The Use of Artificial Intelligence (AI) to Support Dietetic Practice Across Primary Care: A Scoping Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Source of Evidence Selection
2.3. Data Extraction
2.4. Data Analysis and Presentation
3. Results
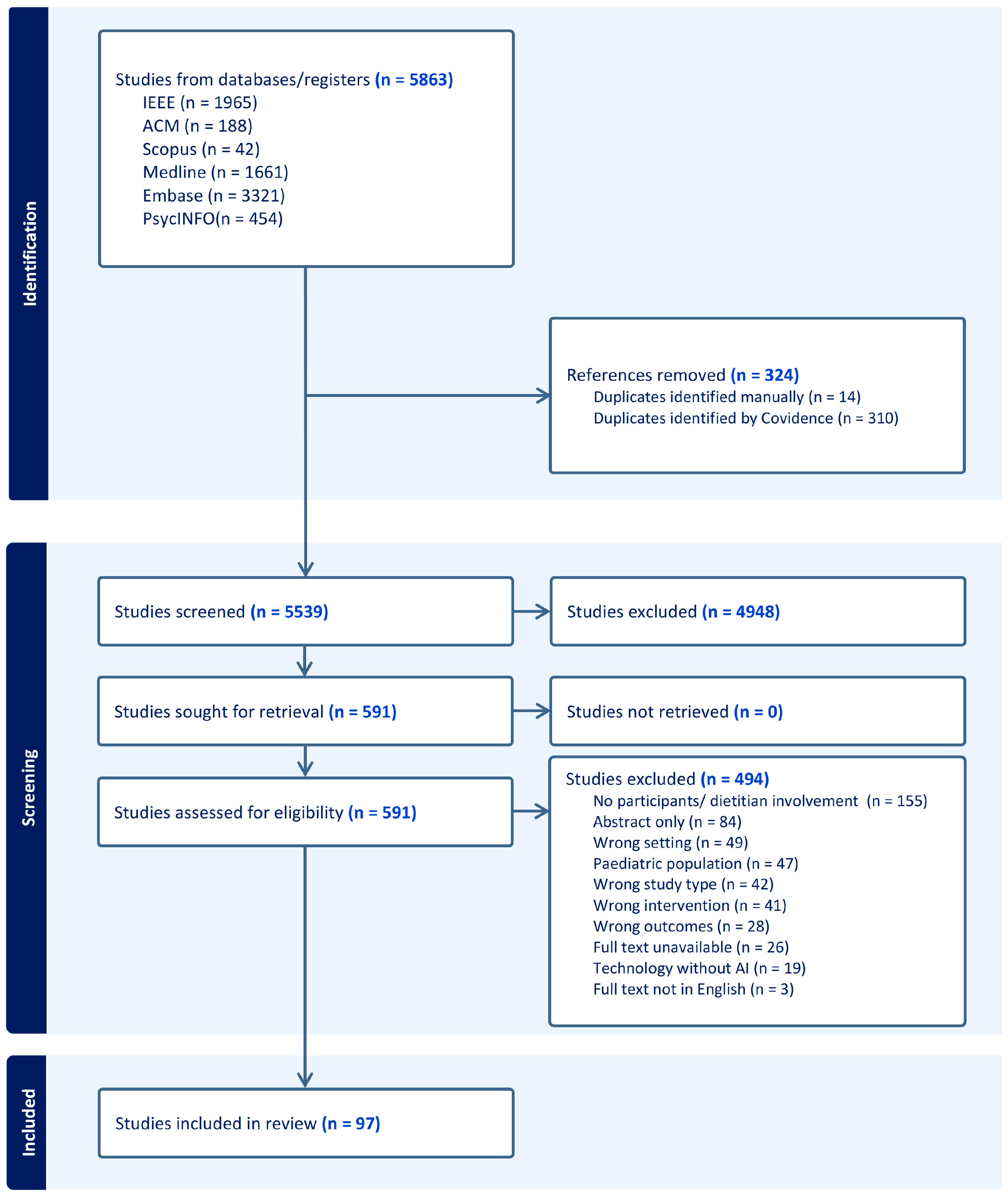
3.1. Findings from Search Strategy
3.2. Characteristics of Included Studies
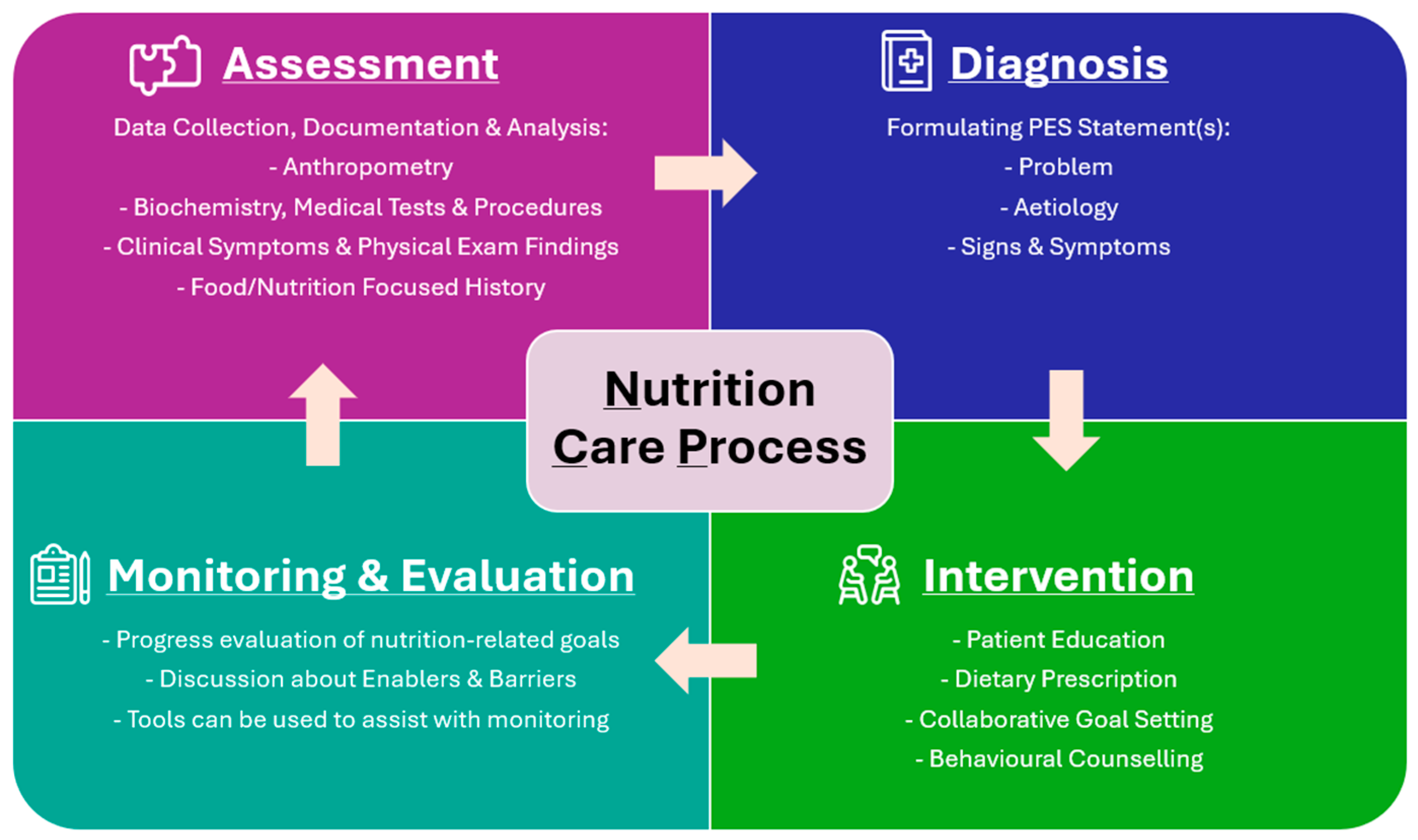
3.3. AI Involvement in the Stages of the Nutrition Care Process (NCP)
3.3.1. Nutrition Assessment
3.3.2. Nutrition Diagnosis
3.3.3. Nutrition Intervention
3.3.4. Monitoring and Evaluation
3.3.5. All Stages of the NCP
3.4. Efficiencies of AI-Integrated Technology Use
3.5. Limitations and Safety Concerns with AI-Integrated Technology Use
3.6. Ethical Considerations
4. Discussion
4.1. Nutrition Assessment
4.2. Nutrition Diagnosis
4.3. Nutrition Intervention
4.4. Monitoring and Evaluation
4.5. Additional Practical Implications
4.5.1. Healthcare Professionals
4.5.2. Reimbursement Models
4.5.3. Patient Safety, Efficacy, and Evaluation Tools
4.5.4. AI-Integrated Technology Development
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 14 September 2025).
- Lane, M.M.; Gamage, E.; Du, S.; Ashtree, D.N.; McGuinness, A.J.; Gauci, S.; Baker, P.; Lawrence, M.; Rebholz, C.M.; Srour, B.; et al. Ultra-processed food exposure and adverse health outcomes: Umbrella review of epidemiological meta-analyses. BMJ 2024, 384, e077310. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yon, D.K.; Nguyen, T.T.; Tanisawa, K.; Son, K.; Zhang, L.; Shu, J.; Peng, W.; Yang, Y.; Branca, F.; et al. Dietary and other lifestyle factors and their influence on non-communicable diseases in the Western Pacific region. Lancet Reg. Health West. Pac. 2024, 43, 100842. [Google Scholar] [CrossRef] [PubMed]
- Swan, W.I.; Vivanti, A.; Hakel-Smith, N.A.; Hotson, B.; Orrevall, Y.; Trostler, N.; Beck Howarter, K.; Papoutsakis, C. Nutrition Care Process and Model Update: Toward Realizing People-Centered Care and Outcomes Management. J. Acad. Nutr. Diet. 2017, 117, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.J.; Ball, L.E.; Ross, L.J.; Barnes, K.A.; Williams, L.T. Effectiveness of Dietetic Consultations in Primary Health Care: A Systematic Review of Randomized Controlled Trials. J. Acad. Nutr. Diet. 2017, 117, 1941–1962. [Google Scholar] [CrossRef]
- Reynolds, R.; Dennis, S.; Hasan, I.; Slewa, J.; Chen, W.; Tian, D.; Bobba, S.; Zwar, N. A systematic review of chronic disease management interventions in primary care. BMC Fam. Pract. 2018, 19, 11. [Google Scholar] [CrossRef]
- Australian Government Department of Health, Disability and Ageing. About Primary Care. Available online: https://www.health.gov.au/topics/primary-care/about (accessed on 14 September 2025).
- O’Connor, R.; Slater, K.; Ball, L.; Jones, A.; Mitchell, L.; Rollo, M.E.; Williams, L.T. The tension between efficiency and effectiveness: A study of dietetic practice in primary care. J. Hum. Nutr. Diet. 2019, 32, 259–266. [Google Scholar] [CrossRef]
- Bond, A.; McCay, K.; Lal, S. Artificial intelligence & clinical nutrition: What the future might have in store. Clin. Nutr. ESPEN 2023, 57, 542–549. [Google Scholar] [CrossRef]
- Korteling, J.E.; van de Boer-Visschedijk, G.C.; Blankendaal, R.A.M.; Boonekamp, R.C.; Eikelboom, A.R. Human- versus Artificial Intelligence. Front. Artif. Intell. 2021, 4, 622364. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, R.; Huang, F.; Lin, Q.; Guo, J.; Zeng, W.; Dong, J. AI nutritionist: Intelligent software as the next generation pioneer of precision nutrition. Comput. Biol. Med. 2024, 178, 108711. [Google Scholar] [CrossRef]
- Chen, J.; Gemming, L.; Hanning, R.; Allman-Farinelli, M. Smartphone apps and the nutrition care process: Current perspectives and future considerations. Patient Educ. Couns. 2018, 101, 750–757. [Google Scholar] [CrossRef]
- Salinari, A.; Machì, M.; Armas Diaz, Y.; Cianciosi, D.; Qi, Z.; Yang, B.; Ferreiro Cotorruelo, M.S.; Villar, S.G.; Dzul Lopez, L.A.; Battino, M.; et al. The Application of Digital Technologies and Artificial Intelligence in Healthcare: An Overview on Nutrition Assessment. Diseases 2023, 11, 97. [Google Scholar] [CrossRef]
- Kelly, J.T.; Collins, P.F.; McCamley, J.; Ball, L.; Roberts, S.; Campbell, K.L. Digital disruption of dietetics: Are we ready? J. Hum. Nutr. Diet. 2021, 34, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Robinson, E.; Raphaeli, O. The future of artificial intelligence in clinical nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 200–206. [Google Scholar] [CrossRef]
- Peters, M.D.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Scoping Reviews (2020). In JBI Manual for Evidence Synthesis; JBI: Adeleide, Australia, 2024. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Abeltino, A.; Bianchetti, G.; Serantoni, C.; Ardito, C.F.; Malta, D.; De Spirito, M.; Maulucci, G. Personalized Metabolic Avatar: A Data Driven Model of Metabolism for Weight Variation Forecasting and Diet Plan Evaluation. Nutrients 2022, 14, 3520. [Google Scholar] [CrossRef]
- Agne, I.; Gedrich, K. Personalized dietary recommendations for obese individuals—A comparison of ChatGPT and the Food4Me algorithm. Clin. Nutr. Open Sci. 2024, 56, 192–201. [Google Scholar] [CrossRef]
- Amft, O.; Kusserow, M.; Troster, G. Automatic Identification of Temporal Sequences in Chewing Sounds. In Proceedings of the 2007 IEEE International Conference on Bioinformatics and Biomedicine (BIBM 2007), Silicon Valley, CA, USA, 2–4 November 2007; pp. 194–201. [Google Scholar]
- Amft, O.; Kusserow, M.; Troster, G. Bite Weight Prediction from Acoustic Recognition of Chewing. IEEE Trans. Biomed. Eng. 2009, 56, 1663–1672. [Google Scholar] [CrossRef]
- Amft, O.; Troster, G. Recognition of dietary activity events using on-body sensors. Artif. Intell. Med. 2008, 42, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Ben Neriah, D.; Geliebter, A. Weight Loss Following Use of a Smartphone Food Photo Feature: Retrospective Cohort Study. JMIR mHealth uHealth 2019, 7, e11917. [Google Scholar] [CrossRef]
- Ben-Yacov, O.; Godneva, A.; Rein, M.; Shilo, S.; Lotan-Pompan, M.; Weinberger, A.; Segal, E. Gut microbiome modulates the effects of a personalised postprandial-targeting (PPT) diet on cardiometabolic markers: A diet intervention in pre-diabetes. Gut 2023, 72, 1486–1496. [Google Scholar] [CrossRef]
- Beyeler, M.; Légeret, C.; Kiwitz, F.; van der Horst, K. Usability and Overall Perception of a Health Bot for Nutrition-Related Questions for Patients Receiving Bariatric Care: Mixed Methods Study. JMIR Hum. Factors 2023, 10, e47913. [Google Scholar] [CrossRef]
- Bohn, T.; Ferrini, K.; Stahl, C. LIFANA—Toward developing a meal recommender system as a dietary support app for the elderly. Int. J. Vitam. Nutr. Res. 2024, 94, 221–238. [Google Scholar] [CrossRef]
- Buchan, M.L.; Goel, K.; Schneider, C.K.; Steullet, V.; Bratton, S.; Basch, E. National Implementation of an Artificial Intelligence-Based Virtual Dietitian for Patients with Cancer. JCO Clin. Cancer Inform. 2024, 8, e2400085. [Google Scholar] [CrossRef]
- Bul, K.; Holliday, N.; Bhuiyan, M.R.A.; Clark, C.C.T.; Allen, J.; Wark, P.A. Usability and Preliminary Efficacy of an Artificial Intelligence-Driven Platform Supporting Dietary Management in Diabetes: Mixed Methods Study. JMIR Hum. Factors 2023, 10, e43959. [Google Scholar] [CrossRef]
- Burgermaster, M.; Son, J.H.; Davidson, P.G.; Smaldone, A.M.; Kuperman, G.; Feller, D.J.; Burt, K.G.; Levine, M.E.; Albers, D.J.; Weng, C.; et al. A new approach to integrating patient-generated data with expert knowledge for personalized goal setting: A pilot study. Int. J. Med. Inform. 2020, 139, 104158. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Li, J.; Chernyshov, G.; Huang, Y.; Kunze, K.; Huang, J.; Starner, T.; Zhang, Q. First Bite/Chew: Distinguish different types of food by first biting/chewing and the corresponding hand movement. In Proceedings of the CHI EA ‘23: Extended Abstracts of the 2023 CHI Conference on Human Factors in Computing Systems, Hamburg, Germany, 19 April 2023; p. 138. [Google Scholar]
- Chen, R.C.; Ting, Y.H.; Chen, J.K.; Lo, Y.W. The nutrients of chronic diet recommended based on domain ontology and decision tree. In Proceedings of the 2015 Conference on Technologies and Applications of Artificial Intelligence (TAAI), Tainan, Taiwan, 20–22 November 2015; pp. 289–295. [Google Scholar]
- Chen, Y.; Hsu, C.Y.; Liu, L.; Yang, S. Constructing a nutrition diagnosis expert system. Expert Syst. Appl. 2012, 39, 2132–2156. [Google Scholar] [CrossRef]
- Chew, H.S.J.; Chew, N.W.S.; Loong, S.S.E.; Lim, S.L.; Tam, W.S.W.; Chin, Y.H.; Chao, A.M.; Dimitriadis, G.K.; Gao, Y.; So, J.B.Y.; et al. Effectiveness of an artificial intelligence-assisted app for improving eating behaviors: Mixed methods evaluation. J. Med. Internet Res. 2024, 26, e46036. [Google Scholar] [CrossRef] [PubMed]
- Chin, E.L.; Simmons, G.; Bouzid, Y.Y.; Kan, A.; Burnett, D.J.; Tagkopoulos, I.; Lemay, D.G. Nutrient Estimation from 24-Hour Food Recalls Using Machine Learning and Database Mapping: A Case Study with Lactose. Nutrients 2019, 11, 3045. [Google Scholar] [CrossRef]
- Chotwanvirat, P.; Prachansuwan, A.; Sridonpai, P.; Kriengsinyos, W. Automated Artificial Intelligence-Based Thai Food Dietary Assessment System: Development and Validation. Curr. Dev. Nutr. 2024, 8, 102154. [Google Scholar] [CrossRef]
- Cohen, R.; Fernie, G.; Fekr, A.R. Contactless Drink Intake Monitoring Using Depth Data. IEEE Access 2023, 11, 12218–12225. [Google Scholar] [CrossRef]
- Davis, C.R.; Murphy, K.J.; Curtis, R.G.; Maher, C.A. A Process Evaluation Examining the Performance, Adherence, and Acceptability of a Physical Activity and Diet Artificial Intelligence Virtual Health Assistant. Int. J. Environ. Res. Public Health 2020, 17, 9137. [Google Scholar] [CrossRef]
- De Marchi, F.; Serioli, M.; Collo, A.; Belotti, E.G.; Alloatti, F.; Biroli, G.; Bolioli, A.; Cantello, R.; Riso, S.; Mazzini, L. A Telehealth Intervention for Nutritional Counseling in Amyotrophic Lateral Sclerosis Patients. J. Clin. Med. 2022, 11, 4286. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Sazonov, E. A Novel Wearable Device for Food Intake and Physical Activity Recognition. Sensors 2016, 16, 1067. [Google Scholar] [CrossRef]
- Fernandes, G.J.; Choi, A.; Schauer, J.M.; Pfammatter, A.F.; Spring, B.J.; Darwiche, A.; Alshurafa, N.I. An Explainable Artificial Intelligence Software Tool for Weight Management Experts (PRIMO): Mixed Methods Study. J. Med. Internet Res. 2023, 25, e42047. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.M.; Farooq, M.; Sazonov, E. Automatic ingestion monitor: A novel wearable device for monitoring of ingestive behavior. IEEE Trans. Bio-Med. Eng. 2014, 61, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.M.; Sazonov, E.S. A robust classification scheme for detection of food intake through non-invasive monitoring of chewing. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 4891–4894. [Google Scholar]
- Garcia, M.B. Plan-Cook-Eat: A Meal Planner App with Optimal Macronutrient Distribution of Calories Based on Personal Total Daily Energy Expenditure. In Proceedings of the 2019 IEEE 11th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment, and Management (HNICEM), Laoag, Philippines, 29 November–1 December 2019; pp. 1–5. [Google Scholar]
- Gonzalez-Flo, E.; Kheirabadi, E.; Rodriguez-Caso, C.; Macia, J. Evolutionary algorithm for the optimization of meal intake and insulin administration in patients with type 2 diabetes. Front. Physiol. 2023, 14, 1149698. [Google Scholar] [CrossRef]
- Hansel, B.; Giral, P.; Gambotti, L.; Lafourcade, A.; Peres, G.; Filipecki, C.; Kadouch, D.; Hartemann, A.; Oppert, J.M.; Bruckert, E.; et al. A Fully Automated Web-Based Program Improves Lifestyle Habits and HbA1c in Patients with Type 2 Diabetes and Abdominal Obesity: Randomized Trial of Patient E-Coaching Nutritional Support (The ANODE Study). J. Med. Internet Res. 2017, 19, e360. [Google Scholar] [CrossRef]
- Hauptmann, H.; Leipold, N.; Madenach, M.; Wintergerst, M.; Lurz, M.; Groh, G.; Bohm, M.; Gedrich, K.; Krcmar, H. Effects and challenges of using a nutrition assistance system: Results of a long-term mixed-method study. User Model. User Adapt. Interact. 2022, 32, 923–975. [Google Scholar] [CrossRef]
- Heremans, E.R.M.; Chen, A.S.; Wang, X.; Cheng, J.; Xu, F.; Martinez, A.E.; Lazaridis, G.; Van Huffel, S.; Chen, J.D.Z. Artificial Neural Network-Based Automatic Detection of Food Intake for Neuromodulation in Treating Obesity and Diabetes. Obes. Surg. 2020, 30, 2547–2557. [Google Scholar] [CrossRef]
- Hezarjaribi, N.; Mazrouee, S.; Ghasemzadeh, H. Speech2Health: A Mobile Framework for Monitoring Dietary Composition from Spoken Data. IEEE J. Biomed. Health Inform. 2018, 22, 252–264. [Google Scholar] [CrossRef]
- Hieronimus, B.; Hammann, S.; Podszun, M.C. Can the AI tools ChatGPT and Bard generate energy, macro- and micro-nutrient sufficient meal plans for different dietary patterns? Nutr. Res. 2024, 128, 105–114. [Google Scholar] [CrossRef]
- Holmes, S.; Moorhead, A.; Bond, R.; Zheng, H.; Coates, V.; McTear, M. WeightMentor, bespoke chatbot for weight loss maintenance: Needs assessment & Development. In Proceedings of the 2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), San Diego, CA, USA, 18–21 November 2019; pp. 2845–2851. [Google Scholar]
- Hossain, D.; Imtiaz, M.H.; Ghosh, T.; Bhaskar, V.; Sazonov, E. Real-Time Food Intake Monitoring Using Wearable Egocentric Camera. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montréal, QC, Canada, 20–24 July 2020; pp. 4191–4195. [Google Scholar]
- Hsiao, M.; Yeh, Y.F.; Hsueh, P.Y.; Lee, S. Intelligent Nutrition Service for Personalized Dietary Guidelines and Lifestyle Intervention. In Proceedings of the 2011 International Joint Conference on Service Sciences, Taipei, Taiwan, 25–27 May 2011; pp. 11–16. [Google Scholar]
- Hsu, C.Y.; Huang, L.C.; Chen, T.M.; Chen, L.F.; Chao, J.C.J. A web-based decision support system for dietary analysis and recommendations. Telemed. e-Health 2011, 17, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Jactel, S.N.; Olson, J.M.; Wolin, K.Y.; Brown, J.; Pathipati, M.P.; Jagiella, V.J.; Korzenik, J.R. Efficacy of a Digital Personalized Elimination Diet for the Self-Management of Irritable Bowel Syndrome and Comorbid Irritable Bowel Syndrome and Inflammatory Bowel Disease. Clin. Transl. Gastroenterol. 2023, 14, e00545. [Google Scholar] [CrossRef]
- Ji, Y.; Plourde, H.; Bouzo, V.; Kilgour, R.D.; Cohen, T.R. Validity and usability of a smartphone image-based dietary assessment app compared to 3-day food diaries in assessing dietary intake among Canadian adults: Randomized controlled trial. JMIR mHealth uHealth 2020, 8, e16953. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lin, Q.; Lu, J.; Hu, C.; Lu, B.; Jiang, N.; Wu, S.; Li, X. Evaluating the Effectiveness of a Generative Pre-trained Transformers-Based Dietary Recommendation System in Managing Potassium Intake for Hemodialysis Patients. J. Ren. Nutr. 2024, 34, 539–545. [Google Scholar] [CrossRef]
- Karakan, T.; Gundogdu, A.; Alagozlu, H.; Ekmen, N.; Ozgul, S.; Tunali, V.; Hora, M.; Beyazgul, D.; Nalbantoglu, O.U. Artificial intelligence-based personalized diet: A pilot clinical study for irritable bowel syndrome. Gut Microbes 2022, 14, 2138672. [Google Scholar] [CrossRef]
- Karmakar, A.; Nishida, M.; Nishimura, M. Eating and Drinking Behavior Recognition Using Multimodal Fusion. In Proceedings of the 2023 IEEE 12th Global Conference on Consumer Electronics (GCCE), Nara, Japan, 10–13 October 2023; pp. 210–213. [Google Scholar]
- Khan, M.T.; Ghaffarzadegan, S.; Feng, Z.; Hasan, T. A Fabric-based Inexpensive Wearable Neckband for Accurate and Reliable Dietary Activity Monitoring. In Proceedings of the 2022 25th International Conference on Computer and Information Technology (ICCIT), Chittagong, Bangladesh, 17–19 December 2022; pp. 994–997. [Google Scholar]
- Kiriakedis, S.; Duty, B.; Chase, T.; Wusirika, R.; Metzler, I. Using ChatGPT-4 to Analyze 24-Hour Urine Results and Generate Custom Dietary Recommendations for Nephrolithiasis. J. Endourol. 2024, 38, 719–724. [Google Scholar] [CrossRef]
- Kirk, D.; Van Eijnatten, E.; Camps, G. Comparison of Answers between ChatGPT and Human Dieticians to Common Nutrition Questions. J. Nutr. Metab. 2023, 2023, 5548684. [Google Scholar] [CrossRef]
- Kobayashi, A.; Mori, S.; Hashimoto, A.; Katsuragi, T.; Kawamura, T. Functional Food Knowledge Graph-based Recipe Recommendation System Focused on Lifestyle-Related Diseases. In Proceedings of the 2024 IEEE 18th International Conference on Semantic Computing (ICSC), Laguna Hills, CA, USA, 5–7 February 2024; pp. 261–268. [Google Scholar] [CrossRef]
- Krishnakumar, A.; Verma, R.; Chawla, R.; Sosale, A.; Saboo, B.; Joshi, S.; Shaikh, M.; Shah, A.; Kolwankar, S.; Mattoo, V. Evaluating glycemic control in patients of South Asian origin with type 2 diabetes using a digital therapeutic platform: Analysis of real-world data. J. Med. Internet Res. 2021, 23, e17908. [Google Scholar] [CrossRef]
- Kwon, O.Y.; Lee, M.K.; Lee, H.W.; Kim, H.; Lee, J.S.; Jang, Y. Mobile App-Based Lifestyle Coaching Intervention for Patients with Nonalcoholic Fatty Liver Disease: Randomized Controlled Trial. J. Med. Internet Res. 2024, 26, e49839. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, J.P.; Fides, A.; Navarro, A.; Guillén, S. Ambient Assisted Nutritional Advisor for elderly people living at home. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 198–203. [Google Scholar]
- Lee, H.A.; Liu, C.Y.; Hsu, C.Y. Precision Nutrition Management in Continuous Care: Leveraging AI for User-Reported Dietary Data Analysis. Stud. Health Technol. Inform. 2024, 315, 256–261. [Google Scholar] [CrossRef]
- Lee, J.; Paudyal, P.; Banerjee, A.; Gupta, S.K.S. FIT-EVE&ADAM: Estimation of Velocity & Energy for Automated Diet Activity Monitoring. In Proceedings of the 16th IEEE International Conference on Machine Learning and Applications, Cancún, Mexico, 21 December 2017; pp. 1071–1074. [Google Scholar]
- Lee, K.S. Joint Audio-Ultrasound Food Recognition for Noisy Environments. IEEE J. Biomed. Health Inform. 2020, 24, 1477–1489. [Google Scholar] [CrossRef]
- Lee, Y.B.; Kim, G.; Jun, J.E.; Park, H.; Lee, W.J.; Hwang, Y.C.; Kim, J.H. An Integrated Digital Health Care Platform for Diabetes Management with AI-Based Dietary Management: 48-Week Results from a Randomized Controlled Trial. Diabetes Care 2023, 46, 959–966. [Google Scholar] [CrossRef]
- Li, X.; Yin, A.; Choi, H.Y.; Chan, V.; Allman-Farinelli, M.; Chen, J. Evaluating the Quality and Comparative Validity of Manual Food Logging and Artificial Intelligence-Enabled Food Image Recognition in Apps for Nutrition Care. Nutrients 2024, 16, 2573. [Google Scholar] [CrossRef]
- Liao, L.L.; Chang, L.C.; Lai, I.J. Assessing the Quality of ChatGPT’s Dietary Advice for College Students from Dietitians’ Perspectives. Nutrients 2024, 16, 1939. [Google Scholar] [CrossRef]
- Lin, Z.; Xie, Y.; Guo, X.; Ren, Y.; Chen, Y.; Wang, C. WiEat: Fine-grained Device-free Eating Monitoring Leveraging Wi-Fi Signals. In Proceedings of the 2020 29th International Conference on Computer Communications and Networks (ICCCN), Honolulu, HI, USA, 3–6 August 2020; pp. 1–9. [Google Scholar]
- Liu, M.; Zhou, B.; Rey, V.F.; Bian, S.; Lukowicz, P. iEat: Automatic wearable dietary monitoring with bio-impedance sensing. Sci. Rep. 2024, 14, 17873. [Google Scholar] [CrossRef]
- Lozano, C.P.; Canty, E.N.; Saha, S.; Broyles, S.T.; Beyl, R.A.; Apolzan, J.W.; Martin, C.K. Validity of an Artificial Intelligence-Based Application to Identify Foods and Estimate Energy Intake Among Adults: A Pilot Study. Curr. Dev. Nutr. 2023, 7, 102009. [Google Scholar] [CrossRef]
- Maher, C.A.; Davis, C.R.; Curtis, R.G.; Short, C.E.; Murphy, K.J. A Physical Activity and Diet Program Delivered by Artificially Intelligent Virtual Health Coach: Proof-of-Concept Study. JMIR mHealth uHealth 2020, 8, e17558. [Google Scholar] [CrossRef]
- Marashi-Hosseini, L.; Jafarirad, S.; Hadianfard, A.M. A fuzzy based dietary clinical decision support system for patients with multiple chronic conditions (MCCs). Sci. Rep. 2023, 13, 12166. [Google Scholar] [CrossRef]
- Martino, F.D.; Delmastro, F.; Dolciotti, C. Malnutrition Risk Assessment in Frail Older Adults using m-Health and Machine Learning. In Proceedings of the ICC 2021—IEEE International Conference on Communications, Online, 14–23 June 2021; pp. 1–6. [Google Scholar]
- Mertes, G.; Ding, L.; Chen, W.; Hallez, H.; Jia, J.; Vanrumste, B. Measuring and Localizing Individual Bites Using a Sensor Augmented Plate During Unrestricted Eating for the Aging Population. IEEE J. Biomed. Health Inform. 2020, 24, 1509–1518. [Google Scholar] [CrossRef]
- Moyen, A.; Rappaport, A.I.; Fleurent-Gregoire, C.; Tessier, A.J.; Brazeau, A.S.; Chevalier, S. Relative Validation of an Artificial Intelligence-Enhanced, Image-Assisted Mobile App for Dietary Assessment in Adults: Randomized Crossover Study. J. Med. Internet Res. 2022, 24, e40449. [Google Scholar] [CrossRef]
- Naja, F.; Taktouk, M.; Matbouli, D.; Khaleel, S.; Maher, A.; Uzun, B.; Alameddine, M.; Nasreddine, L. Artificial intelligence chatbots for the nutrition management of diabetes and the metabolic syndrome. Eur. J. Clin. Nutr. 2024, 78, 887–896. [Google Scholar] [CrossRef]
- Nakaoka, R.; Nakamura, Y.; Matsuda, Y.; Misaki, S.; Yasumoto, K. eat2pic: Food-tech Design as a Healthy Nudge with Smart Chopsticks and Canvas. In Proceedings of the 2021 IEEE International Conference on Pervasive Computing and Communications Workshops and other Affiliated Events (PerCom Workshops), Kassel, Germany, 22–26 March 2021; pp. 389–391. [Google Scholar]
- Nakata, Y.; Sasai, H.; Gosho, M.; Kobayashi, H.; Shi, Y.; Ohigashi, T.; Mizuno, S.; Murayama, C.; Kobayashi, S.; Sasaki, Y. A Smartphone Healthcare Application, CALO mama Plus, to Promote Weight Loss: A Randomized Controlled Trial. Nutrients 2022, 14, 4608. [Google Scholar] [CrossRef]
- Niszczota, P.; Rybicka, I. The credibility of dietary advice formulated by ChatGPT: Robo-diets for people with food allergies. Nutrition 2023, 112, 112076. [Google Scholar] [CrossRef]
- Ocay, A.B.; Fernandez, J.M.; Palaoag, T.D. NutriTrack: Android-based food recognition app for nutrition awareness. In Proceedings of the 2017 3rd IEEE International Conference on Computer and Communications (ICCC), Chengdu, China, 13–16 December 2017; pp. 2099–2104. [Google Scholar]
- Oh, S.W.; Kim, K.K.; Kim, S.S.; Park, S.K.; Park, S. Effect of an Integrative Mobile Health Intervention in Patients with Hypertension and Diabetes: Crossover Study. JMIR mHealth uHealth 2022, 10, e27192. [Google Scholar] [CrossRef]
- Papapanagiotou, V.; Diou, C.; Zhou, L.; van den Boer, J.; Mars, M.; Delopoulos, A. A Novel Chewing Detection System Based on PPG, Audio, and Accelerometry. IEEE J. Biomed. Health Inform. 2017, 21, 607–618. [Google Scholar] [CrossRef]
- Papapanagiotou, V.; Ganotakis, S.; Delopoulos, A. Bite-Weight Estimation Using Commercial Ear Buds. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Online, 1–5 November 2021; pp. 7182–7185. [Google Scholar]
- Papastratis, I.; Konstantinidis, D.; Daras, P.; Dimitropoulos, K. AI nutrition recommendation using a deep generative model and ChatGPT. Sci. Rep. 2024, 14, 14620. [Google Scholar] [CrossRef]
- Papathanail, I.; Abdur Rahman, L.; Brigato, L.; Bez, N.S.; Vasiloglou, M.F.; van der Horst, K.; Mougiakakou, S. The Nutritional Content of Meal Images in Free-Living Conditions-Automatic Assessment with goFOODTM. Nutrients 2023, 15, 3835. [Google Scholar] [CrossRef]
- Papathanail, I.; Vasiloglou, M.F.; Stathopoulou, T.; Ghosh, A.; Baumann, M.; Faeh, D.; Mougiakakou, S. A feasibility study to assess Mediterranean Diet adherence using an AI-powered system. Sci. Rep. 2022, 12, 17008. [Google Scholar] [CrossRef]
- Ponzo, V.; Goitre, I.; Favaro, E.; Merlo, F.D.; Mancino, M.V.; Riso, S.; Bo, S. Is ChatGPT an Effective Tool for Providing Dietary Advice? Nutrients 2024, 16, 469. [Google Scholar] [CrossRef]
- Qarajeh, A.; Tangpanithandee, S.; Thongprayoon, C.; Suppadungsuk, S.; Krisanapan, P.; Aiumtrakul, N.; Garcia Valencia, O.A.; Miao, J.; Qureshi, F.; Cheungpasitporn, W. AI-Powered Renal Diet Support: Performance of ChatGPT, Bard AI, and Bing Chat. Clin. Pract. 2023, 13, 1160–1172. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, A.J.; Hall, R.; Johnston, C.S. A Novel Mobile App (Heali) for Disease Treatment in Participants with Irritable Bowel Syndrome: Randomized Controlled Pilot Trial. J. Med. Internet Res. 2021, 23, e24134. [Google Scholar] [CrossRef]
- Salloum, G.; Semaan, E.; Tekli, J. PIN Prototype for Intelligent Nutrition Assessment and Meal Planning. In Proceedings of the 2018 IEEE International Conference on Cognitive Computing (ICCC), San Francisco, CA, USA, 2–7 July 2018; pp. 110–113. [Google Scholar]
- Samaan, J.S.; Issokson, K.; Feldman, E.; Fasulo, C.; Ng, W.H.; Rajeev, N.; Hollander, B.; Yeo, Y.H.; Vasiliauskas, E. Artificial Intelligence and Patient Education: Examining the Accuracy and Reproducibility of Responses to Nutrition Questions Related to Inflammatory Bowel Disease by GPT-4. medRxiv 2023. [Google Scholar] [CrossRef]
- Sano, A.; Johns, P.; Czerwinski, M. HealthAware: An advice system for stress, sleep, diet and exercise. In Proceedings of the 2015 International Conference on Affective Computing and Intelligent Interaction (ACII), Xi’an, China, 21–24 September 2015; pp. 546–552. [Google Scholar]
- Schiboni, G.; Wasner, F.; Amft, O. A Privacy-Preserving Wearable Camera Setup for Dietary Event Spotting in Free-Living. In Proceedings of the 2018 IEEE International Conference on Pervasive Computing and Communications Workshops (PerCom Workshops), Athens, Greece, 19–23 March 2018; pp. 872–877. [Google Scholar]
- Sefa-Yeboah, S.M.; Osei Annor, K.; Koomson, V.J.; Saalia, F.K.; Steiner-Asiedu, M.; Mills, G.A. Development of a Mobile Application Platform for Self-Management of Obesity Using Artificial Intelligence Techniques. Int. J. Telemed. Appl. 2021, 2021, 6624057. [Google Scholar] [CrossRef]
- Shamanna, P.; Saboo, B.; Damodharan, S.; Mohammed, J.; Mohamed, M.; Poon, T.; Kleinman, N.; Thajudeen, M. Reducing HbA1c in Type 2 Diabetes Using Digital Twin Technology-Enabled Precision Nutrition: A Retrospective Analysis. Diabetes Ther. 2020, 11, 2703–2714. [Google Scholar] [CrossRef]
- Shao, Z.; Fang, S.; Mao, R.; He, J.; Wright, J.L.; Kerr, D.A.; Boushey, C.J.; Zhu, F. Towards Learning Food Portion from Monocular Images with Cross-Domain Feature Adaptation. In Proceedings of the 2021 IEEE 23rd International Workshop on Multimedia Signal Processing (MMSP), Tampere, Finland, 6–8 October 2021; pp. 1–6. [Google Scholar]
- Shoneye, C.L.; Dhaliwal, S.S.; Pollard, C.M.; Boushey, C.J.; Delp, E.J.; Harray, A.J.; Howat, P.A.; Hutchesson, M.J.; Rollo, M.E.; Zhu, F.; et al. Image-Based Dietary Assessment and Tailored Feedback Using Mobile Technology: Mediating Behavior Change in Young Adults. Nutrients 2019, 11, 435. [Google Scholar] [CrossRef]
- Silva, V.C.; Gorgulho, B.; Marchioni, D.M.; Alvim, S.M.; Giatti, L.; de Araujo, T.A.; Alonso, A.C.; Santos, I.D.S.; Lotufo, P.A.; Bensenor, I.M. Recommender System Based on Collaborative Filtering for Personalized Dietary Advice: A Cross-Sectional Analysis of the ELSA-Brasil Study. Int. J. Environ. Res. Public Health 2022, 19, 14934. [Google Scholar] [CrossRef]
- Sowah, R.A.; Bampoe-Addo, A.A.; Armoo, S.K.; Saalia, F.K.; Gatsi, F.; Sarkodie-Mensah, B. Design and Development of Diabetes Management System Using Machine Learning. Int. J. Telemed. Appl. 2020, 2020, 8870141. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, K.; Lan, W.; Gu, Q.; Jiang, G.; Yang, X.; Qin, W.; Han, D. An AI Dietitian for Type 2 Diabetes Mellitus Management Based on Large Language and Image Recognition Models: Preclinical Concept Validation Study. J. Med. Internet Res. 2023, 25, e51300. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Burke, L.E.; Baranowski, T.; Fernstrom, J.D.; Zhang, H.; Chen, H.C.; Bai, Y.; Li, Y.; Li, C.; Yue, Y.; et al. An exploratory study on a chest-worn computer for evaluation of diet, physical activity and lifestyle. J. Healthc. Eng. 2015, 6, 1–22. [Google Scholar] [CrossRef]
- Thames, Q.; Karpur, A.; Norris, W.; Xia, F.; Panait, L.; Weyand, T.; Sim, J. Nutrition5k: Towards Automatic Nutritional Understanding of Generic Food. In Proceedings of the 2021 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Online, 19–25 June 2021; pp. 8899–8907. [Google Scholar]
- Tunali, V.; Arslan, N.C.; Ermis, B.H.; Hakim, G.D.; Gundogdu, A.; Hora, M.; Nalbantoglu, O.U. A Multicenter Randomized Controlled Trial of Microbiome-Based Artificial Intelligence-Assisted Personalized Diet Vs Low Fodmap Diet: A Novel Approach for the Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2024, 119, 1901–1912. [Google Scholar] [CrossRef]
- Turnin, M.C.; Gourdy, P.; Martini, J.; Buisson, J.C.; Chauchard, M.C.; Delaunay, J.; Schirr-Bonnans, S.; Taoui, S.; Poncet, M.F.; Cosma, V.; et al. Impact of a Remote Monitoring Programme Including Lifestyle Education Software in Type 2 Diabetes: Results of the Educ@dom Randomised Multicentre Study. Diabetes Ther. 2021, 12, 2059–2075. [Google Scholar] [CrossRef]
- Valero-Ramon, Z.; Fernandez-Llatas, C.; Martinez-Millana, A.; Traver, V. A Dynamic Behavioral Approach to Nutritional Assessment using Process Mining. In Proceedings of the 2019 IEEE 32nd International Symposium on Computer-Based Medical Systems (CBMS), Córdoba, Spain, 5–7 June 2019; pp. 398–404. [Google Scholar]
- Vasiloglou, M.F.; Lu, Y.; Stathopoulou, T.; Papathanail, I.; Fah, D.; Ghosh, A.; Baumann, M.; Mougiakakou, S. Assessing Mediterranean Diet Adherence with the Smartphone: The Medipiatto Project. Nutrients 2020, 12, 3763. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.P.; Bhatia, D.K. Automated Ingestion Detection for a Health Monitoring System. IEEE J. Biomed. Health Inform. 2014, 18, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kumar, T.S.; Raedt, W.D.; Camps, G.; Hallez, H.; Vanrumste, B. Eat-Radar: Continuous Fine-Grained Intake Gesture Detection Using FMCW Radar and 3D Temporal Convolutional Network with Attention. IEEE J. Biomed. Health Inform. 2024, 28, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Wu, H. Personalized Recommendation Method of “Carbohydrate-Protein” Supplement Based on Machine Learning and Enumeration Method. IEEE Access 2023, 11, 100573–100586. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, S.; Das, A.; Do, E.; Glantz, N.; Bevier, W.; Santiago, R.; Kerr, D.; Gutierrez-Osuna, R.; Mortazavi, B.J. Joint Embedding of Food Photographs and Blood Glucose for Improved Calorie Estimation. In Proceedings of the 2023 IEEE EMBS International Conference on Biomedical and Health Informatics (BHI), Pittsburgh, PA, USA, 15–18 October 2023; pp. 1–4. [Google Scholar]
- Chew, H.S.J.; Ang, W.H.D.; Lau, Y. The potential of artificial intelligence in enhancing adult weight loss: A scoping review. Public Health Nutr. 2021, 24, 1993–2020. [Google Scholar] [CrossRef]
- Mennella, C.; Maniscalco, U.; De Pietro, G.; Esposito, M. Ethical and regulatory challenges of AI technologies in healthcare: A narrative review. Heliyon 2024, 10, e26297. [Google Scholar] [CrossRef]
- Shonkoff, E.; Cara, K.C.; Pei, X.A.; Chung, M.; Kamath, S.; Panetta, K.; Hennessy, E. AI-based digital image dietary assessment methods compared to humans and ground truth: A systematic review. Ann. Med. 2023, 55, 2273497. [Google Scholar] [CrossRef]
- Bailey, R.L. Overview of dietary assessment methods for measuring intakes of foods, beverages, and dietary supplements in research studies. Curr. Opin. Biotechnol. 2021, 70, 91–96. [Google Scholar] [CrossRef]
- Ma, W.; Cai, B.; Wang, Y.; Wang, L.; Sun, M.W.; Lu, C.D.; Jiang, H. Artificial intelligence driven malnutrition diagnostic model for patients with acute abdomen based on GLIM criteria: A cross-sectional research protocol. BMJ Open 2024, 14, e077734. [Google Scholar] [CrossRef]
- Abeltino, A.; Riente, A.; Bianchetti, G.; Serantoni, C.; De Spirito, M.; Capezzone, S.; Esposito, R.; Maulucci, G. Digital applications for diet monitoring, planning, and precision nutrition for citizens and professionals: A state of the art. Nutr. Rev. 2025, 83, e574–e601. [Google Scholar] [CrossRef]
- ZOE Limited. ZOE. Available online: https://zoe.com/en-gb/faqs (accessed on 17 September 2025).
- Bermingham, K.M.; Linenberg, I.; Polidori, L.; Asnicar, F.; Arrè, A.; Wolf, J.; Badri, F.; Bernard, H.; Capdevila, J.; Bulsiewicz, W.J.; et al. Effects of a personalized nutrition program on cardiometabolic health: A randomized controlled trial. Nat. Med. 2024, 30, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health. National Institute of Health: Office of Nutrition Research; National Institute of Health: Bethesda, MD, USA, 2025; p. 24. [Google Scholar]
- Truby, H.; Allman-Farinelli, M.; Beck, E.J.; Beckett, E.L.; Bondonno, C.; Dordevic, A.L.; Livingstone, K.M.; Willcox, J.; Wilkinson, S.A. Advancing the decadal plan for the science of nutrition: Progressing a framework for implementation. Nutr. Diet. 2024, 81, 133–148. [Google Scholar] [CrossRef]
- Salas-Groves, E.; Galyean, S.; Alcorn, M.; Childress, A. Behavior Change Effectiveness Using Nutrition Apps in People with Chronic Diseases: Scoping Review. JMIR mHealth uHealth 2023, 11, e41235. [Google Scholar] [CrossRef]
- Detopoulou, P.; Voulgaridou, G.; Moschos, P.; Levidi, D.; Anastasiou, T.; Dedes, V.; Diplari, E.M.; Fourfouri, N.; Giaginis, C.; Panoutsopoulos, G.I.; et al. Artificial intelligence, nutrition, and ethical issues: A mini-review. Clin. Nutr. Open Sci. 2023, 50, 46–56. [Google Scholar] [CrossRef]
- Safran, E.; Yildirim, S. A cross-sectional study on ChatGPT’s alignment with clinical practice guidelines in musculoskeletal rehabilitation. BMC Musculoskelet. Disord. 2025, 26, 411. [Google Scholar] [CrossRef]
- Desroches, S.; Lapointe, A.; Ratté, S.; Gravel, K.; Légaré, F.; Turcotte, S.; Desroches, S. Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults. Cochrane Database Syst. Rev. 2013, 2013, CD008722. [Google Scholar] [CrossRef]
- Atwal, K. Artificial intelligence in clinical nutrition and dietetics: A brief overview of current evidence. Nutr. Clin. Pract. 2024, 39, 736–742. [Google Scholar] [CrossRef]
- Schubert, T.; Oosterlinck, T.; Stevens, R.D.; Maxwell, P.H.; van der Schaar, M. AI education for clinicians. eClinicalMedicine 2025, 79, 102968. [Google Scholar] [CrossRef]
- Tetik, G.; Türkeli, S.; Pinar, S.; Tarim, M. Health information systems with technology acceptance model approach: A systematic review. Int. J. Med. Inform. 2024, 190, 105556. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.; Münscher, J.C.; Daseking, M.; Telle, N.T. The technology acceptance model and adopter type analysis in the context of artificial intelligence. Front. Artif. Intell. 2025, 7, 1496518. [Google Scholar] [CrossRef]
- Chen, J.; Allman-Farinelli, M. Impact of Training and Integration of Apps into Dietetic Practice on Dietitians’ Self-Efficacy with Using Mobile Health Apps and Patient Satisfaction. JMIR mHealth uHealth 2019, 7, e12349. [Google Scholar] [CrossRef]
- Nilsen, P.; Sundemo, D.; Heintz, F.; Neher, M.; Nygren, J.; Svedberg, P.; Petersson, L. Towards evidence-based practice 2.0: Leveraging artificial intelligence in healthcare. Front. Health Serv. 2024, 4, 1368030. [Google Scholar] [CrossRef]
- Barrimore, S.E.; Cameron, A.E.; Young, A.M.; Hickman, I.J.; Campbell, K.L. Translating Research into Practice: How Confident Are Allied Health Clinicians? J. Allied. Health 2020, 49, 258–262. [Google Scholar]
- Young, A.M.; Olenski, S.; Wilkinson, S.A.; Campbell, K.; Barnes, R.; Cameron, A.; Hickman, I. Knowledge Translation in Dietetics: A Survey of Dietitians’ Awareness and Confidence. Can. J. Diet. Pract. Res. 2020, 81, 49–53. [Google Scholar] [CrossRef]
- Hurt, R.T.; Stephenson, C.R.; Gilman, E.A.; Aakre, C.A.; Croghan, I.T.; Mundi, M.S.; Ghosh, K.; Edakkanambeth, V.J. The Use of an Artificial Intelligence Platform Open Evidence to Augment Clinical Decision-Making for Primary Care Physicians. J. Prim. Care Community Health 2025, 16, 21501319251332215. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Powell, D. Generalist medical AI reimbursement challenges and opportunities. npj Digit. Med. 2025, 8, 125. [Google Scholar] [CrossRef]
- Angus, D.C.; Khera, R.; Lieu, T.; Liu, V.; Ahmad, F.S.; Anderson, B.; Bhavani, S.V.; Bindman, A.; Brennan, T.; Celi, L.A.; et al. AI, Health, and Health Care Today and Tomorrow: The JAMA Summit Report on Artificial Intelligence. JAMA 2025. [Google Scholar] [CrossRef]
- Popover, J.L.; Wallace, S.P.; Feldman, J.; Chastain, G.; Kalathia, C.; Imam, A.; Almasri, M.; Toomey, P.G. Artificial Intelligence in Medicine: A Specialty-Level Overview of Emerging AI Trends. JSLS 2025, 29, e2025.00041. [Google Scholar] [CrossRef]
- Zink, A.; Chernew, M.E.; Neprash, H.T. How Should Medicare Pay for Artificial Intelligence? JAMA Intern. Med. 2024, 184, 863–864. [Google Scholar] [CrossRef]
- Stoyanov, S.R.; Hides, L.; Kavanagh, D.J.; Zelenko, O.; Tjondronegoro, D.; Mani, M. Mobile App Rating Scale: A New Tool for Assessing the Quality of Health Mobile Apps. JMIR mHealth uHealth 2015, 3, e27. [Google Scholar] [CrossRef]
- Liu, X.; Cruz Rivera, S.; Moher, D.; Calvert, M.J.; Denniston, A.K.; Chan, A.W.; Darzi, A.; Holmes, C.; Yau, C.; Ashrafian, H.; et al. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: The CONSORT-AI extension. Nat. Med. 2020, 26, 1364–1374. [Google Scholar] [CrossRef]
- Collins, G.S.; Moons, K.G.M.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Van Calster, B.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; van Smeden, M.; et al. TRIPOD+AI statement: Updated guidance for reporting clinical prediction models that use regression or machine learning methods. BMJ 2024, 385, e078378. [Google Scholar] [CrossRef]
- Kwong, J.C.C.; Khondker, A.; Lajkosz, K.; McDermott, M.B.A.; Frigola, X.B.; McCradden, M.D.; Mamdani, M.; Kulkarni, G.S.; Johnson, A.E.W. APPRAISE-AI Tool for Quantitative Evaluation of AI Studies for Clinical Decision Support. JAMA Netw. Open 2023, 6, e2335377. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.G.; Pantanowitz, L.; Jackson, B.; Palmer, O.; Visweswaran, S.; Pantanowitz, J.; Deebajah, M.; Rashidi, H.H. Ethical and Bias Considerations in Artificial Intelligence/Machine Learning. Mod. Pathol. 2025, 38, 100686. [Google Scholar] [CrossRef]
- Perivolaris, A.; Adams-McGavin, C.; Madan, Y.; Kishibe, T.; Antoniou, T.; Mamdani, M.; Jung, J.J. Quality of interaction between clinicians and artificial intelligence systems. A systematic review. Future Heal. J. 2024, 11, 100172. [Google Scholar] [CrossRef]
- Sosa-Holwerda, A.; Park, O.H.; Albracht-Schulte, K.; Niraula, S.; Thompson, L.; Oldewage-Theron, W. The Role of Artificial Intelligence in Nutrition Research: A Scoping Review. Nutrients 2024, 16, 2066. [Google Scholar] [CrossRef] [PubMed]
- Theodore Armand, T.P.; Nfor, K.A.; Kim, J.I.; Kim, H.C. Applications of Artificial Intelligence, Machine Learning, and Deep Learning in Nutrition: A Systematic Review. Nutrients 2024, 16, 1073. [Google Scholar] [CrossRef]
- Phalle, A.; Gokhale, D. Navigating next-gen nutrition care using artificial intelligence-assisted dietary assessment tools-a scoping review of potential applications. Front. Nutr. 2025, 12, 1518466. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.; Lockwood, C.; Porritt, K.; Pilla, B.; Jordan, Z. JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2024. [Google Scholar] [CrossRef]
- Cant, R.P. Public Health Nutrition: The Accord of Dietitian Providers in Managing Medicare Chronic Care Outpatients in Australia. Int. J. Environ. Res. Public Health 2010, 7, 1841–1854. [Google Scholar] [CrossRef] [PubMed]
- Kassem, H.; Beevi, A.A.; Basheer, S.; Lutfi, G.; Cheikh Ismail, L.; Papandreou, D. Investigation and Assessment of AI’s Role in Nutrition-An Updated Narrative Review of the Evidence. Nutrients 2025, 17, 190. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Population |
|
|
| Concept |
|
|
| Context |
|
|
| Study Design Attributes |
|
|
| Data Extracted | |
|---|---|
| Article details | Article citation, country of publication, discipline |
| Population characteristics | Age, sex, sample size, chronic medical condition |
| Concept characteristics (artificial intelligence (AI)) |
|
| Context characteristics |
|
| Primary outcome |
|
| Secondary outcomes |
|
| Author | Medical Condition | Dietitian Involvement | User/Patient-Focused Outcomes (A: Anthropometry, B: Biochemistry, C: Clinical Symptoms, D: Diet and Food Related, PA: Patient Adherence) | Evaluation Metric | Efficiencies | Limitations/Barriers of Use |
|---|---|---|---|---|---|---|
| Abeltino et al., 2022 [18] | Weight management—overweight | Development of nutrition plans | A: 500 kcal energy deficit/day resulted in an average weight loss of −0.4 ± 0.2 kg/week. 500 kcal energy surplus resulted in an average weight gain of 0.77 ± 0.63 kg/week. | - |
|
|
| Agne et al., 2024 [19] | Weight management—obese | NS | - | - |
|
|
| Amft et al., 2007 [20] | Healthy | NS | D: Classification and determination of sounds patterns for different food types: soft foods (no changes in sound pattern in ordered sequence) and hard foods (clear sequential structure in sound pattern). | Accuracy: 80% |
|
|
| Amft et al., 2009 [21] | Healthy | NS | D: Bite weight prediction using acoustic chewing recordings is a feasible approach for solid foods. Good food recognition results for individual chewing events. Foods with low bite weight had higher prediction errors. | Recall: 80% Precision: 60–70% Accuracy: 94% |
|
|
| Amft et al., 2008 [22] | Healthy | NS | D: Body movements and chewing sounds could be accurately identified using on-body sensors; detection of drinking movements can be used to monitor fluid consumption and avoid dehydration. | Accuracy: Movement: 79% Chewing: 86% Swallowing: 70% |
|
|
| Ben Neriah et al., 2019 [23] | Weight management—overweight, obese | NS | A: Utilisation of the food photo recognition feature embedded within the app was associated with greater % of weight loss. This effect was mediated by increased duration of use and more logged days. PA: Photo users logged more days (6.1 days) compared to non-photo users | - |
| - |
| Ben-Yacov et al., 2023 [24] | Prediabetes | Dietary guidance during intervention and follow- up | B: Greater changes observed in gut microbiota composition with AI-designed postprandial targeting diet compared with the MED diet. | - |
|
|
| Beyeler M et al., 2023 [25] | Weight management—gastric bypass | Provided nutrition counselling at bariatric centre appointments | A: Nil significant difference between the pre- and post-surgery patient groups over several months. | Usability score: 87/100 Usefulness: 5.28/7 Satisfaction: 5.75/7 Learnability: 6.26/7 |
|
|
| Bohn et al., 2024 [26] | Aging population (within healthy weight range) | NS | A, B, C: Use of app and meal recommender system over several months (field trials) did not result in any improvement nor deterioration. No significant changes in health biomarkers (anthropometry, blood pressure) in the target population. PA: Limited adherence (reduction in optimism and perception of healthy changes/outcomes) with increased time of intervention (>3 months). | Consideration of app use: Netherlands: 9% Portugal: 47% |
|
|
| Buchan et al., 2024 [27] | All cancer types—primarily genitourinary, gynaecologic, gastrointestinal, lung | Development of database for algorithm. Manual review and modification of algorithm responses for more complex cases/questions | C: Improved QoL in 82% and better symptom management in 88%. D: Overall beneficial impact on dietary intake. PA: High levels of engagement via testing questions to INA. User retention: 8.8 months; 84% applied advice to guide diet; 47% used recommended recipes. | User satisfaction: 94% |
|
|
| Bul et al., 2023 [28] | Prediabetes, T1DM, T2DM | NS | No mean difference in reported general health status with the use of the platform. A: Reduction in weight and waist circumference. C: Participants were less confident in diabetes management after platform use. D: Participants felt more confident in meal planning and choosing healthy food choices after intervention. PA: Challenges in maintaining user engagement with chronically ill and older populations over longer time periods. | User experience ratings: No additional assistance required for use: 52% User-friendly: 38% Easy to use: 38% | - |
|
| Burgermaster et al., 2020 [29] | T2DM | Conducted interviews, established a standard for comparison, final evaluation comments | D: Inference engine recommendation outputs aligned with clinical diabetes educator (CDE) narrative observations 74% of the time; 63% consistency with the CDE gold standard. | - |
|
|
| Chen et al., 2023 [30] | Healthy | NS | D: Feasible for detection of different types of foods through analysing different bite–chew combinations and hand gestures with accompanying wrist band device. | Accuracy: 93.3% |
| - |
| Chen et al., 2015 [31] | Hypertension | Provided meal proof standard values | - | Accuracy: 100% |
| - |
| Chen et al., 2012. [32] | CKD— haemodialysis (protein energy malnutrition) | Gathering patient information including physical exam findings and diagnoses. Assisted with nutritional diagnosis guidelines to convert into programming rules | - | Rates of diagnosis: Correct diagnosis by expert system: 100% Human misdiagnosis: 3 times |
| - |
| Chew et al., 2024 [33] | Weight management—overweight | NS | C: No significant improvements in anxiety-related symptoms. D: Week-long intervention improvements in overeating habits, snacking, self-regulation of eating habits, depression, and physical activity. | - |
|
|
| Chin et al., 2019 [34] | Healthy | Supervised a team which were estimating lactose values from the ASA24-reported foods and manual look up of foods in NDSR | - | Ranking classifier accuracy: combined XGB model > LASSO and Ridge classifier |
|
|
| Chotwanvirat et al., 2024 [35] | Other | Common practices of Thai Dietitians were applied to arrange ingredients and prepare ready to eat foods | D: Newer system outperformed v4-based system in estimations of fat and protein. No significant difference in measurements of carbohydrates. | - |
|
|
| Cohen et al., 2023 [36] | Other | NS | - | - |
|
|
| Davis et al., 2020 [37] | Weight management—overweight, obese | Gathered a list of frequently asked questions from previous diet and exercise trials | D: High levels of dietary compliance: participants achieved recommended dietary intakes 67% of the time. High levels of compliance to key MED diet food groups. |
|
| |
| De Marchi et al., 2022 [38] | Neurology—amyotrophic lateral Sclerosis | Conduced assessment with multidisciplinary team. Designed a personalised, flexible, normo-caloric dietary plan | A: Weight stabilisation within initial increase for group exposed to the Chabot webapp. Prevention of further weight loss in amyotrophic lateral sclerosis patients. PA: Low rate of drop-out in chatbot use. | - |
| |
| Farooq et al., 2016 [39] | Healthy—no medical conditions that would impact chewing | NS | - | - |
|
|
| Fernandes et al., 2023 [40] | Weight management—obesity | Evaluated whether explanations produced by the tools were understandable and reliable for use | A: Model predicts weight loss as early as 2 weeks into the intervention | Accuracy: 81%; specificity: 86%; sensitivity: 69% |
| - |
| Fontana et al., 2014 [41] | Healthy—no conditions that would impact normal food intake | NS | D: Correctly detected major meals (breakfast, lunch, and dinner), 27 episodes of snacking were incorrectly predicted. | Accuracy: 89.9% |
|
|
| Fontana et al., 2012 [42] | Healthy | NS | - | Accuracy: 90.52% Time resolution: 15 s |
| - |
| Garcia et al., 2019. [43] | Healthy | Served on a panel of human expert validators | D: Behavioural changes towards nutrition and food—participants become more equipped to altering eating habits. Assisted users with building meal preparation ideas and skills for cooking via step-by-step recipes enabling more consistent dietary change and greater awareness of health. Positive feedback on app functionality and ability to generate meal plan with optimal macronutrient distribution with respect to daily calorie intake. PA: Users expressed willingness and trust with interacting with the meal recommendation system. | AQEL (nutrition app quality evaluation tool) |
| - |
| Gonzalez-Flo et al., 2023 [44] | T2DM | NS | B: Regulation of glycaemic control D: Evolutionary algorithm was able to provide tailored assisted regulation of glycaemic control by establish patterns of food intake and insulin doses (basal and postprandial). | - |
| - |
| Hansel et al., 2017 [45] | T2DM obesity | NS | A: Improvement in cardiometabolic and anthropometric risk factors, weight loss, and reduction in waist circumference. B: Improved glycaemic control and aerobic fitness. No significant differences in physiological biomarkers between the two arms at 4 months. No significant differences in terms of change in blood pressure, plasma lipids, aminotransferases, gamma glutamyl aminotransferase, uric acid, fasting glucose, VO2 max, or hs-CRP were observed between the two arms at 4 months. C: No significant difference in blood pressure. D: Improvements in dietary habits and intake of healthier foods (e.g., lipids, saturated fats, sodium and empty calories). | - |
|
|
| Hauptmann et al., 2022 [46] | Weight management—overweight | NS | A: Minimal changes to physique of patients. D: Positive impact on nutritional behaviour evidence by optimal intake of nutrients. Reporting of changes to healthier eating behaviours (e.g., eating more fruits or smaller portion sizes). PA: Greater engagement with visual feedback screens, which led to change towards healthy behaviours compared the recommender features of the app. | - |
|
|
| Heremans et al., 2020 [47] | Dyspepsia | NS | - | Accuracy: Experiment 1: 0.93 Experiment 2: 0.83 |
|
|
| Hezarjaribi et al., 2018 [48] | Healthy | NS | - | Accuracy: 80.6% |
|
|
| Hieronimus et al., 2024 [49] | Other | Data was compared to dietary reference intakes calculated using the USDA DRI calculator used by health professionals | D: Daily meal plans generated contained meals and snacks. AI was able to differentiate between health and unhealthy options. ChatGPT provided food items compliant with specific diets. Bard generated incorrect diet plans. Some micronutrients did not meet recommended values for ChatGPT and Bard. ChatGPT had small portion sizes. | - |
|
|
| Holmes et al., 2019 [50] | Weight management—overweight | NS | A and D: Progress tracking was determined based on the most important features. Self-reporting aspects should retain minimal interactions to maintain convenience. | - |
|
|
| Hossain et al., 2020 [51] | Healthy—no chewing conditions | NS | - | Average precision: 82 ± 3% Average F-score: 74 ± 2% |
|
|
| Hsiao et al., 2011 [52] | Healthy | NS | D: The flexible personalised meal service selection system was able to deliver a tailored meal plan tailored to the user’s requirements. Young adults rely on the internet as the main source for self-learning of healthcare information (e.g., diet). Perceived usefulness, peer influence, social network, and trust in professionals have a positive impact that drives engagement with the system. |
|
| |
| Hsu et al., 2011 [53] | Healthy | Development of database of menus, retrieval of participant data from dietary records, Adjustments of personalised dietary recommendations and nutrition information Evaluation process | D: The recommended menus generated by the fuzzy decision model are reliable and valid. Dietary analysis and recommendations can be used a decision-making tool for dietitians. | - |
| - |
| Jactel et al., 2023 [54] | IBS, Crohn’s disease, ulcerative colitis | Evaluation of nutritional adequacy based on the participant’s list of trigger foods | C: 67.7% achieved total symptomatic resolution by the end of the intervention; 89% reported improves to QoL. Engagement in the program observed improved symptoms and symptomatic resolution in patients with IBS and comorbid IBS/IBD. Significant improvements were reported by 81% at midpoint and persisted for 70% by the end of the study. Measured on IBS symptom severity score and Patient Simple Clinical Colitis Activity Index. PA: Adherence: 89%; retention: 95%. | Patient satisfaction: 92% |
|
|
| Ji et al., 2020 [55] | Healthy | Evaluation of food images and nutrition analysis. Application of the Dietitians of Canada Handy Guide to Servings Sizes | D: Caution should be taken when interpreting results when considering predictions for nutrient content (e.g., carbohydrates, energy, protein, %fat, saturated fatty acids and iron). Significant difference between participant and dietitian data from the app with 12/22 nutrients. Use of data from the app for nutrition assessment would require dietitian review. More effective and stronger levels of validity vs. 3-day food diary when analysing food intake on groups or population levels compared to individuals. PA: High attrition rates (26.5%) due to time or interest in using app. | System usability scale preference for Keenoa app: 34.2%; preference for 3-day food diary: 9.6% |
|
|
| Jin et al., 2024 [56] | End stage renal disease haemodialysis | NS Note: Nephrologists were consulted to assess GPT’s accuracy | B: Significant reduction in patient’s serum potassium levels when individuals followed the system’s recommendations vs. conventional dietary guidance (4.57 +/− 0.76 mmol/L vs. 4.84 +/− 0.94 mmol/L). Not statistically different for pH levels. D: Dietary education provided by GPT tool significantly reduced the proportion of haemodialysis patients with hyperkalaemia from 39.8% to 25%. | Accuracy of outputs compared to the Mayo Clinic Renal Diet Handbook: Overall accuracy: 65% Accuracy for higher potassium foods: 85% Accuracy for lower potassium foods: 48% |
|
|
| Karakan et al., 2022 [57] | IBS (M-subtype) | Designing and administrating diet based on AI recommended micronutrient profiles. Monitoring diet adherence | B: Increase in Faecal bacterium genus, Bacteroides, putatively probiotic genus, and Propionibacterium populations in gut microbiome samples in the personalised nutrition group. C: Change in categorisation of IBS-SSS from severe to moderate was observed only in the intervention group. Change over time of IBS-SSS from pre- to post-intervention was significantly greater in the personalised nutrition group. D: AI-based dietary modification targeting microbiome modulation resulted in significant improvements in symptoms of patients with IBS-M. | Accuracy: 91% |
| - |
| Karmakar et al., 2023 [58] | Healthy | NS | D: Multimodal MS-TCN performs well in recognising common eating and drinking behaviours. | Accuracy: 83.03% F1 scores were noted as reasonable for each individual class tested |
| - |
| Khan et al., 2022 [59] | Healthy | NS | D: System can distinguish between drinking instance, solid food, and other activities. Observed weaker performance in identification of instance of fluid consumption (e.g., drinking water). | Averaged class-wise precision: 84.65%; average recall: 80.81%; average F-measure: 82.61%; average accuracy: 92.65% |
|
|
| Kiriakedis et al., 2024 [60] | Nephrolithiasis | Evaluation of ChatGPT’s responses for accuracy, completeness and appropriateness | B: System had poorer performance when detecting and responding to urine test abnormalities, especially in calcium and citrate levels. Unable to address 30% of abnormalities impacted quality of recommendations offered. Good performance in identification of biomarkers within normal range parameters. D: System could generate personalised recommendations. Performed well in terms of accuracy, completeness, and appropriates for advising hydration. | Likert Scales: Accuracy (5.2/6), completeness (2.4/3), appropriateness (2.6/3) |
|
|
| Kirk D. et al., 2023 [61] | Healthy | Gathered the most asked nutrition questions with corresponding dietitian answers. Graded ChatGPT responses for scientific accuracy, applicability, and comprehensibility | D: Capability of answering commonly asked nutrition questions ChatGPT scored higher in comparison to dietitians in areas of scientific correctness (5/8), accountability (4/8), and comprehensibility (5/8). | Overall grading: ChatGPT responses were higher for 5/8 questions |
|
|
| Kobayashi et al., 2024 [62] | Dyslipidaemia, diabetes | NS | D: Diabetes case: Recommended and non-recommended recipes are highly appropriate for the purpose of health improvement. Dyslipidaemia case: There was no significant difference in the top and bottom 5 recommended recipes. This indicates that the inference system may not have worked as well for this medical condition. Recommendations made by the system were lacking in variety and intrigue. | Precision: >0.99 |
|
|
| Krishnakumar et al., 2021 [63] | T2DM | NS | A: Improvements in weight management of diabetes: weight reduction of 1.32 kg and BMI reduction by 0.47 kg/m2. B: Improvements in management of diabetes. Incremental reduction in blood glucose biochemistry markers (HbA1c, FBG, and PPBG); 63.7% (65/102) patients had improved HbA1c levels after intervention. PA: Greater levels of engagement and retention rate. Over 16 weeks: average duration of time spent with the following: Personal health coach was 106 min. AI powered chatbot: 88 min. | - |
| |
| Kwon et al., 2024 [64] | NAFLD | Consultations with participants on goal setting and evaluation. Provided monthly feedback from dietary intake recorded in app | A: Significant improvements in physiological outcomes (weight loss and BMI) for 6 months B: Significant improvements in liver panel tests (AST, ALT, and GGT) for 6 months. C: Significant improvements in psychological outcomes (self-management, fatigue, depression, and QoL) for 6 months. D: Greater engagement observed higher levels of self-management and knowledge. PA: Mean compliance with respect to using app: 3 months: 82.6%; 6 months: 79.8%. | - |
|
|
| Lázaro et al., 2010 [65] | Aging population (preventing malnutrition) | NS | - | System was able to differentiate the level of complexity of the randomly selected recipes at a higher percentage: 78% |
|
|
| Lee et al., 2024 [66] | Other | NS | D: Utilisation of the platform enhances accessibility and engagement, allowing individuals to obtain nutrition guidance in a seamless manner. Information gathered by the bot was able to synchronise data from external databases and user-reported data to allow for the provision of continuous care. | - |
| - |
| Lee et al., 2017 [67] | Healthy | NS | - | F1 score: 0.96 Overall accuracy: approximately 90% Average accuracy: 99% (identification of plate sections MLP) Average accuracy: 90.40% (estimation of food portion and weight) |
| - |
| Lee et al., 2020 [68] | Healthy | NS | D: Ultrasound modality resulted in lower recognition rate for certain foods that had similarity of softness and crunchiness. | Recognition performance for artificially added noise: 90.13% Recognition performance for noisy environments: 89.67% | - |
|
| Lee et al., 2023 [69] | T2DM | Sending personalised nutrition intervention messages | A: Utilisation of the platform resulted in weight loss. B: Better management of glycaemia levels with significant reductions in HbA1c levels (baseline to 24 and 48 weeks). | - |
|
|
| Li et al., 2024 [70] | Other | Dietetic students assessed nutrition-related apps for their suitability to be applied into the NCP | D: Noom was the highest scoring app. MyFitnessPal and Fastic had the highest degree of accuracy. Generally, energy content was overestimated for Western meals and underestimated for Asian meals. | Accuracy: MyFitnessPal: 97% Fastic: 92% |
|
|
| Liao et al., 2024 [71] | Other | Assessed quality of ChatGPT’s dietary advice | D: Responses by ChatGPT scored highly in readability but lacked understandability, practicality, and completeness. Dietitians commented that the responses often lacked thoroughness and rigour, which can potentially lead to misunderstanding. | Accuracy: 84.38% Objective Nutrition Literacy Test (NL): 7.50% to 37.56% (suboptimal performance) |
|
|
| Lin et al., 2020 [72] | Healthy | NS | D: System can recognise eating motions and provide estimations in chewing and swallowing incidents. | Accuracy: 95% Error: 10% |
|
|
| Liu et al., 2024 [73] | Healthy | NS | - | F1 scores: Four food-related intake activities: 86.4% Classification of seven types of foods: 64.2% |
|
|
| Lozano et al., 2023 [74] | Weight management – overweight | NS | D: Errors in automated energy estimates were relatively high prior to adjustments for beverage-based food items. After adjusting for beverage, the remainder of error was lower (16%). Most energy estimates were driven by the grain products. | Matches for food items: Exact: 46% (118/255); 41% (105/255) were a fair match, and 13% (13/255) were intrusions. |
| |
| Maher et al., 2020 [75] | Weight management—overweight, obese | NS | B: Average loss of weight of 1.3 kg and 2.1 cm from waist circumference. C: No significant changes in blood pressure. D: Adherence to the MED diet increased during the intervention. PA: Adherence to the MED diet increased during the intervention; 70% engagement with Paola. | - |
|
|
| Marashi-Hosseini et al., 2023 [76] | Metabolic syndrome | Commented on results from questionnaire used to establish recommended macronutrient requirements for health condition. Development of gold standard to compare algorithm’s output | D: No significant differences between the diet set by nutrition professionals and the diet recommended by the fuzzy logic model. Proposed system enhanced the reliability, speed, and accuracy of the decision-making of dietitians in setting an optimal diet for patients with multiple chronic conditions | Accuracy: approx. 97% (suitable performance) |
| - |
| Martino et al., 2021 [77] | Aging Population (prediction of malnutrition risk) | NS | D: High levels of accuracy and recall in detection of individual nutritional status through combining data from nutritional intake, dietary habits, and body composition data (models tested: LR with LASSO regularisation, RF, AB, RUS Boost). | Best-performing ML models for malnutrition risk: Accuracy: 94% Recall values: 92% |
|
|
| Mertes et al., 2020 [78] | Healthy—able to self-feed | NS | D: Current model can produce a higher recall and the lowest error. Algorithm is more prone to under-reporting actual food intake. | Precision and Recall: 0.78 |
|
|
| Moyen et al., 2022 [79] | T1DM, T2DM | Manual adjustment of incorrect food intake entries into the app by participants | D: Acceptable agreement between both tools without bias. Moderate-to-strong relative validity compared to ASA24 in terms of macro- and micronutrient intakes for healthy and diabetic adult populations (fibre and iron were reported to be higher and sodium was lower when using Keenoa rather than ASA24). No significant differences in estimated energy intake between tool with similar rates of underreporting and no overreporting observed. | Mean usability score (perceived ease of use): Keenoa: 77% ASA-24: 53% Preference for Keenoa over ASA-24: 74.8% |
|
|
| Naja et al., 2024. [80] | Metabolic syndrome | Evaluation of ChatGPT’s responses in accordance with Nutrition Care Manual with focus on accuracy, clarity, coherence, and practicality | D: Outputs for dietary management of T2DM and metabolic Syndrome were often partially/incomplete or did not align with NCP recommendations. Impacts patient care and accuracy of output. Dietary advice did account for energy balance/intake modification. Assessment of nutritional status was incomplete (did not account for current intake of macronutrients and anthropometric markers). PES statements and appropriate diagnostic terminology were not utilised. | - |
|
|
| Nakaoka et al., 2021 [81] | Other | NS | - | - |
| - |
| Nakata et al., 2022 [82] | Weight management—overweight, obese | NS | A: Significant body weight decreases between intervention and control groups. B: No changes in blood biochemistry measures. D: No differences between two groups for energy. Group that used dietary logs on the app had a decrease in energy intake between the initial and final 2 weeks (−152.3 ± 304.0 kcal). | - |
| - |
| Niszczota et al., 2023 [83] | Food allergies | Generation of prompts and assessment of responses by ChatGPT | D: Potential for outputs with errors that involve inaccuracies in portions or calories of food, meals, or diets. In some cases, there can be severe health consequences: for instance, including almond milk in nut-free diet. Most menus correctly excluded allergens of interest (52 of 56 prompts). Can be used for meal formulation using basic recommendations. Displays cautionary safety labels to raise awareness in users. Miscalculation of energy values for food, meal, or complete menus. Portion sizes and quantities recommended were very specific and impractical. Repetition of products and meals were common, which makes outputs monotonous and challenging. | - | - |
|
| Ocay et al., 2017 [84] | Other | NS | D: Nutritional awareness of respondents was low. After interaction with app, there were high user satisfaction ratings and acceptance (user interface, ease of use, nutritional content estimation, basal metabolic rate calculation, reliability of food recognition, food intake monitoring, and improved diet awareness). Potential to improve user awareness through tracking and dietary monitoring. | Likert scale: User acceptability: 4.43/5 Reliable photo recognition: 5/5 |
| - |
| Oh et al., 2022 [85] | T2DM hypertension | NS | A: No significant differences in body weight, BMI, and body composition between two groups. Any changes in body fat mass were attributed to medicine intake. B: No significant differences HbA1c between the two groups Any changes in HbA1c levels were attributed to an increase in medicine intake. C: No significant difference for blood pressure between both groups. D: Significant low input rate for food intake data with attrition. The notable difficult functions for input of food intake were recording food intake and locating food items from the provided list. Medication input systems experience greater input adherence and were more appealing to users. | Likert Scale: Helpful: 3.4/5 Easy: 2.9/5 Well-functioning: 3.1/5 |
|
|
| Papapanagiotou et al., 2017 [86] | Healthy | NS | D: Utilisation of a combination of signals from all sensors yield improved results compared to results from individual signals (e.g., audio only). | Accuracy: 0.938 Precision: 0.794 Recall: 0.807 F1 score: 0.761 |
|
|
| Papapanagiotou et al., 2021 [87] | Healthy | NS | - | Mean absolute error: <1 (3 out of 4 of the food types) | - |
|
| Papastratis et al., 2024 [88] | Other | NS | D: Meal plans generated were appropriate in terms of energy and nutritional requirements. High levels of accuracy and validated on 3000 virtual user profiles and 1000 real person profiles with 91,000 meal plans generated from the Protein NAP database (large open-source collection of international meals). | Macronutrient accuracies: Average: 87%; fat: 84.04%; SFA: 89.55%; protein: 86.78%; carbohydrates: 83.18% |
|
|
| Papathanail et al., 2023 [89] | Healthy | Carried out 24 h dietary recalls on participants | D: New system has comparable energy and macronutrient estimations performance in comparison to dietitian’s 24 h recall. The re-iteration of the system only requires one image instead of two images. The newer method does not exhibit a statistically significant difference in mean absolute percentage error compared to the two-image system. PA: Discrepancies arose for user compliance with recording meals, omissions, and neglecting the use of reference cards. | Macronutrient estimation errors (system vs. 24 h recall): CHO: 31.27% Protein: 39.17% Fat: 43.24% Misestimation of the new method: Energy: 2.16%; CHO: 0.34%; protein: 3.46%; fat: 0.02% User satisfaction: Tracking food intake: 52.4%; neural: 45.2% recommend to others: 71.4% |
|
|
| Papathanail et al., 2022 [90] | Other | Group identified 31 categories of food items | D: Validation of a fully automatic food and drink recognition system that requires a single image. System able to estimate serving sizes and calculate user’s MDA score. | Mean absolute error of MDA score (system vs. dietitian): 3.5% (non-significant) Mean average precision: 61.8% Positive response from users: 83% |
|
|
| Ponzo et al., 2024 [91] | Dyslipidaemia, hypertension, T2DM, obesity, NAFLD, CKD, sarcopenia | Questions and prompts generated for input were formulated by medical doctors and registered dietitians | D: Reasonable level of accuracy with general dietary advice for non-communicable diseases. Able to provide practical examples of foods to include or exclude from the diet. For some cases, incomplete recommendations (few guidelines missing) were provided for T2DM, obesity, dyslipidaemia, NAFLD, and CKD. Unable to provide suitable guidance if multiple conditions coexisted. | Appropriateness: 55.5–73.3% |
|
|
| Qarajeh et al., 2023 [92] | Other | NS | D: Emerging AI models manifest a diverse range of accuracy in discerning potassium and phosphorus content in foods suitable for CKD patient. ChatGPT 4 performed better in classification of high- and low-potassium and -phosphorous foods compared to ChatGPT3.5 | Accuracy of model compared to Mayo Clinic Renal Diet Handbook: ChatGPT 4: 81% for potassium foods; 77% accuracy for phosphorous foods ChatGPT 3:66% for potassium foods; 85% accuracy for phosphorous foods Bing Chat: 81% for potassium foods; 89% for phosphorous foods Bard AI: 79% for potassium foods; 100% for phosphorous foods |
|
|
| Rafferty et al., 2021 [93] | IBS | Co-designed software with software engineers | C: Improvements in quality-of-life outcomes and bowel-related and habit-related symptoms (2-fold improvement), with 43% of participants in the intervention group no longer meeting the criteria for IBS (Rome IV criteria). QoL improvement scores were correlated with improvements in IBS symptoms scores. D: Both app and control groups had improvements in knowledge and adherence. | Reduction in total IBS symptom severity score: 24% greater for the APP group vs. CON group |
|
|
| Salloum et al., 2018 [94] | Healthy | Evaluation of participant data and findings | A: Body fat percentage and weight recommendations produced by the system: 88.7% agreement with nutrition experts. C: Automation Health State Assessor system: rated > 3.5/4 by 31.2% of nutrition expert ratings. D: Meal plan generator system: experts agree with meal plan suggestions provided, including nutrients of focus and assigning food items. Improvements required for food variability. | - |
| - |
| Samaan et al., 2023. [95] | IBD | Evaluation of ChatGPT responses | D: Comprehensive response to 62.5% of question relating to nutrition and diet needs for surgery, 92.3% questions relating to tube feeding and parenteral nutrition, 64.7% general diet questions, 50% of questions relating to diet for reducing symptoms/inflammation, and 81.8% of questions relating to micronutrients/supplementation needs. | Correct responses: 83% Comprehensive responses: 69% Incorrect/contradicts guidelines responses: 17% High reproducibility in accuracy: 92% |
|
|
| Sano et al., 2015 [96] | Healthy | Evaluation of participant data and findings | D: ~ 40% of the participants ate more balanced meals and increased vegetable intake; ~20% participants report changes to poor dietary habits, i.e., avoiding snacking/eating occasions prior to sleep based on the system’s recommendations. >50% of participants became more aware of their diet, sleep, activity, and stress through tracking behaviours and engaging with surveys; 73% found diet advice was helpful | - |
| - |
| Schiboni et al., 2018 [97] | Healthy | NS | D: Specific orientation and position of the camera limit privacy-infringing content captured in videos. Eliminates the need to integrate error-prone anonymisation to limit privacy concerns. Model performance suggests this is a feasible method. | Average recall > 90% |
|
|
| Sefa-Yeboah et al., 2021 [98] | Weight management—obese | Involved in testing of the program | D: System was also able to generate a personalised nutrition report which includes daily nutritional needs (e.g., macronutrients) and energy intake and offers appropriate recommended meals with high levels of accuracy. Different energy targets were used (1000, 1600, 2000, 2400, 2800, and 3200 kcal) in testing. | - |
|
|
| Shamanna et al., 2020 [99] | T2DM | NS | A and B: Continuous AGP monitoring. Adherence for 3 months resulted in a 1.9% decrease in HbA1c, 6.1% weight decrease, 56.9% reduction in HOMA-IR, significant decline in glucose time below range, and reduction in or elimination of T2DM medication use (most patients). C: All 12 patients on insulin discontinued used within 3 months. Most patients on other T2DM medications such as metformin and DPP-4 inhibitors also ceased medications. Patients on liraglutide stopped the medication. D: Helped patients avoid foods that cause blood glucose spikes and replaced them with foods that do not produce glucose spikes. Daily precision medicine guidance based on continuous glucose monitoring; food intake data ML algorithms can provide benefit to T2DM patients. | - |
| - |
| Shao et al., 2021 [100] | Healthy | Provided the relative energy per food item in each image used. Conducted structured interview with participants for 24 h dietary recall | D: Outperforms participant estimates and can accurately estimate portion size from a single image. Ability to account for all components of a meal (e.g., oils, dressings, sauces) which would generally be overlooked. Higher levels of accuracy with respect to estimation and applied data augmentation (rotating, cropping, and flipping). | Higher levels of accuracy for food image estimation: MAE: 56.33 calories MAPE: 11.47% (significantly outperforms human estimates by 27.56%) |
|
|
| Shoneye et al., 2019 [101] | Healthy | NS | D: Participants in the CHAT intervention reported being shocked and surprised about the feedback on their dietary intake but were receptive to feedback Participants who agreed that dietary feedback made them think about their eating behaviours were more likely to improve their diet during the intervention period (increased vegetable intake by half a serving and decreased intake of energy-dense nutrient-poor foods by half a serving). | - |
|
|
| Silva et al., 2022 [102] | Metabolic syndrome | NS | D: This food recommendation system can analyse an individual’s dietary data and provide personalised dietary recommendations. Items eligible for recommendation included whole cereals, tubers and roots, beans and other legumes, oilseeds, fruits, vegetables, white meats and fish, and low-fat dairy products and milk. Utilisation of user-based collaborative filtering (UBCF) vs. item-based collaborative filtering (IBCF). PA: Human and professional support are still required to ensure patient adherence (such as following through with interventions) and to maximise impacts of interventions. | Precision: 88–91% (similar between UBCF and IBCF) Error metrics: Root means square error (RMSE): 1.49 (UBCF) vs. 1.67 (IBCF) Mean square error (MSE): 2.21 (UBCF) vs. 2.78 (IBCF) |
| - |
| Sowah et al., 2020 [103] | T1DM, T2DM | Clinical requirements and design analysis of the system was based on discussions with collaborators from the Department of Nutrition and Food Science—the diet type of patients was determined to be an essential approach suitable for the diabetes management system | D: Able to predict and label new food images with high levels of accuracy. The meal recommender model and chatbot were able to provide appropriate recommendations that met the user’s caloric needs and could address user questions via a user-friendly interface. | Accuracy: >95% (food recognition and classification for specific caloric intakes) |
| - |
| Sun et al., 2023 [104] | T2DM | Evaluation of ChatGPT response to professional clinical dietitian responses | D: In terms of the ketogenic diet, recommendations mostly followed best practice guidelines, with an overlap rate of 80.7% between dietitian’s recommendations and ChatGPT. Inconsistencies with root vegetables and dry bean food items. | Food recognition model: F1 score: Dino V2: 0.825 Inverse cooking model: 0.477 162/168 favourable reviews of responses from dietitians ChatGPT accuracy: 60.5% Error rates: 64.6% GPT 4.0 accuracy: 74.5% Error rates: 70.6% |
|
|
| Sun et al., 2015 [105] | Healthy | NS | D: Mostly foods with an irregular shape, small size, or poor-quality images resulted in large errors in food identification. | 85/100 food items identified: Presence of Error: 30% Mean absolute relative error (MARE): 16.4% Root mean square error (RMSE): 20.5% |
|
|
| Thames et al., 2021 [106] | Healthy | Provided portion estimations which were then compared | D: Prediction of caloric and macronutrient values of complex, real-world dishes at an accuracy that outperforms professional nutritionists. | Professional nutritionist absolute error: 41% Non-nutritionist’s absolute error: 53% Calorie per gram prediction with model using data from Nutrition5K database; mean Absolute Error: 16.5% |
|
|
| Tunali et al., 2024. [107] | IBS | Face-to-face consultations and delivery of tailored menu plans | C: Microbiome-assisted personalised diet (PD) resulted in significant improvements in IBS-SSS scores (across all subtypes) and IBS-QOL in addressing symptoms (for IBS-C, IBS-D, and IBS-M) (FODMAP diet resulted in improvements in IBS-C and IBS-D). PD led to significant microbiome diversity shifts compared with FODMAP diet. PA: Completion rate of 81%. | - |
| - |
| Turnin et al., 2021 [108] | T2DM | NS | A: Metabolic benefit was correlated with frequency of use of the device, which was reflected in significant weight loss (female participants) and reduction in waist circumference. B: Home telemonitoring and tele education did not significantly improve glycaemic control in T2DM subjects. Slight significant decrease in HbA1c levels (female participants) | - |
| - |
| Valero-Ramon et al., 2019 [109] | Aging population (weight changes and malnutrition screening) | NS | A and D: Models in this study present cases of patients who are malnourished. Indicated malnutrition is related to weight and other factors (e.g., quality and quantity of nutrients and level of activity). These can be used to predict and monitor the risk of malnutrition. | - |
|
|
| Vasiloglou et al., 2020 [110] | Other | Manual calculation of MDA scores using the scoring system outlined in the paper Creation and correction of the Oviva database | D: Proposed system and four experienced DTs indicated similar results/predictions in MDA assessment for free-living conditions. Better performance in multi-label food recognition and serving size estimation compared to baseline method (ResNet101 and ImageNet) by 11% for food recognition and 2% for serving size recognition. | RestNet 101: mAP: 0.47; MAPE: 63%; GCN-based: mAP: 0.58 * MAPE 61% (better performance) |
|
|
| Walker et al., 2014 [111] | Healthy | NS | - | Correctly identified ingestion sounds: >94% False positive: 9% |
|
|
| Wang et al., 2024 [112] | Healthy | NS | - | F1 scores: Eating gestures: 0.896 Drinking gestures: 0.868 |
|
|
| Wang et al., 2023 [113] | Healthy—endurance athletes | NS | D: Most impactful features included carbohydrate intake rate, age, light sleep, activity levels, which are potential areas for targeted interventions, and personalised strategies to optimise CPS intake. BP neural network model displayed slightly more superior performance than the GBRT model. | - |
|
|
| Zhang et al., 2023. [114] | Prediabetes, T2DM | NS | Calorie prediction improvement compared to the following: best CGM model: 10.8%; best image model: 19.5% |
| - |
| Author | Technology | Stages of NCP | Type of AI Technology | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ax | Dx | I | M&E | Image/Audio Recognition | Chatbot | Rec. Sys. | AI Subdomain and Specific Techniques | ||
| Amft et al., 2007 [20] | Wearable sensor: microphone, EMG | ✓ | - | - | - | ✓ | - | - | GA |
| Chen et al., 2023 [30] | First-bite/first-chew wearable system (glasses paired with wristband, inertial measurement units, machine learning microcontroller unit) | ✓ | - | - | - | ✓ | - | - | ML |
| Chotwanvirat et al., 2024 [35] | Institute of Nutrition, Mahidol University iFood app | ✓ | - | - | - | ✓ | - | - | DL |
| Li et al., 2024 [70] | Various free-to-use and paid/premium versions of nutrition-related apps available on the Australian Apple App Store or Google Play Store | ✓ | - | - | - | ✓ | - | - | ML, DL |
| Lozano et al., 2023 [74] | OpenFit mobile app | ✓ | - | - | - | ✓ | - | - | DL—neural network |
| Papapanagiotou et al., 2017 [86] | Chewing detection system: in-ear microphone and photoplethysmography sensor | ✓ | - | - | - | ✓ | - | - | ML—support vector machine |
| Papapanagiotou et al., 2021 [87] | Samsung Galaxy ear buds for bite weight estimation | ✓ | - | - | - | ✓ | - | - | ML, DL—linear regression, support vector regression, and neural network-based estimators |
| Sun et al., 2015 [105] | eButton: food image recognition using sensors (cameras, light sensor, a 3-in-1 inertial measurement unit, accelerometer, gyroscope, magnetometer), audio processor, proximity sensor, barometer, GPS receiver | ✓ | - | - | - | ✓ | - | - | ML—support vector machines (SVMs), hidden Markov model |
| Chen et al., 2012. [32] | Nutritional diagnosis expert system (web-based) | - | ✓ | - | - | - | - | ✓ | Not specified |
| Agne et al., 2024 [19] | ChatGPT Food4Me algorithm | - | - | ✓ | - | - | ✓ | - | NLP, GPT |
| Niszczota et al., 2023 [83] | ChatGPT (Version 3.0) | - | - | ✓ | - | - | ✓ | - | NLP—LLM |
| Ponzo et al., 2024 [91] | ChatGPT (Version 3.5) | - | - | ✓ | - | - | ✓ | - | NLP—LLM |
| Qarajeh et al., 2023 [92] | ChatGPT (Version 3.5), ChatGPT (Version 4), Bard AI (Gemini), Bing Chat | - | - | ✓ | - | - | ✓ | NLP—LLM | |
| Bohn et al., 2024 [26] | LiFANA dietary support app | - | - | ✓ | - | - | - | ✓ | Not specified |
| Chen et al., 2015 [31] | Diet recommendation system for chronic diseases | - | - | ✓ | - | - | - | ✓ | ML—decision tree |
| Hsu et al., 2011 [53] | Web-based food composition system | - | - | ✓ | - | - | - | ✓ | NLP—fuzzy decision model |
| Karakan et al., 2022 [57] | Al algorithm for personalised nutrition strategy accounting for gut microbiome | - | - | ✓ | - | - | - | ✓ | ML |
| Kobayashi et al., 2024 [62] | Food knowledge graph and recipe recommendation system | - | - | ✓ | - | - | - | ✓ | Probabilistic logic programing |
| Marashi-Hosseini et al., 2023 [76] | Clinical decision-making support system: using fuzzy interference system | - | - | ✓ | - | - | - | ✓ | Fuzzy inference system |
| Papastratis et al., 2024 [88] | ChatGPT and deep generative model | - | - | ✓ | - | - | - | ✓ | NLP—LLM, DL |
| Wang et al., 2023 [113] | Personalized recommendation system for “Carbohydrate-Protein” supplements | - | - | ✓ | - | - | - | ✓ | ML, DL—propagation (BP) neural networks, gradient-boosted regression trees (GBRT), enumeration method |
| Bul et al., 2023 [28] | Web-based diabetes nutrition care platform | - | - | ✓ | - | - | ✓ | ✓ | DL, NLP |
| Hieronimus et al., 2024 [49] | ChatGPT (Version 3.5), Bard (Gemini) | - | - | ✓ | - | - | ✓ | ✓ | NLP—LLM |
| Kiriakedis et al., 2024 [60] | ChatGPT (Version 4) by OpenAI | - | - | ✓ | - | - | ✓ | ✓ | NLP—LLM |
| Amft et al., 2009 [21] | Wearable sensor: microphone, EMG, weight scale | - | - | - | ✓ | ✓ | - | - | ML |
| Cohen et al., 2023 [36] | Contactless drinking and fluid intake detection using Lidar camera | - | - | - | ✓ | ✓ | - | - | DL |
| Hossain et al., 2020 [51] | AIM 2.0 wearable sensor system: flexible bend sensor, camera | - | - | - | ✓ | ✓ | - | - | DL—CNN-based image classifier |
| Ocay et al., 2017 [84] | Nutritrack app: food recognition (mobile app) | - | - | - | ✓ | ✓ | - | - | ML |
| Lee et al., 2023 [69] | Integrated digital healthcare platform using AI-based dietary management (mobile app) | - | - | - | ✓ | ✓ | - | ✓ | Not specified |
| Holmes et al., 2019 [50] | WeightMonitor: Chatbot | - | - | - | ✓ | - | ✓ | - | Not specified |
| Lázaro et al., 2010 [65] | PERSONA platform: nutritional management and facilitating tool for interventions | - | - | - | ✓ | - | ✓ | ✓ | Not specified |
| Davis et al., 2020 [37] | Paola: virtual AI health assistant (on Slack communication platform) | - | - | - | ✓ | - | - | ✓ | NLP |
| Lee et al., 2020 [68] | Joint audio–ultrasound food recognition: Doppler sonar at jaw and neck, sensor modules (ultrasonic transmitter) at hyoid bone and jaw | ✓ | ✓ | - | - | ✓ | - | - | DL—DNN |
| Thames et al., 2021 [106] | Nutrition5k: dataset of 5k diverse computer vision algorithm baseline for incorporating depth sensor data to improve nutrition predictions | ✓ | ✓ | - | - | ✓ | - | - | DL—CNN |
| Mertes et al., 2020 [78] | Plate system utilising weight sensors and bite algorithm | - | - | ✓ | ✓ | ✓ | - | - | ML—random forest |
| Vasiloglou et al., 2020 [110] | Medipiatto smartphone app to assess MED diet adherence (mobile app) | - | - | ✓ | ✓ | ✓ | - | DL—CNN | |
| Hsiao et al., 2011 [52] | Diet management system with image recognition, personalised recommendations, and feedback (mobile app) | - | - | ✓ | ✓ | ✓ | - | ✓ | Not specified |
| Kwon et al., 2024 [64] | SMART-liver app: ambient-assisted nutrition advisor | - | - | ✓ | ✓ | ✓ | - | ✓ | Not specified |
| Nakaoka et al., 2021 [81] | Sensor-equipped chopsticks and nudging system (gateway device and digital art canvas) | - | - | ✓ | ✓ | ✓ | - | ✓ | DL |
| Rafferty et al., 2021 [93] | Heali App: AI dietary mobile app enhancing adherence to a low-FODMAP diet | - | - | ✓ | ✓ | ✓ | - | ✓ | Not specified |
| Kirk D. et al., 2023 [61] | ChatGPT by OpenAI (Version 3.0) | - | - | ✓ | ✓ | - | ✓ | - | NLP—LLM |
| Maher et al., 2020 [75] | Paola: AI virtual health coach (via Slack) for Mediterranean-style dietary intervention | - | - | ✓ | ✓ | - | ✓ | - | NLP |
| Samaan et al., 2023 [95] | ChatGPT (Version 4) | - | - | ✓ | ✓ | - | ✓ | - | NLP—LLM |
| Beyeler M. et al., 2023 [25] | HealthBot web-based tool | - | - | ✓ | ✓ | - | ✓ | ✓ | NLP |
| Buchan et al., 2024 [27] | INA: AI-powered virtual assistant platform | - | - | ✓ | ✓ | - | ✓ | ✓ | ML |
| Liao et al., 2024 [71] | ChatGPT (Version 3.5) | - | - | ✓ | ✓ | - | ✓ | ✓ | NPL—LLM |
| Abeltino et al., 2022 [18] | Personal metabolic avatar (PMA) | - | - | ✓ | ✓ | - | - | ✓ | DL |
| Burgermaster et al., 2020 [29] | Patient-generated health data-driven decision-making platform | - | - | ✓ | ✓ | - | - | ✓ | ML |
| Fernandes et al., 2023 [40] | PRIMO: Prime Implicant Maintenance of Outcome tool for weight management experts (mobile app) | - | - | ✓ | ✓ | - | - | ✓ | ML—random forest |
| Garcia et al., 2019 [43] | Pan-Cook-Eat: web-based meal planned recommendation app | - | - | ✓ | ✓ | - | - | ✓ | ML—forward chaining algorithm |
| Gonzalez-Flo et al., 2023 [44] | Evolutionary algorithm to assist with management of T2DM | - | - | ✓ | ✓ | - | - | ✓ | Evolutionary algorithm |
| Hauptmann et al., 2022 [46] | Nutrilize: mobile app | - | - | ✓ | ✓ | - | - | ✓ | ML—content-based algorithm |
| Sano et al., 2015 [96] | HealthAware advice platform (desktop app) | - | - | ✓ | ✓ | - | - | ✓ | ML |
| Tunali et al., 2024 [107] | Enbiosis personalized nutrition model: AI-based personalised low-FODMAP diet | - | - | ✓ | ✓ | - | - | ✓ | ML |
| Turnin et al., 2021 [108] | Remote monitoring programme including lifestyle education software: Nutri-Kiosk, Acti-Kiosk, and Nutri-Educ | - | - | ✓ | ✓ | - | - | ✓ | Not specified |
| Jin et al., 2024 [56] | Generative pretrained transformer-based dietary recommendation system for haemodialysis (web platform) | - | - | ✓ | ✓ | ✓ | ✓ | ✓ | GPT |
| Amft et al., 2008 [22] | Wearable sensor: microphone, EMG, body inertial sensors | ✓ | - | - | ✓ | ✓ | - | - | ML |
| Ben Neriah et al., 2019 [23] | LoseIt! mobile app | ✓ | - | - | ✓ | ✓ | - | - | Not specified |
| Chin et al., 2019 [34] | Machine learning models and data mapping (comparable to ASA24 reports) | ✓ | - | - | ✓ | ✓ | - | - | ML |
| Fontana et al., 2012 [42] | Wearable sensor system: jaw motion sensor, self-report push button | ✓ | - | - | ✓ | ✓ | - | - | ML—linear and RBF support vector machine (SVM) classifiers, pattern recognition |
| Heremans et al., 2020 [47] | Food intake detection via heart-rate variability on ECGs using artificial neural networks | ✓ | - | - | ✓ | ✓ | - | - | ML- ANN |
| Hezarjaribi et al., 2018 [48] | Speech to nutrient information (S2N1): nutrition monitoring system (mobile app) | ✓ | - | - | ✓ | ✓ | - | - | NLP and text mining |
| Karmakar et al., 2023 [58] | Multimodal MS-TCN model: recognition of common dietary behaviours: cameras, inertial measurement units | ✓ | - | - | ✓ | ✓ | - | - | DL—CNN |
| Khan et al., 2022 [59] | Wearable neck band: microphone, radio module | ✓ | - | - | ✓ | ✓ | - | - | ML—random forest classifier |
| Lee et al., 2017 [67] | FIT-EVE&ADAM wearable sensor system: EMG embedded in armband, food image data, thermal cameras | ✓ | - | - | ✓ | ✓ | - | - | NLP—LLM |
| Liu et al., 2024 [73] | iEat: wearable sensor system using wrist worn electrodes | ✓ | - | - | ✓ | ✓ | - | - | DL—neural network |
| Papathanail et al., 2022 [90] | AI-powered system that calculates Mediterranean diet adherence scores (mobile app) | ✓ | - | - | ✓ | ✓ | - | - | DL—CNN |
| Schiboni et al., 2018 [97] | Wearable camera: Raspberry Pi Zero, a camera attached to cap (privacy preserving feature) | ✓ | - | - | ✓ | ✓ | - | - | DL—DNN |
| Shao et al., 2021 [100] | Generative adversarial network, layer normalisation (LN), and group normalisation for food image analysis | ✓ | - | - | ✓ | ✓ | - | - | DL—generative adversarial network (GAN) |
| Krishnakumar et al., 2021 [63] | Wellthy CARE mobile app: e-coaching, decision support system | ✓ | - | - | ✓ | - | ✓ | ✓ | Not specified |
| Chew et al., 2024 [33] | eTRIP app to increase awareness on eating habits | ✓ | - | - | ✓ | ✓ | ✓ | ✓ | Not specified |
| Sowah et al., 2020 [103] | Diabetes management system | ✓ | - | - | ✓ | ✓ | ✓ | ✓ | ML, DL—tensor flow neural network; k-nearest neighbour (KNN) algorithm |
| Farooq et al., 2016 [39] | Wearable sensor system: glasses, piezoelectric strain sensor, accelerometer | ✓ | ✓ | - | ✓ | ✓ | - | - | ML—support vector machine (SVM) classifiers |
| Fontana et al., 2014 [41] | Automatic ingestion monitor: jaw motion sensor, hand gesture sensor, accelerometer | ✓ | ✓ | - | ✓ | ✓ | - | - | ML—ANN, pattern recognition |
| Ji et al., 2020 [55] | Keenoa mobile app | ✓ | ✓ | - | ✓ | ✓ | - | - | ML |
| Lin et al., 2020 [72] | WiEat: device-free eating monitoring system (mobile/laptop app) | ✓ | ✓ | - | ✓ | ✓ | - | - | ML—support vector machine (SVM) |
| Papathanail et al., 2023 [89] | goFOODTM: automatic system food segmentation, recognition, and nutrient estimation | ✓ | ✓ | - | ✓ | ✓ | - | - | DL—CNN |
| Walker et al., 2014 [111] | ID-HMS: wearable sensory system with an external throat microphone system, TMS (microphones, placed over the throat over the laryngeal prominence) | ✓ | ✓ | - | ✓ | ✓ | - | - | ML |
| Wang et al., 2024 [112] | Eat-Radar: Wearable sensor systems with radars and cameras to capture gestures in relation to food intake | ✓ | ✓ | - | ✓ | ✓ | - | - | ML, DL—3D temporal convoluted network |
| Zhang et al., 2023 [114] | Multi-modality model for processing food image extractions and metabolic readings (e.g., CGM) for calorie prediction | ✓ | ✓ | ✓ | ✓ | - | - | ML, DL | |
| Oh et al., 2022 [85] | LIBIT app: integrative mHealth program | ✓ | ✓ | ✓ | ✓ | - | ✓ | Not specified | |
| Martino et al., 2021 [77] | DoEatWell (m-health app) and decision-making support system: clinical decision-making support tool for malnutrition screening | ✓ | ✓ | - | ✓ | - | - | ✓ | ML |
| Nakata et al., 2022 [82] | CALO mama Plus: weight loss coaching mobile app | ✓ | - | ✓ | ✓ | ✓ | - | ✓ | Not specified |
| Ben-Yacov et al., 2023 [24] | Algorithm that provides personalised postprandial-targeting (PPT) diet | ✓ | - | ✓ | ✓ | - | - | ✓ | ML |
| Hansel et al., 2017 [45] | ANODE: e-coaching program is a web-based nutritional support tool | ✓ | - | ✓ | ✓ | - | - | ✓ | Not specified |
| Sefa-Yeboah et al., 2021 [98] | Mobile- and web-based genetic-algorithm-based platform for obesity management | - | ✓ | ✓ | ✓ | ✓ | - | ✓ | GA—genetic algorithm |
| Silva et al., 2022 [102] | Personalised Dietary Recommender System using user-based and item-based collaborative filtering | - | ✓ | ✓ | ✓ | ✓ | - | ✓ | ML |
| Moyen et al., 2022 [79] | Keenoa (mobile app linked to web platform) compared to ASA24 | ✓ | ✓ | ✓ | ✓ | ✓ | - | - | ML |
| Lee et al., 2024 [66] | Nutritional intake model management system | ✓ | ✓ | ✓ | ✓ | ✓ | - | ✓ | DL—CNN |
| Shoneye et al., 2019 [101] | Technology-assisted dietary assessment or TADA mobile food record app (mobile app) | ✓ | ✓ | ✓ | ✓ | ✓ | - | ✓ | Not specified |
| De Marchi et al., 2022 [38] | e-health Chatbot (webapp app) | ✓ | ✓ | ✓ | ✓ | - | ✓ | - | Not specified |
| Naja et al., 2024. [80] | ChatGPT (Version 3) | ✓ | ✓ | ✓ | ✓ | - | ✓ | ✓ | NLP |
| Jactel et al., 2023 [54] | Machine learning-designed personalised elimination diet | ✓ | ✓ | ✓ | ✓ | - | ✓ | ML | |
| Salloum et al., 2018 [94] | Personal Intelligent Nutrition (PIN): automates patient health assessment and meal plans | ✓ | ✓ | ✓ | ✓ | - | - | ✓ | Fuzzy logic |
| Shamanna et al., 2020 [99] | Twin Precision Nutrition (TPN) program underpinned by Twin Precision Technology | ✓ | ✓ | ✓ | ✓ | - | - | ✓ | ML |
| Valero-Ramon et al., 2019 [109] | Process mining algorithm to support malnutrition assessment | ✓ | ✓ | ✓ | ✓ | - | - | ✓ | ML—process mining |
| Sun et al., 2023 [104] | Artificial intelligence (AI)-based nutritionist program: ChatGPT and GPT 4.0 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NLP—LLM; DL |
| Ethics: Privacy (n = 11) | Safety in Nutrition Practice (n = 6) |
|---|---|
Privacy Preservation: Captured and Accessed
| Warnings and Disclaimers
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, K.; Mekhail, S.; Chan, V.; Li, X.; Yin, A.; Choi, H.Y.; Allman-Farinelli, M.; Chen, J. The Use of Artificial Intelligence (AI) to Support Dietetic Practice Across Primary Care: A Scoping Review of the Literature. Nutrients 2025, 17, 3515. https://doi.org/10.3390/nu17223515
Ngo K, Mekhail S, Chan V, Li X, Yin A, Choi HY, Allman-Farinelli M, Chen J. The Use of Artificial Intelligence (AI) to Support Dietetic Practice Across Primary Care: A Scoping Review of the Literature. Nutrients. 2025; 17(22):3515. https://doi.org/10.3390/nu17223515
Chicago/Turabian StyleNgo, Kaitlyn, Simone Mekhail, Virginia Chan, Xinyi Li, Annabelle Yin, Ha Young Choi, Margaret Allman-Farinelli, and Juliana Chen. 2025. "The Use of Artificial Intelligence (AI) to Support Dietetic Practice Across Primary Care: A Scoping Review of the Literature" Nutrients 17, no. 22: 3515. https://doi.org/10.3390/nu17223515
APA StyleNgo, K., Mekhail, S., Chan, V., Li, X., Yin, A., Choi, H. Y., Allman-Farinelli, M., & Chen, J. (2025). The Use of Artificial Intelligence (AI) to Support Dietetic Practice Across Primary Care: A Scoping Review of the Literature. Nutrients, 17(22), 3515. https://doi.org/10.3390/nu17223515






