Longitudinal Association Between Menopausal Transition and Obstructive Sleep Apnea with Effect Modification by Salt Intake: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
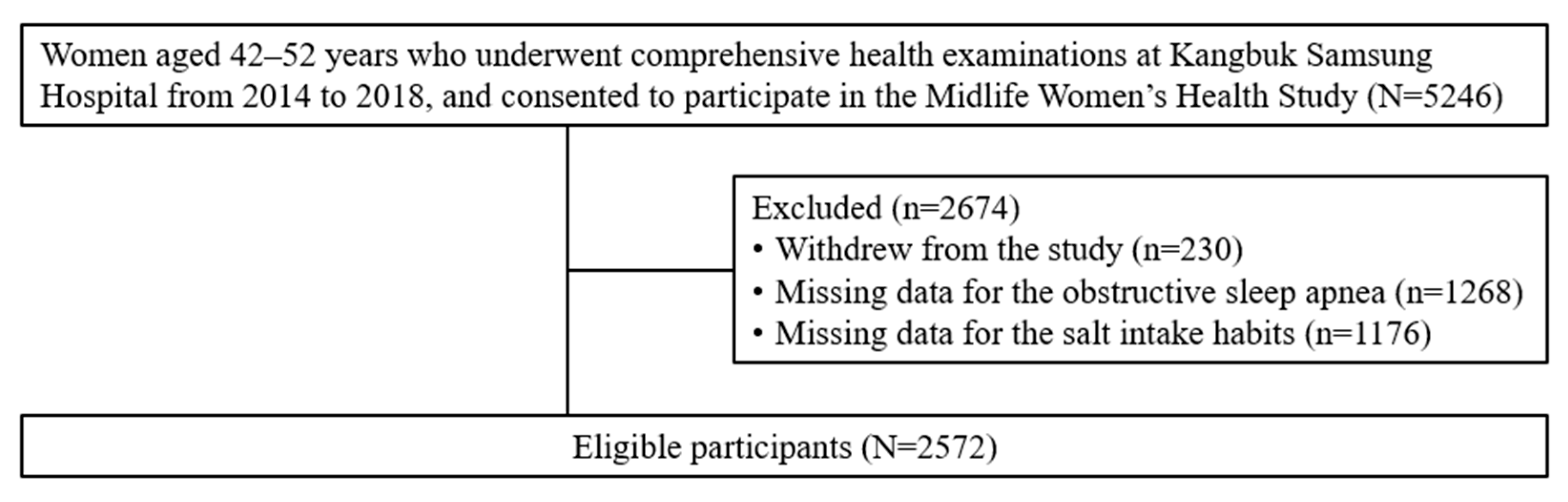
2.1. Study Population
2.2. Measurement
2.3. Definition of Menopausal Stages
2.4. Assessment of Salt Intake Habits
2.5. Definition of OSA
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CI | confidence interval |
| GEE | generalized estimating equation |
| HEPA | health-enhancing physical activity |
| NIH | National Institute of Health |
| OSA | Obstructive sleep apnea |
| STRAW | Stages of Reproductive Aging Workshop |
References
- Dantas, A.B.d.A.; Gonçalves, F.M.; Martins, A.A.; Alves, G.Â.; Stechman-Neto, J.; Corrêa, C.d.C.; Santos, R.S.; Nascimento, W.V.; de Araujo, C.M.; Taveira, K.V.M. Worldwide prevalence and associated risk factors of obstructive sleep apnea: A meta-analysis and meta-regression. Sleep Breath 2023, 27, 2083–2109. [Google Scholar] [CrossRef] [PubMed]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Cunningham, J.; Hunter, M.; Budgeon, C.; Murray, K.; Knuiman, M.; Hui, J.; Hillman, D.; Singh, B.; James, A. The prevalence and comorbidities of obstructive sleep apnea in middle-aged men and women: The Busselton Healthy Ageing Study. J. Clin. Sleep Med. 2021, 17, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Strenth, C.; Wani, A.; Alla, R.; Khan, S.; Schneider, F.D.; Thakur, B. Obstructive Sleep Apnea and Its Cardiac Implications in the United States: An Age-Stratified Analysis Between Young and Older Adults. J. Am. Heart Assoc. 2024, 13, e033810. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.; Ayappa, I.; Ayas, N.; Beaudin, A.E.; Hoyos, C.; Kushida, C.A.; Kaminska, M.; Mullins, A.; Naismith, S.L.; Osorio, R.S.; et al. The link between obstructive sleep apnea and neurocognitive impairment: An official American thoracic society workshop report. Ann. Am. Thorac. Soc. 2022, 19, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, S.; Bardwell, W.A.; Guglielmi, O.; Chiorri, C.; Bonanni, E.; Magnavita, N. Association of anxiety and depression in obstructive sleep apnea patients: A systematic review and meta-analysis. Behav. Sleep Med. 2020, 18, 35–57. [Google Scholar] [CrossRef]
- BaHammam, A.S. The gender gap in obstructive sleep apnea: Unmasking the disproportionate costs on women. Sleep 2025, 48, zsaf068. [Google Scholar] [CrossRef]
- Bouloukaki, I.; Tsiligianni, I.; Schiza, S. Evaluation of obstructive sleep apnea in female patients in primary care: Time for improvement? Med. Princ. Pract. 2021, 30, 508–514. [Google Scholar] [CrossRef]
- Zhou, P.; Li, H.; Li, H.; Chen, Y.; Lv, Y. A possible important regulatory role of estrogen in obstructive sleep apnea hypoventilation syndrome. Front. Med. 2025, 12, 1369393. [Google Scholar] [CrossRef] [PubMed]
- Perger, E.; Mattaliano, P.; Lombardi, C. Menopause and sleep apnea. Maturitas 2019, 124, 35–38. [Google Scholar] [CrossRef]
- Li, T.; Song, L.; Li, G.; Li, F.; Wang, X.; Chen, L.; Rong, S.; Zhang, L. Eating habit of adding salt to foods and incident sleep apnea: A prospective cohort study. Respir. Res. 2023, 24, 5. [Google Scholar] [CrossRef]
- Fiori, C.Z.; Martinez, D.; Montanari, C.C.; Lopez, P.; Camargo, R.; Sezerá, L.; Gonçalves, S.C.; Fuchs, F.D. Diuretic or sodium-restricted diet for obstructive sleep apnea—A randomized trial. Sleep 2018, 41, zsy016. [Google Scholar] [CrossRef]
- Han, Y.-J.; Jang, E.-H.; Lee, S. Sodium intake trend and current intake level by meal provision place among the citizens of Seoul. Nutr. Res. Pract. 2023, 17, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.R.; Chang, Y.; Park, J.; Cho, Y.; Kim, C.; Kwon, M.-J.; Kang, J.; Kwon, R.; Lim, G.-Y.; Ahn, J.; et al. Early-onset vasomotor symptoms and development of depressive symptoms among premenopausal women. J. Affect. Disord. 2024, 354, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Namgoung, S.; Chang, Y.; Woo, C.; Kim, Y.; Kang, J.; Kwon, R.; Lim, G.; Choi, H.R.; Kim, K.; Kim, H.; et al. Metabolically healthy and unhealthy obesity and risk of vasomotor symptoms in premenopausal women: Cross-sectional and cohort studies. BJOG Int. J. Obstet. Gynaecol. 2022, 129, 1926–1934. [Google Scholar] [CrossRef]
- Jang, Y.; Chang, Y.; Park, J.; Kim, C.; Jeon, S.W.; Kang, J.; Kwon, R.; Lim, G.-Y.; Kim, K.-H.; Kim, H.; et al. Menopausal stage transitions and their associations with overall and individual sleep quality in middle-aged Korean women. J. Affect. Disord. 2025, 368, 82–89. [Google Scholar] [CrossRef]
- Park, J.; Chang, Y.; Choi, H.R.; Kim, J.H.; Seo, S.W.; Ryu, H.J.; Cho, Y.; Kim, C.; Kwon, R.; Lim, G.-Y.; et al. Overactive bladder and cognitive impairment in middle-aged women: A cross-sectional study. Maturitas 2024, 187, 108042. [Google Scholar] [CrossRef]
- Agaku, I.T.; King, B.A.; Dube, S.R. Current cigarette smoking among adults—United States, 2005–2012. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 29–34. [Google Scholar]
- Liu, Y.; Colditz, G.A.; Rosner, B.; Berkey, C.S.; Collins, L.C.; Schnitt, S.J.; Connolly, J.L.; Chen, W.Y.; Willett, W.C.; Tamimi, R.M. Alcohol intake between menarche and first pregnancy: A prospective study of breast cancer risk. J. Natl. Cancer Inst. 2013, 105, 1571–1578. [Google Scholar] [CrossRef]
- Chun, M.Y. Validity and reliability of Korean version of international physical activity questionnaire short form in the elderly. Korean J. Fam. Med. 2012, 33, 144. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Yang, Y.J.; Kim, B.; Kang, J.-H. Validity and reliability of Korean version of International Physical Activity Questionnaire (IPAQ) short form. Korean J. Fam. Med. 2007, 28, 532–541. [Google Scholar]
- Park, S.E.; Ko, S.-H.; Kim, J.Y.; Kim, K.; Moon, J.H.; Kim, N.H.; Han, K.D.; Choi, S.H.; Cha, B.S. Diabetes fact sheets in Korea 2024. Diabetes Metab. J. 2025, 49, 24–33. [Google Scholar] [CrossRef]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.; Rebar, R.W.; Sherman, S.; Sluss, P.M.; de Villiers, T.J. Executive summary of the Stages of Reproductive Aging Workshop + 10: Addressing the unfinished agenda of staging reproductive aging. J. Clin. Endocrinol. Metab. 2012, 97, 1159–1168. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, J.E.; Chang, Y.; Kim, M.K.; Sung, E.; Shin, H.; Ryu, S. Dietary sodium and potassium intake in relation to non-alcoholic fatty liver disease. Br. J. Nutr. 2016, 116, 1447–1456. [Google Scholar] [CrossRef]
- Kim, H.J.; Paik, H.Y.; Lee, S.Y.; Shim, J.E.; Kim, Y.S. Salt usage behaviors are related to urinary sodium excretion in normotensive Korean adults. Asia Pac. J. Clin. Nutr. 2007, 16, 122–128. [Google Scholar] [PubMed]
- Chung, F.; Abdullah, H.R.; Liao, P. STOP-Bang questionnaire: A practical approach to screen for obstructive sleep apnea. Chest 2016, 149, 631–638. [Google Scholar] [CrossRef]
- Ong, T.H.; Raudha, S.; Fook-Chong, S.; Lew, N.; Hsu, A.A.L. Simplifying STOP-BANG: Use of a simple questionnaire to screen for OSA in an Asian population. Sleep Breath 2010, 14, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Vana, K.D.; Silva, G.E.; Goldberg, R. Predictive abilities of the STOP-Bang and Epworth Sleepiness Scale in identifying sleep clinic patients at high risk for obstructive sleep apnea. Res. Nurs. Health 2013, 36, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.-I.; Kim, D.-H.; Kim, J.-S.; Shin, W.C. Usefulness of using alternative body-mass index and neck circumference criteria for STOP-Bang questionnaire in screening South Korean obstructive sleep apnea patients. Sleep Med. Res. 2020, 11, 38–43. [Google Scholar] [CrossRef]
- Kim, B.; Lee, E.M.; Chung, Y.-S.; Kim, W.-S.; Lee, S.-A. The utility of three screening questionnaires for obstructive sleep apnea in a sleep clinic setting. Yonsei Med. J. 2015, 56, 684. [Google Scholar] [CrossRef]
- Pavarangkul, T.; Jungtrakul, T.; Chaobangprom, P.; Nitiwatthana, L.; Jongkumchok, W.; Morrakotkhiew, W.; Kachenchart, S.; Chindaprasirt, J.; Limpawattana, P.; Srisaenpang, S.; et al. The Stop-Bang Questionnaire as a Screening Tool for obstructive sleep apnea-induced hypertension in Asian population. Neurol. Int. 2016, 8, 6104. [Google Scholar] [CrossRef]
- Kim, H.C.; Lee, H.; Lee, H.-H.; Ahn, S.V.; Lee, J.-M.; Cheon, D.Y.; Jhee, J.H.; Yoon, M.; Shin, M.-H.; Heo, J.; et al. Korea hypertension fact sheet 2024: Nationwide population-based analysis with a focus on young adults. Clin. Hypertens. 2025, 31, e11. [Google Scholar] [CrossRef]
- Zorn, C.J. Generalized estimating equation models for correlated data: A review with applications. Am. J. Political Sci. 2001, 45, 470–490. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, J.; Li, R.; You, J. Semiparametric longitudinal model with irregular time autoregressive error process. Stat. Sin. 2015, 25, 507–527. [Google Scholar] [CrossRef]
- Chesnaye, N.C.; Stel, V.S.; Tripepi, G.; Dekker, F.W.; Fu, E.L.; Zoccali, C.; Jager, K.J. An introduction to inverse probability of treatment weighting in observational research. Clin. Kidney J. 2022, 15, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.R.; Saaresranta, T.; Riha, R.L. Sex differences in obstructive sleep apnoea. Eur. Respir. Rev. 2019, 28, 190030. [Google Scholar] [CrossRef]
- Geer, J.H.; Hilbert, J. Gender Issues in Obstructive Sleep Apnea. Yale J. Biol. Med. 2021, 94, 487–496. [Google Scholar]
- Chen, L.; Pivetta, B.; Nagappa, M.; Saripella, A.; Islam, S.; Englesakis, M.; Chung, F. Validation of the STOP-Bang questionnaire for screening of obstructive sleep apnea in the general population and commercial drivers: A systematic review and meta-analysis. Sleep Breath 2021, 25, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.; Yan, E.; Chung, F. The STOP-Bang questionnaire: A narrative review on its utilization in different populations and settings. Sleep Med. Rev. 2024, 78, 102007. [Google Scholar] [CrossRef]
- White, L.H.; Bradley, T.D. Bradley, Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J. Physiol. 2013, 591, 1179–1193. [Google Scholar] [CrossRef]
- Kazi, R.N.A. Silent Effects of High Salt: Risks Beyond Hypertension and Body’s Adaptation to High Salt. Biomedicines 2025, 13, 746. [Google Scholar] [CrossRef]
- Anderson, D.E.; Parsons, B.A.; McNeely, J.D.; Miller, E.R. Salt sensitivity of blood pressure is accompanied by slow respiratory rate: Results of a clinical feeding study. J. Am. Soc. Hypertens. 2007, 1, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Balcan, B.; Akdeniz, B.; Peker, Y.; Collaborators, T.T. Obstructive Sleep Apnea and Pulmonary Hypertension: A Chicken-and-Egg Relationship. J. Clin. Med. 2024, 13, 2961. [Google Scholar] [CrossRef]
- Brown, J.; Yazdi, F.; Jodari-Karimi, M.; Owen, J.G.; Reisin, E. Obstructive Sleep Apnea and Hypertension: Updates to a Critical Relationship. Curr. Hypertens. Rep. 2022, 24, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Saaresranta, T.; Anttalainen, U.; Polo, O. Sleep disordered breathing: Is it different for females? ERJ Open Res. 2015, 1, 00063–2015. [Google Scholar] [CrossRef]
- Moccia, P.; Belda-Montesinos, R.; Monllor-Tormos, A.; Chedraui, P.; Cano, A. Body weight and fat mass across the menopausal transition: Hormonal modulators. Gynecol. Endocrinol. 2021, 38, 99–104. [Google Scholar] [CrossRef]
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium intake and hypertension. Nutrients 2019, 11, 1970. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A.; et al. Menopause transition and cardiovascular disease risk: Implications for timing of early prevention: A scientific statement from the American Heart Association. Circulation 2020, 142, e506–e532. [Google Scholar] [CrossRef]
- Shen, W.; Cai, L.; Wang, B.; Li, J.; Sun, Y.; Chen, Y.; Xia, F.; Wang, N.; Lu, Y. Associations of a proinflammatory diet, habitual salt intake, and the onset of type 2 diabetes: A prospective cohort study from the UK Biobank. Diabetes Obes. Metab. 2024, 26, 2119–2127. [Google Scholar] [CrossRef]
- Shaw, P.A.; Deffner, V.; Keogh, R.H.; Tooze, J.A.; Dodd, K.W.; Küchenhoff, H.; Kipnis, V.; Freedman, L.S. Epidemiologic analyses with error-prone exposures: Review of current practice and recommendations. Ann. Epidemiol. 2018, 28, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Freedman, L.S.; Commins, J.M.; Moler, J.E.; Arab, L.; Baer, D.J.; Kipnis, V.; Midthune, D.; Moshfegh, A.J.; Neuhouser, M.L.; Prentice, R.L.; et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am. J. Epidemiol. 2014, 180, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Soh, Y.C.; Fairley, A.; Alawad, M.; Lee, S.S.; Su, T.T.; Stephan, B.C.M.; Reidpath, D.; Robinson, L.; Yasin, S.; Siervo, M.; et al. Assessing sodium intake in middle-aged and older adults with elevated blood pressure: Validation of spot urine excretion and dietary survey-derived estimates. Nutrients 2024, 16, 1461. [Google Scholar] [CrossRef]
- Stayner, L.; Pearce, N.; Nøhr, E.A.; Freeman, L.B.; Deffner, V.; Ferrari, P.; Freedman, L.S.; Kogevinas, M.; Kromhout, H.; Lewis, S.; et al. Information bias: Misclassification and mismeasurement of exposure and outcome. In Statistical Methods in Cancer Research Volume V: Bias Assessment in Case–Control and Cohort Studies for Hazard Identification; International Agency for Research on Cancer: Lyon, France, 2024. [Google Scholar]
- Lee, M.-K.; Choi, J.H.; Lee, J.Y. Validity of Modified STOP-Bang Questionnaire as a Screening Tool for Obstructive Sleep Apnea. Ann. Otol. Rhinol. Laryngol. 2024, 133, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, W.; Liang, C.; Yan, X.; Zhang, H.; Dai, H.; Yu, H.; Zhang, H.; An, H.; Zhao, Y. Accuracy and modification of the STOP-bang questionnaire for screening patients with obstructive sleep apnea in China. J. Sleep Res. 2023, 32, e13781. [Google Scholar] [CrossRef] [PubMed]
| Variable | Frequency (%) |
|---|---|
| Age at baseline 1 | 48.3 ± 3.5 |
| Risk factors for OSA | |
| Snoring | 300 (11.7) |
| Tiredness | 1373 (53.4) |
| Observed apnea | 57 (2.2) |
| Presence of hypertension | 160 (6.2) |
| BMI (≥30.0 kg/m2) | 57 (2.2) |
| Age (≥50 years) | 871 (33.6) |
| Neck circumference (≥41 cm) | 79 (3.1) |
| Menopausal stages | |
| Pre-menopause | 942 (36.6) |
| Early transition | 515 (20.0) |
| Late transition | 500 (19.4) |
| Post-menopause | 615 (23.9) |
| Age at menarche | |
| <12 years | 114 (4.4) |
| 12–13 years | 922 (35.9) |
| 14–16 years | 1446 (56.2) |
| ≥17 years | 90 (3.5) |
| Smoking | |
| Never | 2235 (86.9) |
| Current/Former | 323 (12.6) |
| Unknown | 14 (0.5) |
| Alcohol consumption | |
| <10 g/day | 2404 (93.5) |
| ≥10 g/day | 168 (6.5) |
| Parity | |
| Nulliparous | 186 (7.2) |
| Parous | 2340 (91.0) |
| Unknown | 46 (1.8) |
| Marital status | |
| Married/co-habiting | 2408 (93.6) |
| Unmarried | 101 (3.9) |
| Divorced/Separated/Widowed | 63 (2.5) |
| Education | |
| ≤High school | 473 (18.4) |
| ≥College | 2060 (80.1) |
| Unknown | 39 (1.5) |
| Physical activity 2 | |
| Inactivity | 872 (33.9) |
| Moderate activity | 1259 (49.0) |
| HEPA | 441 (17.2) |
| History of diabetes mellitus 3 | |
| Yes | 74 (2.9) |
| Medication for hyperlipidemia | |
| Yes | 112 (4.3) |
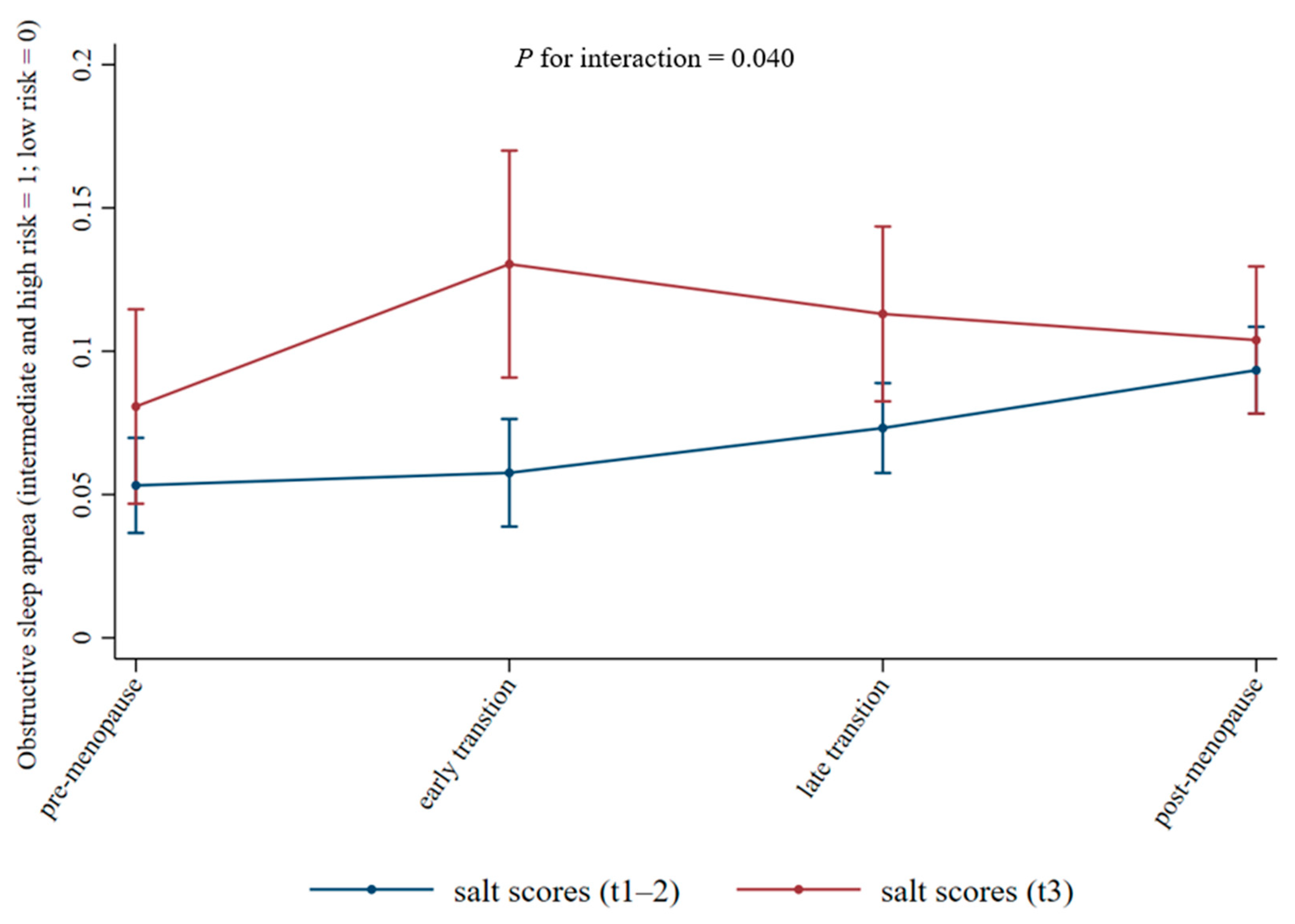
| Variable | Coefficient (95% CI) | p for Interaction | |
|---|---|---|---|
| Salt Intake Scores (Tertiles 1–2) | Salt Intake Scores (Tertile 3) | ||
| Menopausal transition | 0.040 | ||
| Pre-menopause | Ref | Ref | |
| Early transition | 0.10 (−0.38–0.58) | 0.64 (0.02–1.27) | |
| Late transition | 0.40 (−0.02–0.82) | 0.45 (−0.19–1.08) | |
| Post-menopause | 0.72 (0.26–1.17) | 0.33 (−0.32–0.98) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Jang, Y.; Chang, Y.; Ryu, S. Longitudinal Association Between Menopausal Transition and Obstructive Sleep Apnea with Effect Modification by Salt Intake: A Prospective Cohort Study. Nutrients 2025, 17, 3612. https://doi.org/10.3390/nu17223612
Shin S, Jang Y, Chang Y, Ryu S. Longitudinal Association Between Menopausal Transition and Obstructive Sleep Apnea with Effect Modification by Salt Intake: A Prospective Cohort Study. Nutrients. 2025; 17(22):3612. https://doi.org/10.3390/nu17223612
Chicago/Turabian StyleShin, Sujeong, Yoonyoung Jang, Yoosoo Chang, and Seungho Ryu. 2025. "Longitudinal Association Between Menopausal Transition and Obstructive Sleep Apnea with Effect Modification by Salt Intake: A Prospective Cohort Study" Nutrients 17, no. 22: 3612. https://doi.org/10.3390/nu17223612
APA StyleShin, S., Jang, Y., Chang, Y., & Ryu, S. (2025). Longitudinal Association Between Menopausal Transition and Obstructive Sleep Apnea with Effect Modification by Salt Intake: A Prospective Cohort Study. Nutrients, 17(22), 3612. https://doi.org/10.3390/nu17223612






