Supplementation with hArg During the Rapid Growth of the Placenta Modulates Final Placental Angiogenesis and Pregnancy Outcomes
Abstract
1. Introduction
2. Materials and Methods
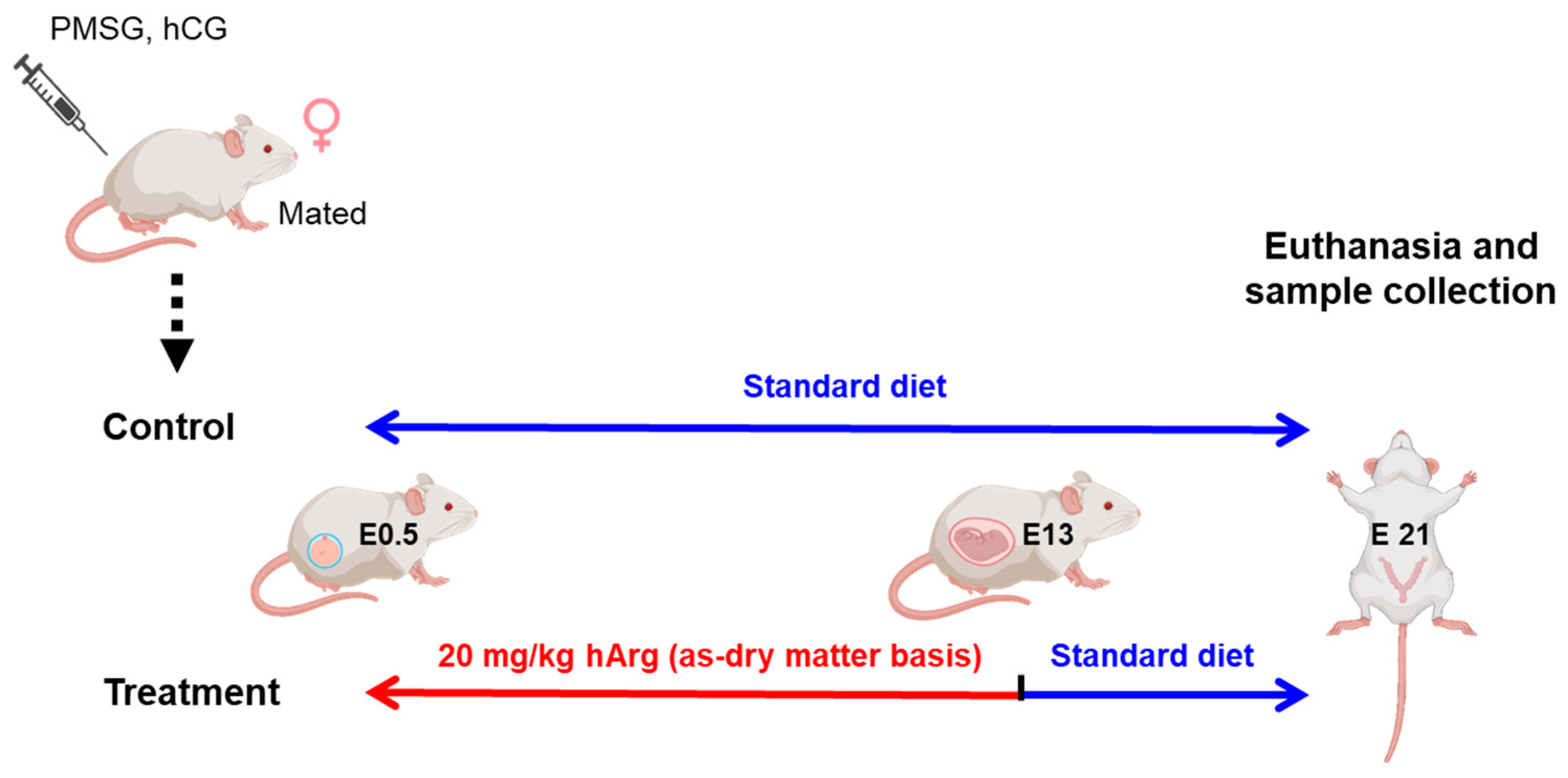
2.1. Experimental Animal Design
2.2. Tissue Collection
2.3. Pregnancy Outcome
2.4. Amino Acid Analysis
2.5. Assessment of Reproductive Hormones
2.6. Histologic Analysis of the Placenta
2.7. Immunofluorescence Analysis of the Placenta
2.8. Quantitative Polymerase Chain Reaction (qPCR)
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
3.1. Maternal hArg Supplementation Ameliorates Pregnancy Outcomes at E21
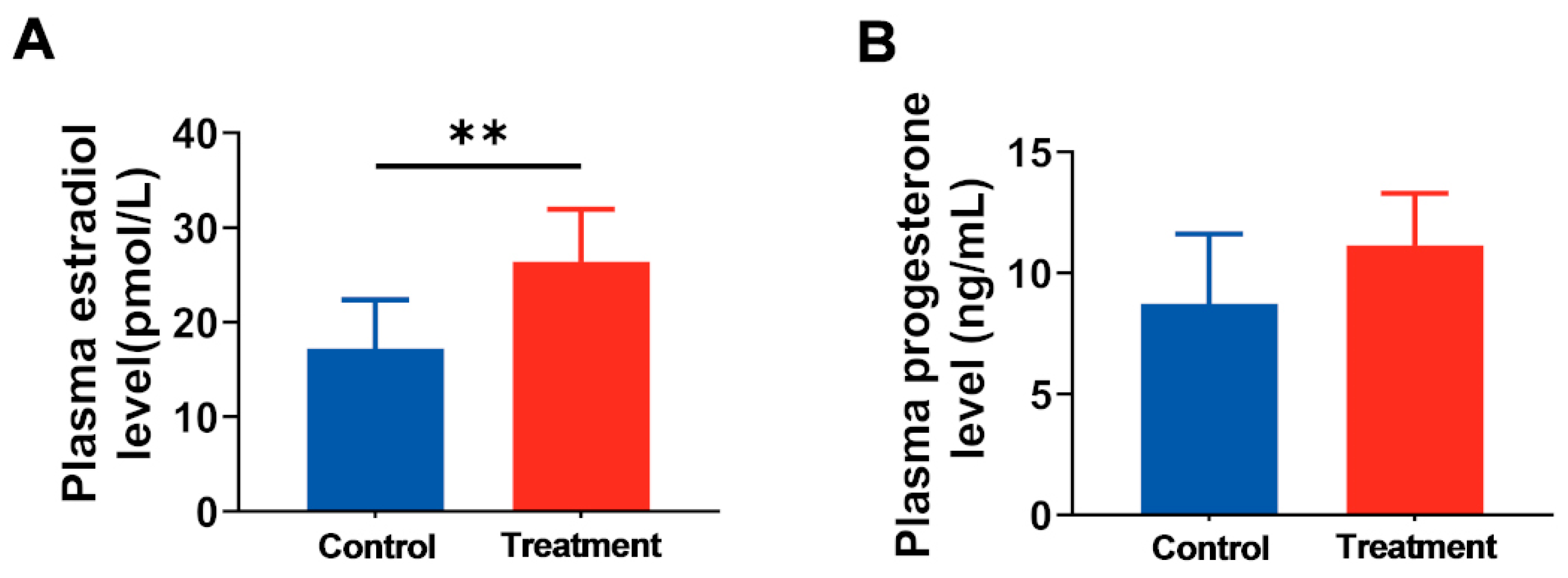
3.2. Maternal hArg Supplementation Affected the Reproductive Endocrine System of Pregnant Rats
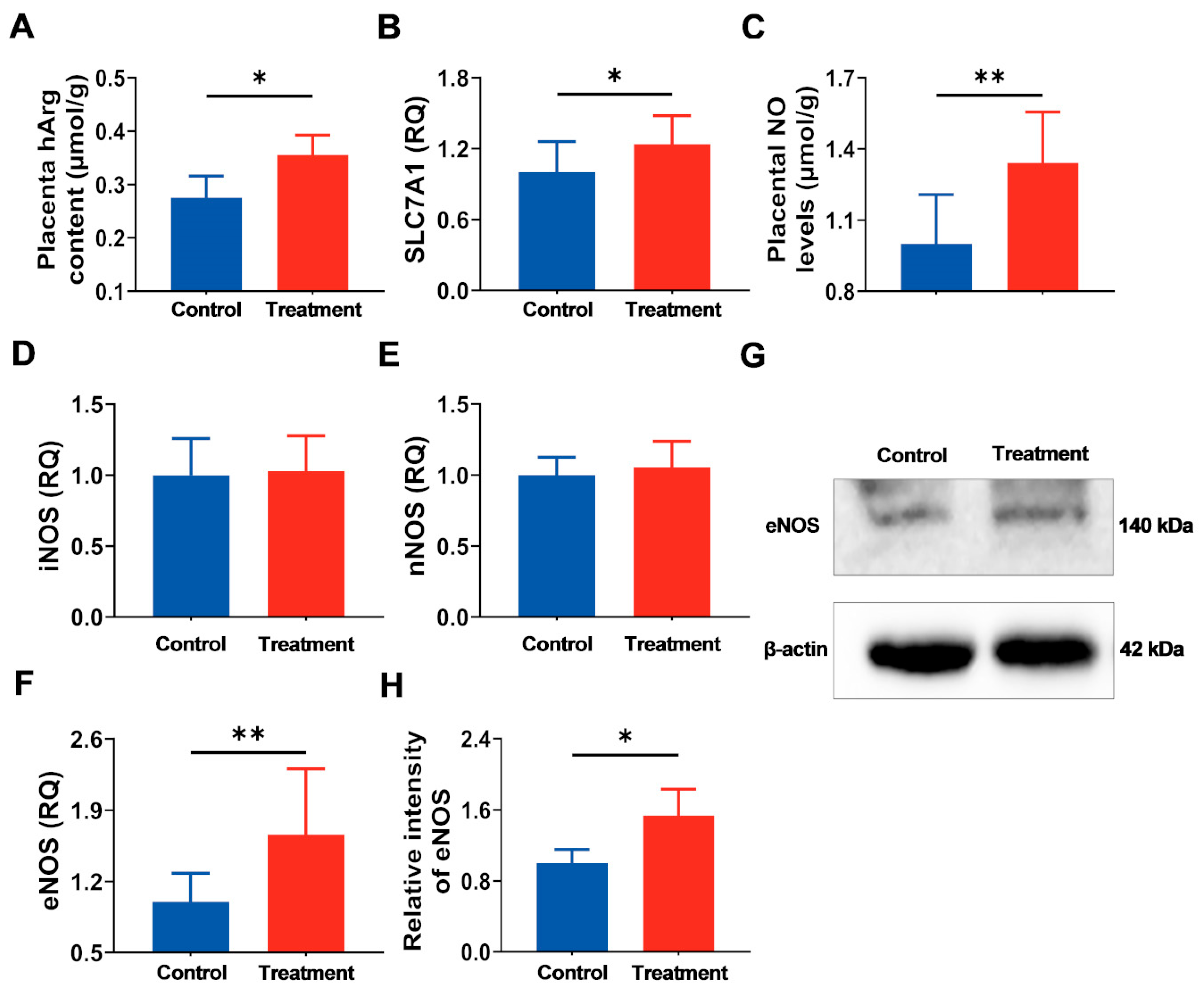
3.3. Supplementation of Maternal hArg Promotes hArg Transport and Metabolism in the Placenta
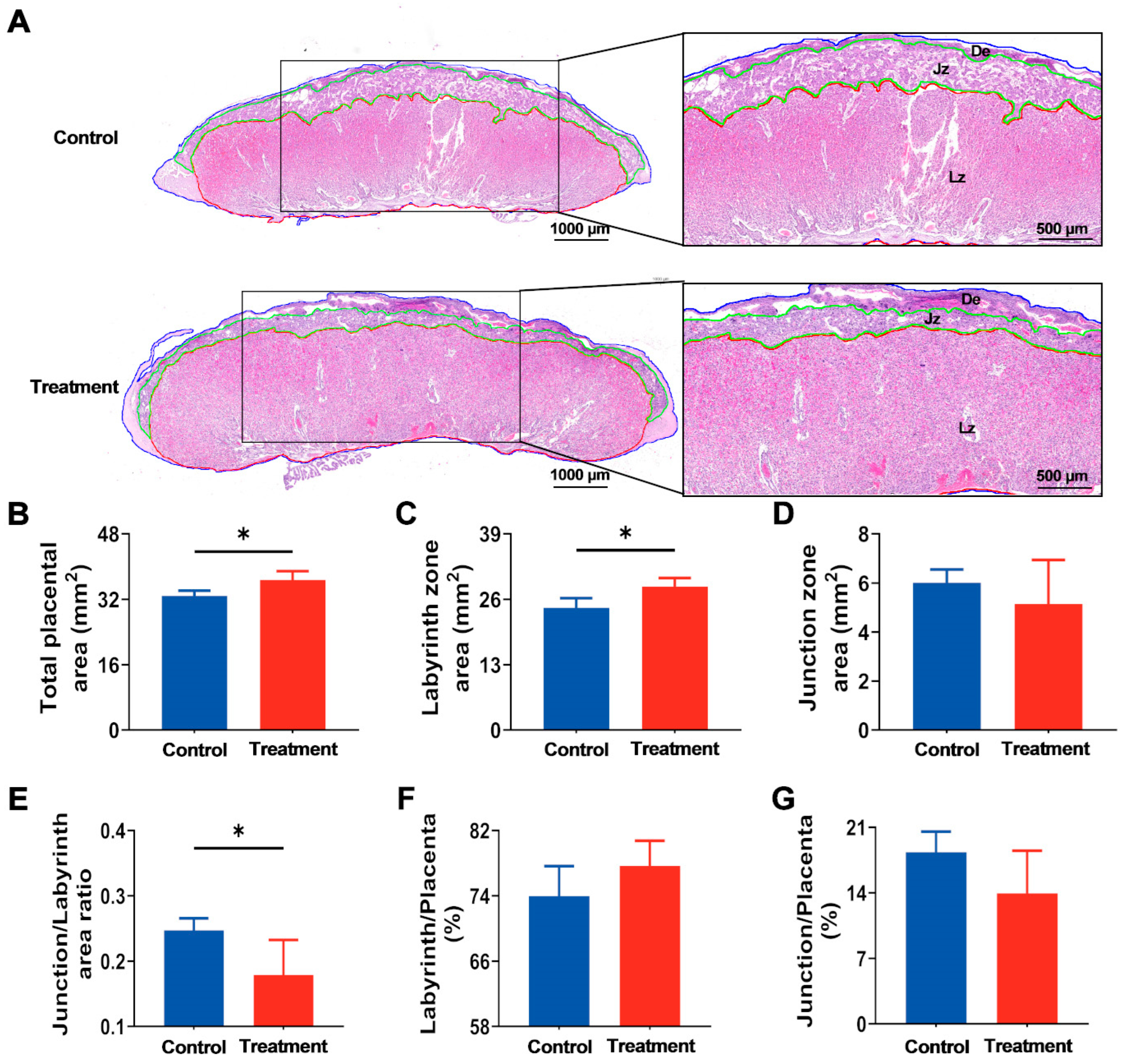
3.4. Maternal hArg Supplementation Enhanced Placental Development at E21
3.5. Maternal hArg Supplementation Facilitates Placental Angiogenesis
3.6. The hArg Activates the PI3K-AKT Pathway, Optimizing the Environment for Angiogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Embryonic day 9.5 | E9.5 |
| LZ | Labyrinthine zone |
| hArg | Homoarginine |
| Arg | Arginine |
| NO | Nitric oxide |
| E2 | Estradiol |
| PROG | Progesterone |
| JZ | Junction zone |
| CD31 | Cluster of differentiation 31 |
| VEGFA | Vascular endothelial growth factor A |
| eNOS | Endothelial nitric oxide synthase |
| PI3K | Phosphoinositide 3-kinase |
| AKT | AKT serine/threonine kinase |
| pAkt | Phosphorylated AKT |
| SLC7A1 | Solute carrier family 7 member 1 |
| iNOS | Inducible nitric oxide synthase |
| nNOS | Neuronal nitric oxide synthase |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| PGF | Placental growth factor |
References
- Zhang, H.; Zhang, X.; Wang, Y.; Zhao, X.; Zhang, L.; Li, J.; Zhang, Y.; Wang, P.; Liang, H. Dietary Folic Acid Supplementation Attenuates Maternal High-Fat Diet-Induced Fetal Intrauterine Growth Retarded via Ameliorating Placental Inflammation and Oxidative Stress in Rats. Nutrients 2023, 15, 3263. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.H.; Huang, S.B.; Song, T.X.; Yin, Y.L.; Tan, C.Q. Placental Angiogenesis in Mammals: A Review of the Regulatory Effects of Signaling Pathways and Functional Nutrients. Adv. Nutr. 2021, 12, 2415–2434. [Google Scholar] [CrossRef]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Feng, Q.T.; Li, X.; Qin, X.; Yu, C.; Jin, Y.; Qian, X.J. PTEN inhibitor improves vascular remodeling and cardiac function after myocardial infarction through PI3k/Akt/VEGF signaling pathway. Mol. Med. 2020, 26, 111. [Google Scholar] [CrossRef]
- Blundell, C.; Tess, E.R.; Schanzer, A.S.R.; Coutifaris, C.; Su, E.J.; Parry, S.; Huh, D. A microphysiological model of the human placental barrier. Lab. Chip 2016, 16, 3065–3073. [Google Scholar] [CrossRef]
- Maltepe, E.; Fisher, S.J. Placenta: The forgotten organ. Annu. Rev. Cell Dev. Biol. 2015, 31, 523–552. [Google Scholar] [CrossRef] [PubMed]
- Starks, R.R.; Abu Alhasan, R.; Kaur, H.; Pennington, K.A.; Schulz, L.C.; Tuteja, G. Transcription Factor PLAGL1 Is Associated with Angiogenic Gene Expression in the Placenta. Int. J. Mol. Sci. 2020, 21, 8317. [Google Scholar] [CrossRef] [PubMed]
- Hemberger, M.; Hanna, C.W.; Dean, W. Mechanisms of early placental development in mouse and humans. Nat. Rev. Genet. 2020, 21, 27–43. [Google Scholar] [CrossRef]
- Yue, H.; Ji, X.; Zhang, Y.; Li, G.; Sang, N. Gestational exposure to PM2.5 impairs vascularization of the placenta. Sci. Total Environ. 2019, 665, 153–161. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, X.; Geng, X.; Yue, H.; Li, G.; Sang, N. Maternal PM2.5 exposure and abnormal placental nutrient transport. Ecotox Environ. Safe 2021, 207, 111281. [Google Scholar] [CrossRef]
- Choe, C.U.; Atzler, D.; Wild, P.S.; Carter, A.M.; Boger, R.H.; Ojeda, F.; Simova, O.; Stockebrand, M.; Lackner, K.; Nabuurs, C.; et al. Homoarginine levels are regulated by L-arginine: Glycine amidinotransferase and affect stroke outcome: Results from human and murine studies. Circulation 2013, 128, 1451–1461. [Google Scholar] [CrossRef]
- März, W.; Meinitzer, A.; Drechsler, C.; Pilz, S.; Krane, V.; Kleber, M.E.; Fischer, J.; Winkelmann, B.R.; Böhm, B.O.; Ritz, E.; et al. Homoarginine, Cardiovascular Risk, and Mortality. Circulation 2010, 122, 967–975. [Google Scholar] [CrossRef]
- Pilz, S.; Tomaschitz, A.; Meinitzer, A.; Drechsler, C.; Ritz, E.; Krane, V.; Wanner, C.; Böhm, B.O.; März, W. Low serum homoarginine is a novel risk factor for fatal strokes in patients undergoing coronary angiography. Stroke 2011, 42, 1132–1134. [Google Scholar] [CrossRef]
- Tomaschitz, A.; Meinitzer, A.; Pilz, S.; Rus-Machan, J.; Genser, B.; Drechsler, C.; Grammer, T.; Krane, V.; Ritz, E.; Kleber, M.E.; et al. Homoarginine, kidney function and cardiovascular mortality risk. Nephrol. Dial. Transpl. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2014, 29, 663–671. [Google Scholar] [CrossRef]
- Vogl, L.; Pohlhammer, J.; Meinitzer, A.; Rantner, B.; Stadler, M.; Peric, S.; Hammerer-Lercher, A.; Klein-Weigel, P.; Fraedrich, G.; Kronenberg, F.; et al. Serum concentrations of L-arginine and L-homoarginine in male patients with intermittent claudication: A cross-sectional and prospective investigation in the CAVASIC Study. Atherosclerosis 2015, 239, 607–614. [Google Scholar] [CrossRef]
- Atzler, D.; McAndrew, D.J.; Cordts, K.; Schneider, J.E.; Zervou, S.; Schwedhelm, E.; Neubauer, S.; Lygate, C.A. Dietary Supplementation with Homoarginine Preserves Cardiac Function in a Murine Model of Post-Myocardial Infarction Heart Failure. Circulation 2017, 135, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Atzler, D.; McAndrew, D.J.; Crabtree, M.; Hale, A.; Bailey, J.; Cordts, K.; Schneider, J.E.; Zervou, S.; Schwedhelm, E.; Neubauer, S.; et al. Homoarginine Supplementation Improves Cardiac Function in a Murine Model of Ischaemic Heart Failure. Circulation 2015, 132, A13341. [Google Scholar] [CrossRef]
- Faller, K.M.E.; Atzler, D.; McAndrew, D.J.; Zervou, S.; Whittington, H.J.; Simon, J.N.; Aksentijevic, D.; Ten Hove, M.; Choe, C.-U.; Isbrandt, D.; et al. Impaired cardiac contractile function in arginine:glycine amidinotransferase knockout mice devoid of creatine is rescued by homoarginine but not creatine. Cardiovasc. Res. 2018, 114, 417–430. [Google Scholar] [CrossRef]
- Nitz, K.; Lacy, M.; Bianchini, M.; Wichapong, K.; Kücükgöze, I.A.; Bonfiglio, C.A.; Migheli, R.; Wu, Y.T.; Burger, C.; Li, Y.F.; et al. The Amino Acid Homoarginine Inhibits Atherogenesis by Modulating T-Cell Function. Circ. Res. 2022, 131, 701–712. [Google Scholar] [CrossRef]
- Valtonen, P.; Laitinen, T.; Lyyra-Laitinen, T.; Raitakari, O.T.; Juonala, M.; Viikari, J.S.A.; Heiskanen, N.; Vanninen, E.; Punnonen, K.; Heinonen, S. Serum L-Homoarginine Concentration is Elevated During Normal Pregnancy and is Related to Flow-Mediated Vasodilatation. Circ. J. 2008, 72, 1879–1884. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C.; Meinitzer, A.; Pilz, S.; Krane, V.; Tomaschitz, A.; Ritz, E.; März, W.; Wanner, C. Homoarginine, heart failure, and sudden cardiac death in haemodialysis patients. Eur. J. Heart Fail. 2011, 13, 852–859. [Google Scholar] [CrossRef]
- Wu, G.Y.; Bazer, F.W.; Davis, T.A.; Jaeger, L.A.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Meininger, C.J.; Spencer, T.E.; Yin, Y.L. Important roles for the arginine family of amino acids in swine nutrition and production. Livest. Sci. 2007, 112, 8–22. [Google Scholar] [CrossRef]
- Yu, G.; Wang, J.; Liu, Y.; Luo, T.; Meng, X.; Zhang, R.; Huang, B.; Sun, Y.; Zhang, J. Metabolic perturbations in pregnant rats exposed to low-dose perfluorooctanesulfonic acid: An integrated multi-omics analysis. Environ. Int. 2023, 173, 107851. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Z.; Zhang, H.; Gao, Z.; Xia, S.; Sun, X.; Zhou, L.; Li, Z.; Ma, P.; Wu, B.J.B. Role of StAR Gene in Sex Steroid Hormone Regulation and Gonadal Development in Ark Shell Scapharca broughtonii. Biology 2025, 14, 925. [Google Scholar] [CrossRef]
- Karetnikova, E.S.; Jarzebska, N.; Markov, A.G.; Weiss, N.; Lentz, S.R.; Rodionov, R.N. Is Homoarginine a Protective Cardiovascular Risk Factor? Arter. Thromb. Vasc. Biol. 2019, 39, 869–875. [Google Scholar] [CrossRef]
- Ain, R.; Konno, T.; Canham, L.N.; Soares, M.J. Phenotypic analysis of the rat placenta. In Placenta and Trophoblast; Methods in Molecular Medicine; Humana: Totowa, NJ, USA, 2006; Volume 121, pp. 295–313. [Google Scholar] [CrossRef]
- Al-Ghorbani, M.; Vigneshwaran, V.; Ranganatha, V.L.; Prabhakar, B.T.; Khanum, S.A. Synthesis of oxadiazole-morpholine derivatives and manifestation of the repressed CD31 Microvessel Density (MVD) as tumoral angiogenic parameters in Dalton’s Lymphoma. Bioorg Chem. 2015, 60, 136–146. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Dahlen, C.R.; Ward, A.K.; Crouse, M.S.; Borowicz, P.P.; Davila-Ruiz, B.J.; Kanjanaruch, C.; Bochantin, K.A.; McLean, K.J.; McCarthy, K.L.; et al. Role of the placenta in developmental programming: Observations from models using large animals. Anim. Reprod. Sci. 2023, 257, 107322. [Google Scholar] [CrossRef] [PubMed]
- Lefkou, E.; Varoudi, K.; Pombo, J.; Jurisic, A.; Jurisic, Z.; Contento, G.; Girardi, G. Triple therapy with pravastatin, low molecular weight heparin and low dose aspirin improves placental haemodynamics and pregnancy outcomes in obstetric antiphospholipid syndrome in mice and women through a nitric oxide-dependent mechanism. Biochem. Pharmacol. 2020, 182, 114217. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.B.; Wang, X.H.; Wang, C.C.; Li, W.Y.; Liu, H.Y. L-arginine supplementation improved neonatal outcomes in pregnancies with hypertensive disorder or intrauterine growth restriction: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2022, 41, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Farsetti, D.; Pometti, F.; Vasapollo, B.; Novelli, G.P.; Nardini, S.; Lupoli, B.; Lees, C.; Valensise, H. Nitric oxide donor increases umbilical vein blood flow and fetal oxygenation in fetal growth restriction. A pilot study. Placenta 2024, 151, 59–66. [Google Scholar] [CrossRef]
- Khalil, A.A.; Tsikas, D.; Akolekar, R.; Jordan, J.; Nicolaides, K.H. Asymmetric dimethylarginine, arginine and homoarginine at 11–13 weeks’ gestation and preeclampsia: A case-control study. J. Hum. Hypertens. 2013, 27, 38–43. [Google Scholar] [CrossRef]
- Berlinguer, F.; Porcu, C.; Molle, G.; Cabiddu, A.; Dattena, M.; Gallus, M.; Pasciu, V.; Succu, S.; Sotgiu, F.D.; Paliogiannis, P.; et al. Circulating Concentrations of Key Regulators of Nitric Oxide Production in Undernourished Sheep Carrying Single and Multiple Fetuses. Animals 2020, 10, 65. [Google Scholar] [CrossRef]
- Atzler, D.; Schönhoff, M.; Cordts, K.; Ortland, I.; Hoppe, J.; Hummel, F.C.; Gerloff, C.; Jaehde, U.; Jagodzinski, A.; Böger, R.H.; et al. Oral supplementation with L-homoarginine in young volunteers. Br. J. Clin. Pharmacol. 2016, 82, 1477–1485. [Google Scholar] [CrossRef]
- Greene, J.M.; Dunaway, C.W.; Bowers, S.D.; Rude, B.J.; Feugang, J.M.; Ryan, P.L. Dietary L-Arginine Supplementation during Gestation in Mice Enhances Reproductive Performance and Transcription Activity in the Fetoplacental Unit. J. Nutr. 2012, 142, 456–460. [Google Scholar] [CrossRef]
- He, W.H.; Jin, M.M.; Liu, A.P.; Zhou, Y.; Hu, X.L.; Zhu, Y.M.; Liu, A.X. Estradiol promotes trophoblast viability and invasion by activating SGK1. Biomed. Pharmacother. 2019, 117, 109092. [Google Scholar] [CrossRef] [PubMed]
- Berkane, N.; Liere, P.; Oudinet, J.P.; Hertig, A.; Lefevre, G.; Pluchino, N.; Schumacher, M.; Chabbert-Buffet, N. From Pregnancy to Preeclampsia: A Key Role for Estrogens. Endocr. Rev. 2017, 38, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.C.L.; Gilbert, B.M.; Bernal, C.; Crissman, K.R.; Sones, J.L. Estrogen and Progesterone Receptors Are Dysregulated at the BPH/5 Mouse Preeclamptic-Like Maternal-Fetal Interface. Biology 2024, 13, 192. [Google Scholar] [CrossRef]
- Spencer, T.; Johnson, G.; Bazer, F.; Wu, G. Regulation of placental nitric oxide synthesis by estrogen and progesterone in pigs. In Biology of Reproduction; Soc Study Reproduction: Madison, WI, USA, 2003; pp. 363–364. [Google Scholar]
- Li, S.; Ye, X.Y.; Wen, X.L.; Yang, X.F.; Wang, L.; Gao, K.G.; Xiao, H.; Jiang, Z.Y. Arginine and its metabolites stimulate proliferation, differentiation, and physiological function of porcine trophoblast cells through β-catenin and mTOR pathways. BMC Vet. Res. 2024, 20, 167. [Google Scholar] [CrossRef]
- Gao, K.; Jiang, Z.; Lin, Y.; Zheng, C.; Zhou, G.; Chen, F.; Yang, L.; Wu, G. Dietary L-arginine supplementation enhances placental growth and reproductive performance in sows. Amino Acids 2012, 42, 2207–2214. [Google Scholar] [CrossRef]
- Chafai, A.; Fromm, M.F.; König, J.; Maas, R. The prognostic biomarker L-homoarginine is a substrate of the cationic amino acid transporters CAT1, CAT2A and CAT2B. Sci. Rep. 2017, 7, 4767. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, N.; Yamamasu, S.-I.; Tachibana, D.; Nishio, J.; Nakai, Y.; Shintaku, H.; Ishiko, O. Activity of synthetic enzymes of tetrahydrobiopterin in the human placenta. Int. J. Mol. Med. 2004, 13, 117–120. [Google Scholar] [CrossRef]
- Menichini, D.; Feliciello, L.; Neri, I.; Facchinetti, F. L-Arginine supplementation in pregnancy: A systematic review of maternal and fetal outcomes. J. Matern. Fetal Neona 2023, 36, 2217465. [Google Scholar] [CrossRef]
- Al-Hijji, J.; Andolf, E.; Laurini, R.; Batra, S. Nitric oxide synthase activity in human trophoblast, term placenta and pregnant myometrium. Reprod. Biol. Endocrinol. 2003, 1, 51. [Google Scholar] [CrossRef] [PubMed]
- Krol, M.; Kepinska, M. Human Nitric Oxide Synthase-Its Functions, Polymorphisms, and Inhibitors in the Context of Inflammation, Diabetes and Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 22, 56. [Google Scholar] [CrossRef]
- Savvidou, M.D.; Vallance, P.J.; Nicolaides, K.H.; Hingorani, A.D. Endothelial nitric oxide synthase gene polymorphism and maternal vascular adaptation to pregnancy. Hypertension 2001, 38, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Caton, J.S.; Redmer, D.A.; Grazul-Bilska, A.T.; Vonnahme, K.A.; Borowicz, P.P.; Luther, J.S.; Wallace, J.M.; Wu, G.; Spencer, T.E. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J. Physiol. 2006, 572, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.B.; Ran, S.J.; Wu, L.; Dai, T.C.; Peng, J.; Zhou, Y.F. Maternal supplementation spermidine during gestation improves placental angiogenesis and reproductive performance of high prolific sows. J. Nutr. Biochem. 2025, 136, 109792. [Google Scholar] [CrossRef]
- Bao, J.J.; Zou, Y.; Liu, Y.Y.; Yuan, L.; Garfield, R.E.; Liu, H.S. Nicotine protects fetus against LPS-induced fetal growth restriction through ameliorating placental inflammation and vascular development in late pregnancy in rats. Biosci. Rep. 2019, 39, BSR20190386. [Google Scholar] [CrossRef]
- Coskun, R.; Chang, Z.L.; Rodriguez, A.M.; Liu, H.X.; Cheng, J.Y.; Alippe, Y.; Diamond, M.S.; Gordon, J.I. Effects of the gut microbiota on placental angiogenesis and intrauterine growth in gnotobiotic mice. Proc. Natl. Acad. Sci. USA 2025, 122, e2426341122. [Google Scholar] [CrossRef]
- Demarez, C.; De Assis, L.V.M.; Krohn, M.; Ramella, N.; Schwaninger, M.; Oster, H.; Astiz, M. The trophoblast clock controls transport across placenta in mice. Development 2021, 148, 197673. [Google Scholar] [CrossRef]
- Bai, G.D.; Jiang, X.; Qin, J.W.; Zou, Y.B.; Zhang, W.T.; Teng, T.; Shi, B.M.; Sun, H.Y. Perinatal exposure to glyphosate-based herbicides impairs progeny health and placental angiogenesis by disturbing mitochondrial function. Environ. Int. 2022, 170, 107579. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, Y.H.; Zhang, X.; Zhang, X.Y.; Huang, X.Y.; Lu, Y.X.; Li, Y.J.; Brännström, M.; Sferruzzi-Perri, A.N.; Shao, L.R.; et al. Defective Uterine Spiral Artery Remodeling and Placental Senescence in a Pregnant Rat Model of Polycystic Ovary Syndrome. Am. J. Pathol. 2023, 193, 1916–1935. [Google Scholar] [CrossRef]
- Hu, C.J.; Wu, Z.F.; Huang, Z.H.; Hao, X.Y.; Wang, S.Q.; Deng, J.P.; Yin, Y.L.; Tan, C.Q. Nox2 impairs VEGF-A-induced angiogenesis in placenta via mitochondrial ROS-STAT3 pathway. Redox Biol. 2021, 45, 102051. [Google Scholar] [CrossRef]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef] [PubMed]
- Tarallo, V.; Trotta, S.M.; Panico, S.; D’Orsi, L.; Mercadante, G.; Cicatiello, V.; De Falco, S. PlGF and VEGF-A/PlGF Heterodimer are Crucial for Recruitment and Activation of Immune Cells During Choroid Neovascularization. Investig. Ophth. Vis. Sci. 2024, 65, 12. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Q.; Tao, R.Y.; Chen, L.; Xiong, Y.; Xue, H.; Hu, L.C.; Yan, C.C.; Xie, X.D.; Lin, Z.; Panayi, A.C.; et al. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J. Nanobiotechnol. 2021, 19, 150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Z.; Qin, X.-H.; Zhang, J. MicroRNA-183 inhibition exerts suppressive effects on diabetic retinopathy by inactivating BTG1-mediated PI3K/Akt/VEGF signaling pathway. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E1050–E1060. [Google Scholar] [CrossRef]
- Fei, Y.; Ling, Z.C.; Tong, Q.; Wang, J. Apoptotic Extracellular Vesicles from Supernumerary Tooth-Derived Pulp Stem Cells Transfer COL1A1 to Promote Angiogenesis via PI3K/Akt/VEGF Pathway. Int. J. Nanomed. 2024, 19, 6811–6828. [Google Scholar] [CrossRef]
- Amadori, R.; Aquino, C.I.; Colagiorgio, S.; Osella, E.; Surico, D.; Remorgida, V. What may happen if you are pregnant during COVID-19 lockdown? A retrospective study about peripartum outcomes. Minerva Obs. Gynecol. 2021, 74, 319–324. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Stamilio, D.M.; Ural, S.; Macones, G.A.; Odibo, A.O. Relationship between abnormal fetal testing and adverse perinatal outcomes in intrauterine growth restriction. Am. J. Obs. Gynecol. 2007, 196, 48–51. [Google Scholar] [CrossRef]
- Bucher, V.; Mitchell, A.R.; Gudmundsson, P.; Atkinson, J.; Wallin, N.; Asp, J.; Sennström, M.; Hildén, K.; Edvinsson, C.; Ek, J.; et al. Prediction of adverse maternal and perinatal outcomes associated with pre-eclampsia and hypertensive disorders of pregnancy: A systematic review and meta-analysis. Eclinicalmedicine 2024, 76, 102861. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Yong, F.; Liu, L.; Ren, N.; Han, R.; Zhang, T.; Che, D. Supplementation with hArg During the Rapid Growth of the Placenta Modulates Final Placental Angiogenesis and Pregnancy Outcomes. Nutrients 2025, 17, 3563. https://doi.org/10.3390/nu17223563
Li H, Yong F, Liu L, Ren N, Han R, Zhang T, Che D. Supplementation with hArg During the Rapid Growth of the Placenta Modulates Final Placental Angiogenesis and Pregnancy Outcomes. Nutrients. 2025; 17(22):3563. https://doi.org/10.3390/nu17223563
Chicago/Turabian StyleLi, Huijuan, Feng Yong, Lixue Liu, Na Ren, Rui Han, Tianrui Zhang, and Dongsheng Che. 2025. "Supplementation with hArg During the Rapid Growth of the Placenta Modulates Final Placental Angiogenesis and Pregnancy Outcomes" Nutrients 17, no. 22: 3563. https://doi.org/10.3390/nu17223563
APA StyleLi, H., Yong, F., Liu, L., Ren, N., Han, R., Zhang, T., & Che, D. (2025). Supplementation with hArg During the Rapid Growth of the Placenta Modulates Final Placental Angiogenesis and Pregnancy Outcomes. Nutrients, 17(22), 3563. https://doi.org/10.3390/nu17223563






