental exposures [
2]. Therapeutic approaches range from proton pump inhibitors and dietary modifications to topical corticosteroids and emerging biologic agents, all aiming to improve long-term outcomes. Esophageal dilation may be considered for patients with strictures and food impaction [
3].
The KD is a high-fat, low-carbohydrate, and adequate protein diet therapy that is used in the dietary management of drug-resistant or refractory epilepsy. Classic ketogenic diets are usually prescribed in ketogenic ratios and are typically defined by grams of fat to combined grams of net carbohydrate (total carbohydrate minus dietary fiber) and grams of protein. Alternatively, modified Atkins diets (MAD) are designed more leniently with limited net carbohydrates and ample fats. These diets prompt the body into a state of nutritional ketosis, which occurs when the body metabolizes fat in the absence of sufficient carbohydrate [
4]. KDs were first studied in patients with epilepsy in the 1920s [
5] and have now been used successfully for over 100 years though its mechanism of action is still poorly understood. The anti-seizure efficacy of KDs in refractory epilepsy has been reported at 30–70% of patients responding with a greater than 50% reduction in seizure frequency and 1–33% experiencing seizure freedom [
6,
7,
8]. These diets can be implemented orally or with tube feedings with a variety of commercially available ketogenic formulas. The medically managed KD for refractory epilepsy is highly restrictive and requires close monitoring by an experienced ketogenic team, including registered dietitians, to ensure nutritional adequacy and optimized seizure control.
As more patients with refractory epilepsy follow a restricted KD, we are increasingly encountering cases of EoE within this group similar to the general population. Alongside standard drug treatments, dietary modifications are becoming a key part of managing EoE. These dietary interventions include adopting elimination diets to limit exposure to common allergens, using specialized formulas that avoid major allergens or incorporate hydrolyzed proteins, and employing amino acid-based feeds or allergen-free home-blenderized diets. What is not clear is how these dietary interventions should be applied to those following a KD. In some instances, discontinuing the ketogenic regimen may be considered to expand food choices.
In our study, we identify the various dietary management strategies that have been used for patients with EoE who are on a KD, aiming to provide insights that can help clinicians tailor care for this complex and extremely rare patient population.
2. Materials and Methods
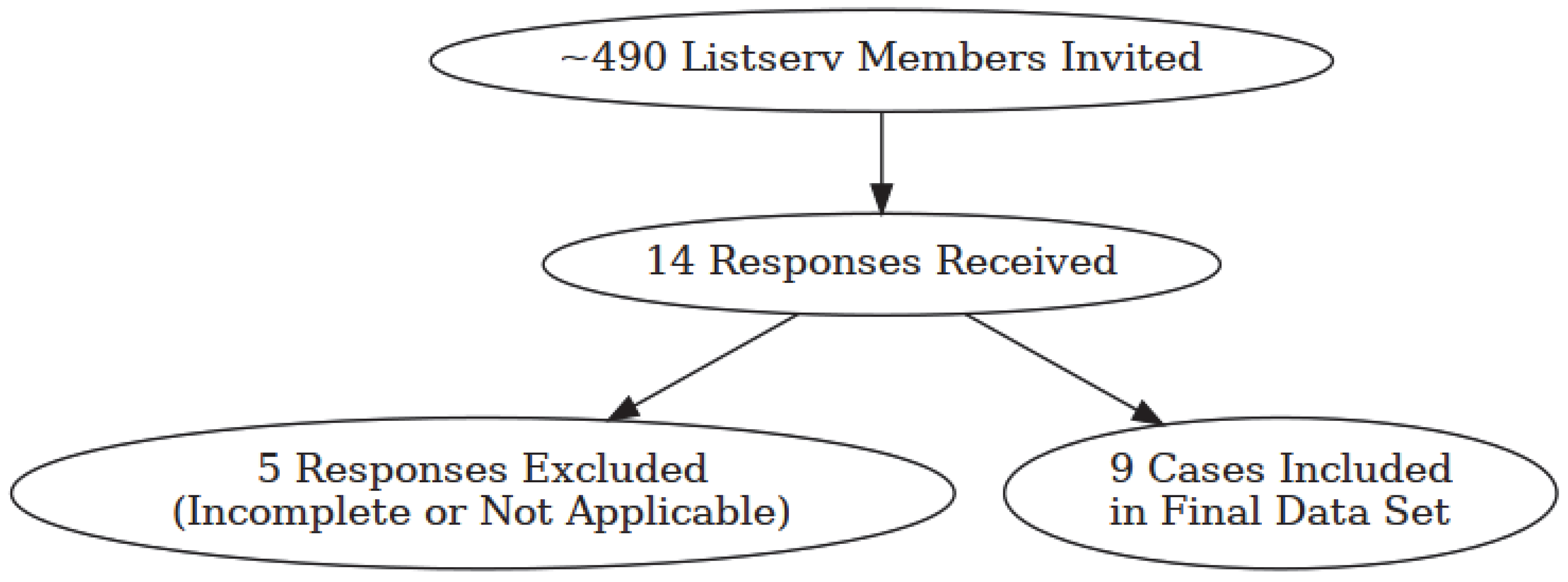
This was a retrospective clinician reported case series. An online survey was conducted via Survey Monkey® (San Mateo, CA, USA) online survey tool and was directed to healthcare professionals. Surveys were sent to registered dietitians specializing in ketogenic therapy for refractory epilepsy in order to contact those who have had a patient also diagnosed with EoE. A survey invitation was sent to the International Keto Dietitians Listserv (ketodietitians Google group email listserv), which at the time the survey was conducted included approximately 490 members. Members of this listserv were practitioners, primarily registered dietitians, currently practicing in the specialty field of ketogenic diet therapy. We aimed to collect a conservative number of at least 5 cases given the presumed rarity of patients on KD who are also diagnosed with EoE. Data was collected between September and December 2024.
The survey included 11 questions and collected information from respondents about their experience in the management of patients on ketogenic therapy who also had a diagnosis of EoE.
The following data was collected:
Patient age at the time of EoE diagnosis;
Patient assigned sex at birth;
Epilepsy diagnosis;
Mode of nutrition (oral, tube-fed or both);
Diet prior to EoE diagnosis;
Diagnosing endoscopy findings;
Elected treatment for EoE;
Diet after EoE diagnosis;
Follow-up endoscopy findings (if applicable);
Patient outcome/response to treatment.
A subjective determination of “improved”, “stable”, or “unsatisfactory response” of EoE symptoms was made based on reported patient outcome/response to treatment description. We did not assess seizure control outcomes. All questions required a response in order to be included in the data set.
Demographic and baseline characteristics are presented in descriptive summaries and, where applicable, a mean and/or median is expressed. Counts and proportions are presented for categorical variables.
The study protocol was reviewed by the Orlando Health IRB and it was determined since subjects are healthcare professionals reflecting on their experiences with the target population that the study would be exempt status. Subjects consented by electing to participate in the survey. No protected health information was collected.
3. Results
A total of 14 responses were received, and 9 reported cases were analyzed. Five survey responses were excluded, as they were incomplete (
n = 4) or not applicable (
n = 1). See
Figure 1 for a breakdown in invitations sent to final data set. All respondents were registered dietitian/nutritionists. All completed and relevant surveys reported were included in the data analysis. These included a total of nine cases of patients with a diagnosis of EoE, confirmed by endoscopy, who were also receiving a KD. Two patients were diagnosed with EoE prior to starting the KD. All other patients (78%,
n = 7/9) were diagnosed with EoE while already on the KD.
A summary of patient characteristics is reported in
Table 1. Of the cases reported, 56% were female and 89% were children. The median age of the patients was 9 years old (ranging from 2 to 30 years). Indications for KD therapy are also included in
Table 1. Patients were fed through different feeding methods with 44% being fed by feeding tube, 33% being fed by both mouth and feeding tube, and 22% being fed by mouth only.
Treatment for EoE was multifaceted, with the majority having some form of dietary management, followed by protein pump inhibitor therapy, swallowed steroids, inhaled steroids, and muscarinic agonist.
Of the KD interventions provided, the tube fed patients, including two of the patients fed by mouth and feeding tube, were transitioned to an extensively hydrolyzed whey protein-based ketogenic formula (n = 3), plant-based ketogenic formulas with modulars (n = 2), or an amino-acid based protein powder with modulars (n = 1). The orally fed patients, including one of the patients fed by mouth and feeding tube, were continued on an oral diet with elimination or restriction of one food, either dairy (n = 2) or nuts (n = 1). One patient was treated only with medications with no specific dietary intervention described.
All patients, except two, reported improved EoE symptom outcomes, including feeding tolerance (
n = 5), normalized esophagogastroduodenoscopy (
n = 2), improved clinically (
n = 1), positive weight gain (
n = 2), and resolution of EoE symptoms (
n = 1). Of the two patients without reported improvement, one reported stable symptoms when continued on a classic KD by mouth and feeding tube. The other continued with episodes of reflux and inflammation on a dairy-restricted KD and protein pump inhibitor therapy. A table of individual patient descriptions are reported in
Table 2. To summarize, 7/9 cases showed symptom improvement, 1/9 case reported stable symptoms and 1/9 case appeared to have worsening/unsatisfactory response. See
Table 3 for a summary of case outcomes.
4. Discussion
This case series describes various strategies by which a KD may be implemented in a patient who is diagnosed with EoE. These included ketogenic formulas with extensively hydrolyzed whey-based protein, plant-based formulas free of most major allergens, amino acid-based modular approach, and restricted oral diets which included elimination or avoidance of dairy and nuts. All cases reported were able to either initiate or continue with ketogenic therapy for their refractory epilepsy, despite their EoE diagnosis.
The rationale for selecting one dietary approach over another in EoE remains largely speculative, as no standardized diet therapy has been universally established. However, dietary interventions commonly focus on the elimination of major food allergens, including cow’s milk, eggs, peanuts, tree nuts, soy, wheat, fish, shellfish, and sesame. For patients who are tube-fed and needing to follow a ketogenic diet, commercially available ketogenic formulas offer treatment alternatives that can exclude many of these allergens. In this study, the most frequently used formula was an extensively hydrolyzed whey-based ketogenic product. Although derived from milk, the protein in this formula is broken down into very small peptides, which may be tolerated by some individuals with milk sensitivities. Additionally, two different plant-based ketogenic formulas were utilized, both of which are free from most major food allergens. While amino acid-based ketogenic formulas are not currently available commercially, they can be custom made using individual amino acids and other nutritional modulars to create a nutritionally complete formula. One case in this study employed this approach presumably to avoid reactions to intact protein sources. For orally fed patients, dietary management typically involves the elimination of major allergens through a process of trial and error. In this cohort, individuals receiving oral nutrition had chosen to exclude dairy and/or nuts from their diets, one of whom was not successful in controlling EoE symptoms.
Another potential dietary approach to the KD for a patient diagnosed with EoE not described with this patient set is a home blenderized tube-fed diet free of major allergens. Although this approach requires more effort from families and carries an increased risk of foodborne illness, when carefully managed, it could offer another viable option for patients with EoE.
Most patients received some form of dietary intervention when diagnosed with EoE. The elected course of intervention may be best guided by what is tolerated and feasible for the patient, including a medication-only approach as was reported in one subject here. Interestingly, the two patients who continued to experience EoE symptoms were on some form of oral diet, suggesting that it may be more challenging to control allergen exposure in patients fed orally with conventional foods. Conversely, some patients on oral and tube feeding regimens were reported to have positive outcomes. When it comes to ketogenic formulas, it is not clear if there is an advantage to selecting specific protein sources to best manage the immune response that occurs with EoE. In this series, a variety of nutrition approaches were used. Most of these patients were reported to tolerate their ketogenic feeding and some of them reported a normalized EGD. Overall, the data collected is not sufficient to determine whether any one protein source offered distinct clinical advantages.
There is a need for standardized nutrition interventions for EoE, including for patients who also require KDs for the management of refractory epilepsy. One clear takeaway from this series is that a diagnosis of EoE does not necessitate the discontinuation or avoidance of ketogenic therapy. Some evidence suggests that reducing inflammation could be an important disease-modifying effect of a KD [
9]. Reducing inflammation would have a beneficial effect in patients with refractory epilepsy, as well as in those patients diagnosed concurrently with EoE. In mice models, the KD, with elevated plasma ketones, showed reduced pain and inflammation thought to be related to increased levels of adenosine and/or GABA, two powerful inhibitory neurotransmitters in the nervous system [
10].
Additionally, KD alters the gut microbiome, reducing microbe populations that promote pro-inflammatory cytokine signaling and increasing taxa associated with anti-inflammatory profiles [
11]. This microbial shift may also influence Th2-driven pathways, which are central to EoE pathogenesis. Dysbiosis in EoE has been linked to enhanced Th2 responses and eosinophilic infiltration of the esophagus [
12]. By promoting a gut environment less conducive to Th2 polarization and reducing systemic inflammatory mediators, KD may plausibly mitigate the chronic inflammation characteristic of EoE. However, this mechanistic rationale would be highly influenced by a variety of factors including age, fiber intake, pharmaceutical use, host genetics, among many others, making its overall impact challenging to predict.
This study has several limitations. First, its retrospective design introduces the potential for recall bias and limits the accuracy of the data collected. The small sample size (n = 9) restricts the ability to conduct statistical analyses and limits the generalizability of the findings. Additionally, follow-up EGD results were unavailable for some cases post-diet intervention, although these individuals were reported to have tolerated diet changes. The absence of long-term outcome data prevents assessment of the sustained efficacy of the interventions. Furthermore, there was considerable variability in both medical treatments and dietary approaches across cases, making it difficult to isolate the effects of any single intervention. The concurrent use of medications, such as PPIs and corticosteroids, further complicates attribution of clinical improvement solely to dietary modifications. Lastly, the sample was drawn from a specialized dietitian listserv, which may have introduced selection bias toward more successful or memorable cases, potentially skewing the results.
Further research with a larger patient pool and standardized follow-up, including evaluation of seizure control outcomes following dietary changes for EoE, is needed to validate and expand upon these findings. Capturing data on medical and dietary interventions, diet tolerance, inflammatory markers, endoscopic/histologic scores, long-term outcomes, adverse events, and impact on seizure control in this population would establish the evidence needed to clarify effective interventions and help guide practice.
5. Conclusions
There are different dietary strategies to approach KD implementation in those diagnosed with EoE. Our findings provide a foundation for further investigation and highlight the potential for use of KDs for the dietary management of refractory epilepsy in patients also diagnosed with EoE. These results suggest EoE is not a contraindication to a KD, but ideal nutrition intervention in this group needs further exploration.
Author Contributions
Conceptualization, R.J., K.U., A.P. and V.P.; methodology, R.J., K.U., A.P. and V.P.; investigation, R.J. and K.U.; resources, A.P. and V.P.; data curation, R.J. and K.U.; writing—original draft preparation, R.J. and K.U.; writing—review and editing, R.J., K.U., A.P. and V.P.; visualization, R.J., K.U., A.P. and V.P.; supervision, R.J., K.U., A.P. and V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Orlando Health as Exempt Status (Reference # 24.091.07, 12 September 2024).
Informed Consent Statement
Patient consent was not required as the survey was directed to healthcare practitioners caring for patients and was voluntary with no direct benefits from participating in this study. No identifiable data or PHI was requested for case subjects. No expected physical, psychological, social, economic, or legal risks were expected for participating in this survey. The participants were not requested to sign a consent to participate in the survey, but they did receive a copy of the study information and, if they agreed to participate in the study, were provided a link to the survey in the study information sheet.
Data Availability Statement
Data set available upon request from the authors. The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors would like to thank the members of the International Keto Dietitians Listserv (ketodietitians Google group email listserv) for sharing their experiences with this unique patient population. All product selection and care decisions were made by clinicians independently and collected retrospectively; no company influenced inclusion, analysis, or results.
Conflicts of Interest
Akash Pandey is a paid presenter for Kate Farms® Medical, Mead Johnson & Company LLC, Alcresta® Therapeutics, Mirum Pharma, and Ipsen Pharma. Rebecca Jennings is employed by Ajinomoto Cambrooke, Inc. The remaining authors have no conflicts of interest.
References
- Dellon, E.S.; Hirano, I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology 2018, 154, 319–332.e3. [Google Scholar] [CrossRef]
- Khokhar, D.; Marella, S.; Idelman, G.; Chang, J.W.; Chehade, M.; Hogan, S.P. Eosinophilic esophagitis: Immune mechanisms and therapeutic targets. Clin. Exp. Allergy 2022, 52, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.C.; Dellon, E.S. Eosinophilic Esophagitis. Med. Clin. N. Am. 2019, 103, 29–42. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G.; et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018, 3, 175–192. [Google Scholar] [CrossRef]
- Wheless, J.W. History of the ketogenic diet. Epilepsia 2008, 49 (Suppl. 8), 3–5. [Google Scholar] [CrossRef] [PubMed]
- Neal, E.G.; Chaffe, H.; Schwartz, R.H.; Lawson, M.S.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J.H. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia 2009, 50, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Turner, Z.; Doerrer, S.C.; Stanfield, A.; Kossoff, E.H. Complications During Ketogenic Diet Initiation: Prevalence, Treatment, and Influence on Seizure Outcomes. Pediatr. Neurol. 2017, 68, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, M.R.; Hosseini, S.A.; Zamani, G.R.; Mohammadi, M.; Tavassoli, A.; Badv, R.S.; Heidari, M.; Karimi, P.; Malamiri, R.A. The efficacy of the ketogenic diet in infants and young children with refractory epilepsies using a formula-based powder. Acta Neurol. Belg. 2017, 117, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Masino, S.A.; Rho, J.M. Mechanisms of Ketogenic Diet Action. In Jasper’s Basic Mechanisms of the Epilepsies, 4th ed.; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2012. [Google Scholar]
- Ruskin, D.N.; Kawamura, M.; Masino, S.A. Reduced Pain and Inflammation in Juvenile and Adult Rats Fed a Ketogenic Diet. PLoS ONE 2009, 4, e8349. [Google Scholar] [CrossRef] [PubMed]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef] [PubMed]
- Angerami Almeida, K.; de Queiroz Andrade, E.; Burns, G.; Hoedt, E.C.; Mattes, J.; Keely, S.; Collison, A. The microbiota in eosinophilic esophagitis: A systematic review. J. Gastroenterol. Hepatol. 2022, 37, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).