The Role of Bacopa monnieri in Alzheimer’s Disease: Mechanisms and Potential Clinical Use—A Review
Abstract
1. Introduction
2. AD
2.1. AD Risk Factors
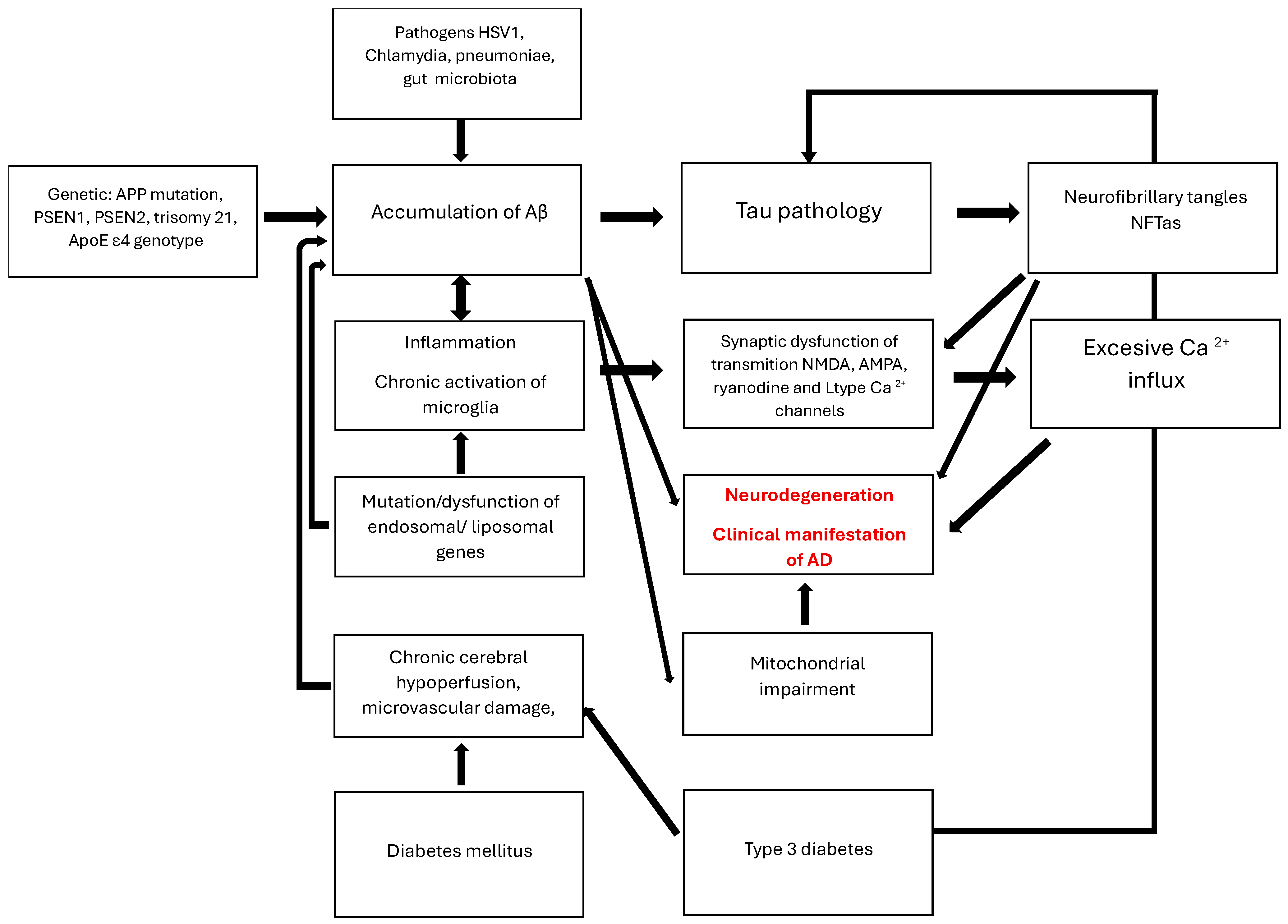
2.2. AD Pathogenesis and Treatment
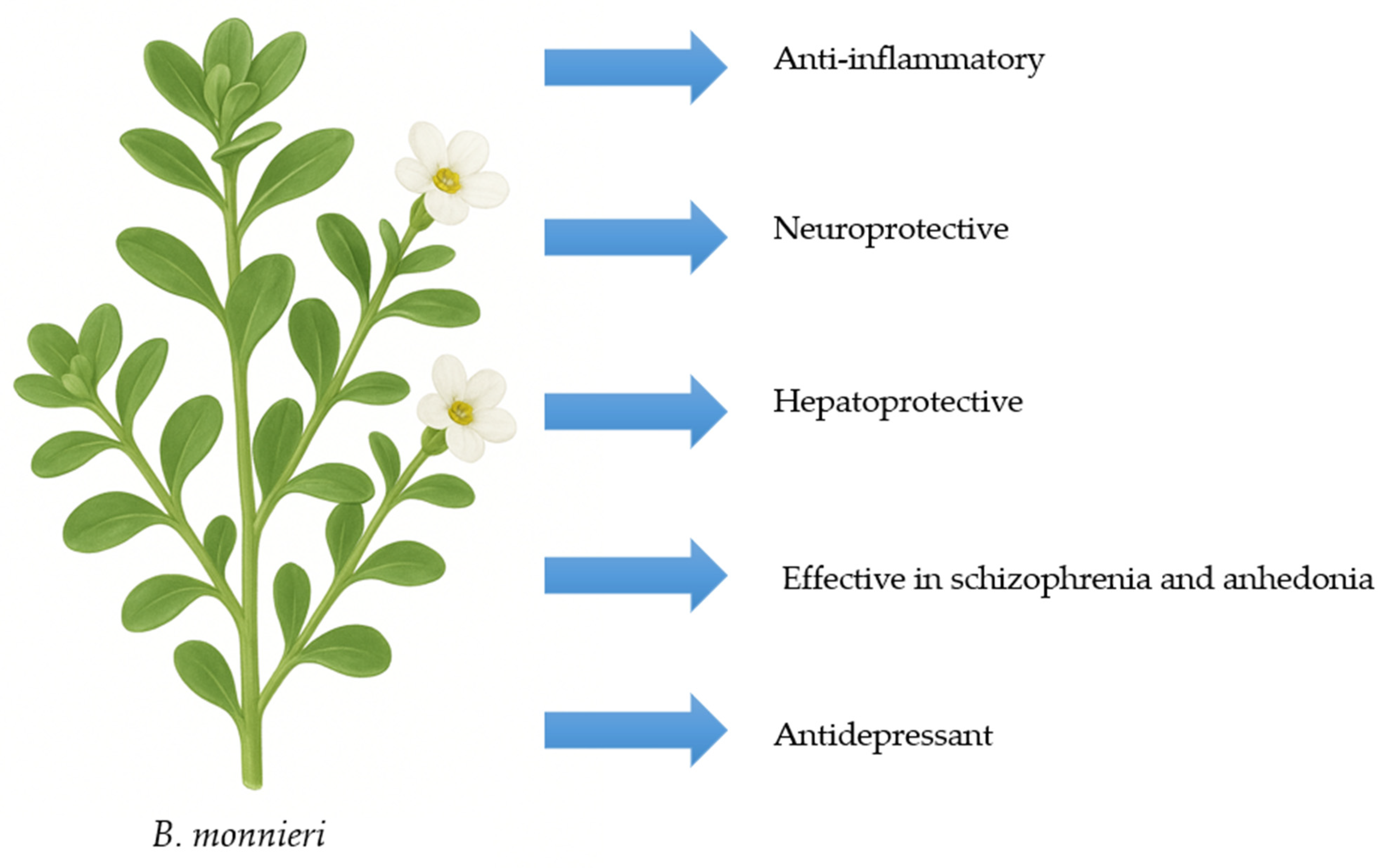
3. Characteristics of B. monnieri
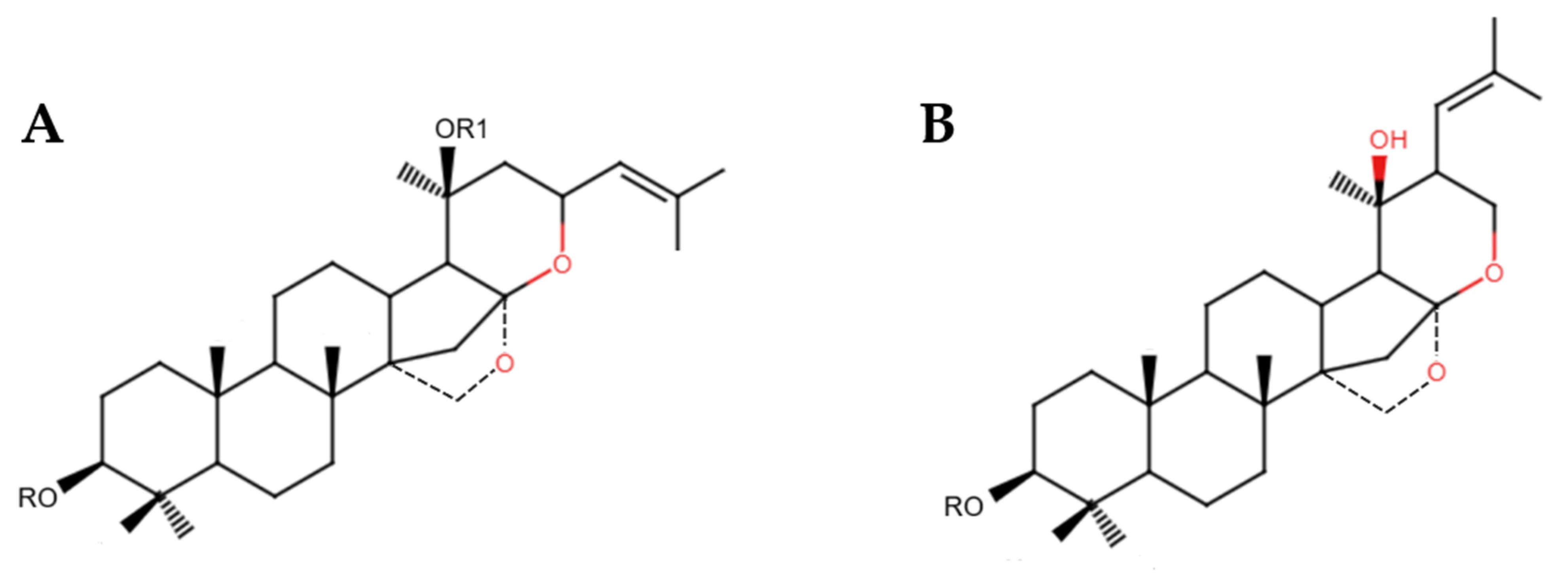
3.1. Active Substances
3.2. Molecular Mechanisms
3.3. Safety and Toxicity
4. B. monnieri in AD
4.1. Preclinical Trials
4.2. Clinical Trials
5. Limitations and Directions for Future Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shalini, V.T.; Neelakanta, S.J.; Sriranjini, J.S. Neuroprotection with Bacopa monnieri—A review of experimental evidence. Mol. Biol. Rep. 2021, 48, 2653–2668. [Google Scholar] [CrossRef] [PubMed]
- Fatima, U.; Roy, S.; Ahmad, S.; Ali, S.; Elkady, W.M.; Khan, I.; Alsaffar, R.M.; Adnan, M.; Islam, A.; Hassan, M.I. Pharmacological attributes of Bacopa monnieri extract: Current updates and clinical manifestation. Front. Nutr. 2022, 9, 972379. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Ghaffari Jolfayi, A.; Fazlollahi, A.; Morsali, S.; Sarkesh, A.; Daei Sorkhabi, A.; Golabi, B.; Aletaha, R.; Motlagh Asghari, K.; Hamidi, S.; et al. Alzheimer’s disease: A comprehensive review of epidemiology, risk factors, symptoms diagnosis, management, caregiving, advanced treatments and associated challenges. Front. Med. 2024, 11, 1474043. [Google Scholar] [CrossRef] [PubMed]
- Thakor, V.S.; Tyagi, A.; Lee, J.M., Jr.; Coffman, F.; Mittal, R. Alois Alzheimer (1864–1915): The Father of Modern Dementia Research and the Discovery of Alzheimer’s Disease. Cureus 2024, 16, e71731. [Google Scholar] [CrossRef]
- Hippius, H.; Neundörfer, G. The discovery of Alzheimer’s disease. Dialogues Clin. Neurosci. 2003, 5, 101–108. [Google Scholar] [CrossRef]
- Wilmoth, J.R. Human longevity in historical perspective. In Physiological Basis of Aging and Geriatrics; CRC Press: Boca Raton, FL, USA, 2007; pp. 23–34. [Google Scholar]
- Cogswell, P.M.; Andrews, T.J.; Barakos, J.A.; Barkhof, F.; Bash, S.; Benayoun, M.D.; Chiang, G.C.; Franceschi, A.M.; Jack, C.R., Jr.; Pillai, J.J.; et al. Alzheimer Disease Anti-Amyloid Immunotherapies: Imaging Recommendations and Practice Considerations for Monitoring of Amyloid-Related Imaging Abnormalities. AJNR Am. J. Neuroradiol. 2025, 46, 24–32. [Google Scholar] [CrossRef]
- Reitz, C.; Mayeux, R. Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 2014, 88, 640–651. [Google Scholar] [CrossRef]
- Chang, Z.; Wang, Z.; Luo, L.; Xie, Z.; Yue, C.; Bian, X.; Yang, H.; Wang, P. Case report: Double mutations in a patient with early-onset Alzheimer’s disease in China, PSEN2 and IDE variants. Front. Neurosci. 2024, 18, 1423892. [Google Scholar] [CrossRef]
- Roses, A.D. On the Discovery of the Genetic Association of Apolipoprotein E Genotypes and Common Late-Onset Alzheimer Disease. J. Alzheimer’s Dis. 2006, 9, 361–366. [Google Scholar] [CrossRef]
- Avitan, I.; Halperin, Y.; Saha, T.; Bloch, N.; Atrahimovich, D.; Polis, B.; Samson, A.O.; Braitbard, O. Towards a Consensus on Alzheimer’s Disease Comorbidity? J. Clin. Med. 2021, 10, 4360. [Google Scholar] [CrossRef]
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.C.; Belder, C.; Ballard, C.; Kales, H.C.; Mummery, C.; Caramelli, P.; Ciccarelli, O.; Frederiksen, K.S.; Gomez-Isla, T.; Ismail, Z.; et al. Treatment for Alzheimer’s disease. Lancet 2025, 406, 1408–1423. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Supnet, C.; Sun, S.; Zhang, H.; Good, L.; Popugaeva, E.; Bezprozvanny, I. The role of ryanodine receptor type 3 in a mouse model of Alzheimer disease. Channels 2014, 8, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.S.; Cashman, N.R. Passive immunotherapies targeting Aβ and tau in Alzheimer’s disease. Neurobiol. Dis. 2020, 144, 105010. [Google Scholar] [CrossRef]
- Reardon, S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature 2023, 613, 227–228. [Google Scholar] [CrossRef]
- Wicker, A.; Shriram, J.; Decourt, B.; Sabbagh, M.N. Passive Anti-amyloid Beta Monoclonal Antibodies: Lessons Learned over Past 20 Years. Neurol. Ther. 2024, 13, 1571–1595. [Google Scholar] [CrossRef]
- Albert, M.; Mairet-Coello, G.; Danis, C.; Lieger, S.; Caillierez, R.; Carrier, S.; Skrobala, E.; Landrieu, I.; Michel, A.; Schmitt, M.; et al. Prevention of tau seeding and propagation by immunotherapy with a central tau epitope antibody. Brain 2019, 142, 1736–1750. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Sun, J.; Shen, H.M.; Wang, J.; Yang, C. Impairment of the autophagy-lysosomal pathway in Alzheimer’s diseases: Pathogenic mechanisms and therapeutic potential. Acta Pharm. Sin. B 2022, 12, 1019–1040. [Google Scholar] [CrossRef]
- Kelliny, S.; Zhou, X.F.; Bobrovskaya, L. Alzheimer’s Disease and Frontotemporal Dementia: A Review of Pathophysiology and Therapeutic Approaches. J. Neurosci. Res. 2025, 103, e70046. [Google Scholar] [CrossRef]
- Kleinridders, A. Deciphering Brain Insulin Receptor and Insulin-Like Growth Factor 1 Receptor Signalling. J. Neuroendocrinol. 2016, 28, 11. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Wijesekara, N.; Fraser, P.E.; De Felice, F.G. The Link Between Tau and Insulin Signaling: Implications for Alzheimer’s Disease and Other Tauopathies. Front. Cell Neurosci. 2019, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Donahoo, W.T.; DeKosky, S.T.; Lee, Y.A.; Kotecha, P.; Svensson, M.; Bian, J.; Guo, J. GLP-1RA and SGLT2i Medications for Type 2 Diabetes and Alzheimer Disease and Related Dementias. JAMA Neurol. 2025, 82, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Özsoy, Ş.; Özşahin Delibaş, E.A.; Yiğittürk, G. Edaravone ameliorates memory, hippocampal morphology, and inflammation in a rat model of Alzheimer’s disease. Int. J. Comput. Exp. Sci. Eng. 2023, 9, 288–295. [Google Scholar]
- Jeyasri, R.; Muthuramalingam, P.; Suba, V.; Ramesh, M.; Chen, J.T. Bacopa monnieri and Their Bioactive Compounds Inferred Multi-Target Treatment Strategy for Neurological Diseases: A Cheminformatics and System Pharmacology Approach. Biomolecules 2020, 10, 536. [Google Scholar] [CrossRef]
- Karati, D.; Mukherjee, S.; Roy, S. The antioxidant potential of bacoside and its derivatives in Alzheimer’s disease: The molecular mechanistic paths and therapeutic prospects. Toxicol. Rep. 2025, 14, 101945. [Google Scholar] [CrossRef]
- Murthy, H.N. Biotechnological production of bacosides from cell and organ cultures of Bacopa monnieri. Appl. Microbiol. Biotechnol. 2022, 106, 1799–1811. [Google Scholar] [CrossRef]
- Jeena, G.S.; Kumar, S.; Bharti, S.; Singh, N.; Joshi, A.; Lahane, V.; Meghani, R.; Yadav, A.K.; Shukla, S.; Tripathi, V.; et al. Engineering Bacopa monnieri for improved bacoside content and its neurological evaluation. Appl. Microbiol. Biotechnol. 2025, 109, 83. [Google Scholar] [CrossRef]
- Gościniak, A.; Stasiłowicz-Krzemień, A.; Szeląg, M.; Pawlak, J.; Skiera, I.; Kwiatkowska, H.; Nowak, N.; Bernady, K.; Trzaskoma, P.; Zimak-Krótkopad, O.; et al. Bacopa monnieri: Preclinical and Clinical Evidence of Neuroactive Effects, Safety of Use and the Search for Improved Bioavailability. Nutrients 2025, 17, 1939. [Google Scholar] [CrossRef]
- Pandey, A.; Madan, S.; Sandhiya, K.; Sharma, R.; Raturi, A.; Bhatt, A.; Gaurav, N. Comparison of Antioxidant, Phytochemical Profiling of Bacopa monnieri (Brahmi). Scientifictemper 2022, 13, 286–293. [Google Scholar]
- Nemetchek, M.D.; Stierle, A.A.; Stierle, D.B.; Lurie, D.I. The Ayurvedic plant Bacopa monnieri inhibits inflammatory pathways in the brain. J. Ethnopharmacol. 2017, 197, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Keegan, A.P.; Stough, C.; Paris, D.; Luis, C.A.; Abdullah, L.; Ait-Ghezala, G.; Crawford, F.; Mullan, M. Bacopa monnieri supplementation has no effect on serum brain-derived neurotrophic factor levels but beneficially modulates nuclear factor kappa B and cyclic AMP response element-binding protein levels in healthy elderly subjects. J. Clin. Transl. Res. 2023, 9, 50–58. [Google Scholar] [PubMed]
- Dubey, T.; Kushwaha, P.; Thulasiram, H.V.; Chandrashekar, M.; Chinnathambi, S. Bacopa monnieri reduces Tau aggregation and Tau-mediated toxicity in cells. Int. J. Biol. Macromol. 2023, 234, 123171. [Google Scholar] [CrossRef] [PubMed]
- Sadaqat, M.; Qasim, M.; ul Tahir, M.T.; Qamar, M.; Masoud, M.S.; Ashfaq, U.A.; Noor, F.; Fatima, K.; Allemailem, K.S.; Alrumaihi, F.; et al. Advanced network pharmacology study reveals multi-pathway and multi-gene regulatory molecular mechanism of Bacopa monnieri in liver cancer based on data mining, molecular modeling, and microarray data analysis. Comput. Biol. Med. 2023, 161, 107059. [Google Scholar] [CrossRef]
- Altaf, A.; Kiran, A.; Sarwar, M.; Maqbool, T.; Sharif, S.; Iqbal, H.; Farooq, S.; Ali, Q.; Han, S.; Ahmad, A. Therapeutic potential of Bacopa monnieri extracts against hepatocellular carcinoma through in-vitro and computational studies. PLoS ONE 2025, 20, e0321445. [Google Scholar] [CrossRef]
- Ayilara, G.O.; Owoyele, B.V. Effectiveness of Bacopa Monnieri (Brahmi) in the management of schizophrenia: A systematic review. Nutr. Neurosci. 2025, 28, 788–795. [Google Scholar] [CrossRef]
- Micheli, L.; Spitoni, S.; Di Cesare Mannelli, L.; Bilia, A.R.; Ghelardini, C.; Pallanti, S. Bacopa monnieri as augmentation therapy in the treatment of anhedonia, preclinical and clinical evaluation. Phytother. Res. 2020, 34, 2331–2340. [Google Scholar] [CrossRef]
- Zaazaa, A.M.; Daoud, N.N.; El-Gendy, O.A.; Al-Shafei, A.I. Neuroprotective role of Bacopa monnieri extract in modulating depression in an experimental rat model. J. Affect. Disord. 2022, 308, 229–235. [Google Scholar] [CrossRef]
- Siwek, M.; Woroń, J.; Wrzosek, A.; Gupało, J.; Chrobak, A.A. Harder, better, faster, stronger? Retrospective chart review of adverse events of interactions between adaptogens and antidepressant drugs. Front. Pharmacol. 2023, 14, 1271776. [Google Scholar] [CrossRef]
- Acquarulo, B.; Tandon, P.; Macica, C.M. Suspected cholinergic toxicity due to cevimeline hydrochloride and Bacopa monnieri interaction: A case report. J. Med. Case Rep. 2022, 16, 253. [Google Scholar] [CrossRef]
- Dixit, H.; Selvaa Kumar, C.; Dasgupta, D.; Gadewal, N. Molecular docking analysis of hyperphosphorylated tau protein with compounds derived from Bacopa monnieri and Withania somnifera. Bioinformation 2021, 17, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Zanotta, D.; Puricelli, S.; Bonoldi, G. Cognitive effects of a dietary supplement made from extract of Bacopa monnieri, astaxanthin, phosphatidylserine, and vitamin E in subjects with mild cognitive impairment: A noncomparative, exploratory clinical study. Neuropsychiatr. Dis. Treat. 2014, 10, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Abdul Manap, A.S.; Vijayabalan, S.; Madhavan, P.; Chia, Y.Y.; Arya, A.; Wong, E.H.; Rizwan, F.; Bindal, U.; Koshy, S. Bacopa monnieri, a Neuroprotective Lead in Alzheimer Disease: A Review on Its Properties, Mechanisms of Action, and Preclinical and Clinical Studies. Drug Target Insights 2019, 13, 1177392819866412. [Google Scholar] [CrossRef] [PubMed]
- Mehla, J.; Gupta, P.; Pahuja, M.; Diwan, D.; Diksha, D. Indian Medicinal Herbs and Formulations for Alzheimer’s Disease, from Traditional Knowledge to Scientific Assessment. Brain Sci. 2020, 10, 964. [Google Scholar] [CrossRef]
- Witter, S.; Witter, R.; Vilu, R.; Samoson, A. Medical Plants and Nutraceuticals for Amyloid-β Fibrillation Inhibition. J. Alzheimers Dis. Rep. 2018, 2, 239–252. [Google Scholar] [CrossRef]
- Brimson, J.M.; Prasanth, M.I.; Plaingam, W.; Tencomnao, T. Bacopa monnieri (L.) wettst. Extract protects against glutamate toxicity and increases the longevity of Caenorhabditis elegans. J. Tradit. Complement. Med. 2019, 10, 460–470. [Google Scholar] [CrossRef]
- Valotto Neto, L.J.; de Araujo, M.R.; Moretti, R.C., Jr.; Mendes Machado, N.; Joshi, R.K.; dos Santos Buglio, D.; Barbalho Lamas, C.; Direito, R.; Fornari Laurindo, L.; Tanaka, M.; et al. Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis. Antioxidants 2024, 13, 393. [Google Scholar] [CrossRef]
- Palollathil, A.; Najar, M.A.; Amrutha, S.; Pervaje, R.; Modi, P.K.; Prasad, T.S.K. Bacopa monnieri confers neuroprotection by influencing signaling pathways associated with interleukin 4, 13 and extracellular matrix organization in Alzheimer’s disease: A proteomics-based perspective. Neurochem. Int. 2024, 180, 105864. [Google Scholar] [CrossRef]
- Petcharat, K.; Singh, M.; Ingkaninan, K.; Attarat, J.; Yasothornsrikul, S. Bacopa monnieri protects SH-SY5Y cells against tert-Butyl hydroperoxide-induced cell death via the ERK and PI3K pathways. Siriraj Med. J. 2015, 67, 20–26. [Google Scholar]
- Roy, S.; Chakravarty, S.; Talukdar, P.; Talapatra, N. Identification of Bioactive Compounds Present in Bacopa monnieri Linn. Against Caspase-3 and Tau Protein Kinase I to Prevent Alzheimer’s Disease: An in Silico Study. Pharma Innov. J. 2019, 8, 855–861. [Google Scholar]
- Seth, B.; Sahoo, K.K.; Aravind, K.R.; Sahu, B.B.; Singh, V.R.; Patra, N. Statistical optimization of bacoside A biosynthesis in plant cell suspension cultures using response surface methodology. 3 Biotech 2020, 10, 264. [Google Scholar] [CrossRef]
- Basheer, A.; Agarwal, A.; Mishra, B.; Gupta, A.; Padma Srivastava, M.V.; Kirubakaran, R.; Vishnu, V. Use of Bacopa monnieri in the Treatment of Dementia Due to Alzheimer Disease: Systematic Review of Randomized Controlled Trials. Interact. J. Med. Res. 2022, 11, e38542. [Google Scholar] [CrossRef]
- Prabhakar, S.; Vishnu, V.Y.; Modi, M.; Mohanty, M.; Sharma, A.; Medhi, B.; Mittal, B.R.; Khandelwal, N.; Goyal, M.K.; Lal, V.; et al. Efficacy of Bacopa monnieri (Brahmi) and Donepezil in Alzheimer’s Disease and Mild Cognitive Impairment: A Randomized Double-Blind Parallel Phase 2b Study. Ann. Indian Acad. Neurol. 2020, 23, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Mishra, A.K.; Mishra, U. Brahmi (Bacopa monnieri Linn) in the treatment of dementias—A pilot study. Future Healthc. J. 2019, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Raghav, S.; Singh, H.; Dalal, P.K.; Srivastava, J.S.; Asthana, O.P. Randomized controlled trial of standardized Bacopa monniera extract in age-associated memory impairment. Indian J. Psychiatry 2006, 48, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Grossberg, G.T. Memantine: A review of studies into its safety and efficacy in treating Alzheimer’s disease and other dementias. Clin. Interv. Aging 2009, 4, 367–377. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, L.; Deng, G.; Liu, J.; Chen, Q.; Chen, Z. Systemic delivery to central nervous system by engineered PLGA nanoparticles. Am. J. Transl. Res. 2016, 8, 749–764. [Google Scholar]
- van Vlerken, L.E.; Vyas, T.K.; Amiji, M.M. Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm. Res. 2007, 24, 1405–1414. [Google Scholar] [CrossRef]
- Sharma, S.; Parmar, A.; Kori, S.; Sandhir, R. PLGA-based nanoparticles: A new paradigm in biomedical applications. Trends Anal. Chem. 2016, 80, 30–40. [Google Scholar] [CrossRef]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef]
- Sah, H.; Thoma, L.A.; Desu, H.R.; Sah, E.; Wood, G.C. Concepts and practices used to develop functional PLGA-based nanoparticulate systems. Int. J. Nanomed. 2013, 8, 747–765. [Google Scholar] [CrossRef] [PubMed]

| Author | Study Type | Study Group | Results |
|---|---|---|---|
| Nemetchek et al. [33] | In vitro | N9 microglial cell line from CBA mice | Different types of B. monnieri extracts effectively inhibit the release of TNF-α and IL-6 from cells. Inhibition of caspase-1 and -3, and MMP-3. |
| Brimson et al. [48] | In vitro | HT-22 cell line and wild C. elegans | Anti-glutamate toxicity action. |
| Witter et al. [47] | In vitro | Lyophilized Aβ40 and lyophilized MAβ40 | Inhibition of Aβ fibrillation. |
| Palollathil et al. [50] | In vitro | IMR-32 cells (ATCC CCL-127) | Antioxidative action via free radical scavenging, neuroprotective effect, improvement in extracellular matrix organization, IL-4, and IL-13 signaling. |
| Petcharat et al. [51] | In vitro | SH-SY5Y neuroblastoma cells | Increase in ERK1/2 and Akt phosphorylation. |
| Roy et al. [52] | In silico | N/A | Bacosaponines demonstrate higher receptor affinity compared to donepezil. |
| Factor | Effect of B. monnieri | Mechanism | References |
|---|---|---|---|
| Oxydative stress | ↓ | Lipid peroxidation decreases, and ROS scavenging. | [45] |
| Tau protein tangling | ↓ | Inhibition occurs by interacting with the R2 repeat domain of the hyperphosphorylated protein. | [43] |
| Aβ aggregation | ↓ | The binding of bacoside A to amyloid oligomers prevents it from aggregating. | [45,46,47] |
| Neuroinflammation | ↓ | Decrease in TNF-α and IL-6 levels. Inhibition of caspase-1, caspase-3 and MMP-3. | [33,45,46,47] |
| Neurodegeneration | ↓ | Decrease in apoptosis by increasing activation (phosphorylation) of ERK1/2 and PI3K pathways. | [33,51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiciński, M.; Fajkiel-Madajczyk, A.; Wójcicki, J.; Ozorowski, M.; Szambelan, M. The Role of Bacopa monnieri in Alzheimer’s Disease: Mechanisms and Potential Clinical Use—A Review. Nutrients 2025, 17, 3538. https://doi.org/10.3390/nu17223538
Wiciński M, Fajkiel-Madajczyk A, Wójcicki J, Ozorowski M, Szambelan M. The Role of Bacopa monnieri in Alzheimer’s Disease: Mechanisms and Potential Clinical Use—A Review. Nutrients. 2025; 17(22):3538. https://doi.org/10.3390/nu17223538
Chicago/Turabian StyleWiciński, Michał, Anna Fajkiel-Madajczyk, Jakub Wójcicki, Mateusz Ozorowski, and Monika Szambelan. 2025. "The Role of Bacopa monnieri in Alzheimer’s Disease: Mechanisms and Potential Clinical Use—A Review" Nutrients 17, no. 22: 3538. https://doi.org/10.3390/nu17223538
APA StyleWiciński, M., Fajkiel-Madajczyk, A., Wójcicki, J., Ozorowski, M., & Szambelan, M. (2025). The Role of Bacopa monnieri in Alzheimer’s Disease: Mechanisms and Potential Clinical Use—A Review. Nutrients, 17(22), 3538. https://doi.org/10.3390/nu17223538





