Age-Related Anabolic Resistance: Nutritional and Exercise Strategies, and Potential Relevance to Life-Long Exercisers
Abstract
1. Introduction
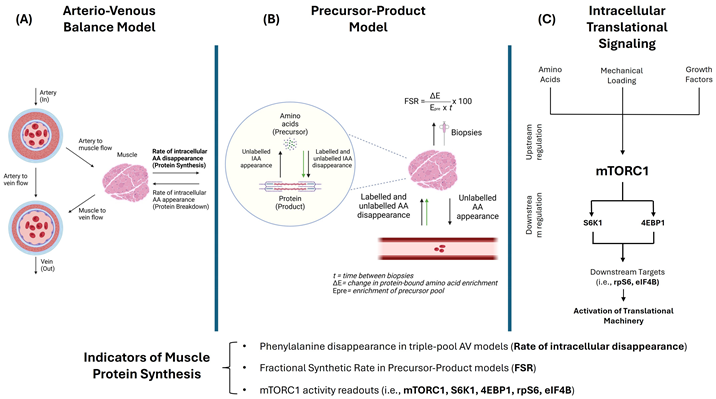 (A) Indirect methods for studying muscle protein metabolism mainly include three-pool arteriovenous (AV) balance models, where enrichment in labeled amino acids (AAs) (e.g., phenylalanine (Phe)) in arterial, venous, and intracellular water pools permits calculations of MPS, MPB, and net protein balance aided by measurements of blood flow to the muscle [30,31]. (B) Direct assessments of muscle protein turnover can be performed using muscle biopsies through the classic precursor-product model, where infused labeled AAs are the precursor—typically intracellular AA enrichment used as surrogate for amino acyl-tRNA—and proteins are the product. This model, considered the gold standard method for assessing MPS [32], consists of estimating muscle fractional synthesis rates (FSR) of mixed or individual types of proteins (e.g., myofibrillar FSR) through calculating the incorporation of a labeled AA tracer (green arrows) into muscle proteins between biopsies in a given amount of time [33]. Since these methods are more tedious and require maintaining an isotopic steady state, the use of deuterated water (D2O) for non-substrate specific 2H labeling has garnered great interest as a reliable method for estimating protein turnover over longer periods of time in free-living conditions [34,35]. (C) Expression and phosphorylation levels of signaling proteins involved in translational processes are frequently reported to provide insights into MPS. Muscle cell growth signaling is at least partially governed by the serine/threonine kinase mechanistic target of rapamycin (mTOR), with the distinct complex 1 (mTORC1) being responsible for integrating environmental cues to switch between states of anabolism and catabolism [36,37,38]. Inability of older muscles to respond to nutrient sensing and mechanical loading through mTOR signaling pathways has been proposed to underlie age-related anabolic resistance [13,39]. Although not considered “first-line” trigger for initiating muscle hypertrophy, growth factors, mainly insulin and insulin-like growth factor (IGF-1), play important roles in mediating hypertrophic signals through mTOR [37]. Activation of the translational machinery involves mTORC1-dependent phosphorylation of key substrates, including ribosomal protein S6 kinase-1 (S6K1) at Thr389 and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) at Thr37/46. Additional downstream targets such as ribosomal protein S6 (rpS6) and eukaryotic initiation factor 4B (eIF4B), along with phosphorylation of mTOR at Ser2448 and Akt at Thr308, serve as molecular readouts of mTORC1 activity and are frequently reported in studies evaluating MPS responses [40,41]. Figure created with BioRender.com.
(A) Indirect methods for studying muscle protein metabolism mainly include three-pool arteriovenous (AV) balance models, where enrichment in labeled amino acids (AAs) (e.g., phenylalanine (Phe)) in arterial, venous, and intracellular water pools permits calculations of MPS, MPB, and net protein balance aided by measurements of blood flow to the muscle [30,31]. (B) Direct assessments of muscle protein turnover can be performed using muscle biopsies through the classic precursor-product model, where infused labeled AAs are the precursor—typically intracellular AA enrichment used as surrogate for amino acyl-tRNA—and proteins are the product. This model, considered the gold standard method for assessing MPS [32], consists of estimating muscle fractional synthesis rates (FSR) of mixed or individual types of proteins (e.g., myofibrillar FSR) through calculating the incorporation of a labeled AA tracer (green arrows) into muscle proteins between biopsies in a given amount of time [33]. Since these methods are more tedious and require maintaining an isotopic steady state, the use of deuterated water (D2O) for non-substrate specific 2H labeling has garnered great interest as a reliable method for estimating protein turnover over longer periods of time in free-living conditions [34,35]. (C) Expression and phosphorylation levels of signaling proteins involved in translational processes are frequently reported to provide insights into MPS. Muscle cell growth signaling is at least partially governed by the serine/threonine kinase mechanistic target of rapamycin (mTOR), with the distinct complex 1 (mTORC1) being responsible for integrating environmental cues to switch between states of anabolism and catabolism [36,37,38]. Inability of older muscles to respond to nutrient sensing and mechanical loading through mTOR signaling pathways has been proposed to underlie age-related anabolic resistance [13,39]. Although not considered “first-line” trigger for initiating muscle hypertrophy, growth factors, mainly insulin and insulin-like growth factor (IGF-1), play important roles in mediating hypertrophic signals through mTOR [37]. Activation of the translational machinery involves mTORC1-dependent phosphorylation of key substrates, including ribosomal protein S6 kinase-1 (S6K1) at Thr389 and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) at Thr37/46. Additional downstream targets such as ribosomal protein S6 (rpS6) and eukaryotic initiation factor 4B (eIF4B), along with phosphorylation of mTOR at Ser2448 and Akt at Thr308, serve as molecular readouts of mTORC1 activity and are frequently reported in studies evaluating MPS responses [40,41]. Figure created with BioRender.com.2. Methodology
3. Evidence on Anabolic Resistance Associated with Aging
3.1. Anabolic Resistance to Nutritional Stimuli
3.2. Anabolic Resistance to Acute and Long-Term Exercise
3.3. Anabolic Resistance to Combinations of Exercise and Nutrition
3.4. Anabolic Resistance in Master Athletes
4. Factors Contributing to Anabolic Resistance and Their Relevance to Master Athletes
4.1. Physical Inactivity
4.2. Compromised Insulin Signaling
4.3. Adiposity
4.4. Low-Grade Inflammation
5. Optimizing Nutrition to Support Muscle Protein Synthesis in Healthy Master Athletes
5.1. Protein
5.2. EAA/Leucine
5.3. HMB
5.4. Omega-3 Fatty Acids
5.5. Optimal Energy Intake
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef]
- Kim, I.Y.; Park, S.; Jang, J.; Wolfe, R.R. Understanding Muscle Protein Dynamics: Technical Considerations for Advancing Sarcopenia Research. Ann. Geriatr. Med. Res. 2020, 24, 157–165. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef]
- Morton, R.W.; Traylor, D.A.; Weijs, P.J.M.; Phillips, S.M. Defining anabolic resistance: Implications for delivery of clinical care nutrition. Curr. Opin. Crit. Care 2018, 24, 124–130. [Google Scholar] [CrossRef]
- Cartee, G.D.; Hepple, R.T.; Bamman, M.M.; Zierath, J.R. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016, 23, 1034–1047. [Google Scholar] [CrossRef]
- Breen, L.; Stokes, K.A.; Churchward-Venne, T.A.; Moore, D.R.; Baker, S.K.; Smith, K.; Atherton, P.J.; Phillips, S.M. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J. Clin. Endocrinol. Metab. 2013, 98, 2604–2612. [Google Scholar] [CrossRef]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef]
- Timmerman, K.L.; Dhanani, S.; Glynn, E.L.; Fry, C.S.; Drummond, M.J.; Jennings, K.; Rasmussen, B.B.; Volpi, E. A moderate acute increase in physical activity enhances nutritive flow and the muscle protein anabolic response to mixed nutrient intake in older adults. Am. J. Clin. Nutr. 2012, 95, 1403–1412. [Google Scholar] [CrossRef]
- Biolo, G.; Pišot, R.; Mazzucco, S.; Di Girolamo, F.G.; Situlin, R.; Lazzer, S.; Grassi, B.; Reggiani, C.; Passaro, A.; Rittweger, J.; et al. Anabolic resistance assessed by oral stable isotope ingestion following bed rest in young and older adult volunteers: Relationships with changes in muscle mass. Clin. Nutr. 2017, 36, 1420–1426. [Google Scholar] [CrossRef]
- Banks, N.F.; Rogers, E.M.; Church, D.D.; Ferrando, A.A.; Jenkins, N.D.M. The contributory role of vascular health in age-related anabolic resistance. J. Cachexia Sarcopenia Muscle 2022, 13, 114–127. [Google Scholar] [CrossRef]
- Beals, J.W.; Skinner, S.K.; McKenna, C.F.; Poozhikunnel, E.G.; Farooqi, S.A.; van Vliet, S.; Martinez, I.G.; Ulanov, A.V.; Li, Z.; Paluska, S.A.; et al. Altered anabolic signalling and reduced stimulation of myofibrillar protein synthesis after feeding and resistance exercise in people with obesity. J. Physiol. 2018, 596, 5119–5133. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 422–424. [Google Scholar] [CrossRef]
- Conn, V.S.; Koopman, R.J.; Ruppar, T.M.; Phillips, L.J.; Mehr, D.R.; Hafdahl, A.R. Insulin Sensitivity Following Exercise Interventions: Systematic Review and Meta-Analysis of Outcomes Among Healthy Adults. J. Prim. Care Community Health 2014, 5, 211–222. [Google Scholar] [CrossRef]
- Jayedi, A.; Soltani, S.; Emadi, A.; Zargar, M.-S.; Najafi, A. Aerobic Exercise and Weight Loss in Adults: A Systematic Review and Dose-Response Meta-Analysis. JAMA Netw. Open 2024, 7, e2452185. [Google Scholar] [CrossRef]
- Zheng, G.; Qiu, P.; Xia, R.; Lin, H.; Ye, B.; Tao, J.; Chen, L. Effect of Aerobic Exercise on Inflammatory Markers in Healthy Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Aging Neurosci. 2019, 11, 98. [Google Scholar] [CrossRef]
- Reaburn, P.; Dascombe, B. Endurance performance in masters athletes. Eur. Rev. Aging Phys. Act. 2008, 5, 31–42. [Google Scholar] [CrossRef]
- Daly, L.S.; Van Hooren, B.; Jakeman, P. Physiological Characteristics of a 92-Year-Old 4-time World Champion Indoor Rower. J. Appl. Physiol. 2023, 135, 1415–1420. [Google Scholar] [CrossRef]
- McKendry, J.; Breen, L.; Shad, B.J.; Greig, C.A. Muscle morphology and performance in master athletes: A systematic review and meta-analyses. Ageing Res. Rev. 2018, 45, 62–82. [Google Scholar] [CrossRef]
- Gries, K.J.; Trappe, S.W. The aging athlete: Paradigm of healthy aging. Int. J. Sports Med. 2022, 43, 661–678. [Google Scholar] [CrossRef]
- Moore, D.R. Protein Requirements for Master Athletes: Just Older Versions of Their Younger Selves. Sports Med. 2021, 51, 13–30. [Google Scholar] [CrossRef]
- McKendry, J.; Shad, B.J.; Smeuninx, B.; Oikawa, S.Y.; Wallis, G.; Greig, C.; Phillips, S.M.; Breen, L. Comparable Rates of Integrated Myofibrillar Protein Synthesis Between Endurance-Trained Master Athletes and Untrained Older Individuals. Front. Physiol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Doering, T.M.; Jenkins, D.G.; Reaburn, P.R.; Borges, N.R.; Hohmann, E.; Phillips, S.M. Lower Integrated Muscle Protein Synthesis in Masters Compared with Younger Athletes. Med. Sci. Sports Exerc. 2016, 48, 1613–1618. [Google Scholar] [CrossRef]
- Aragon, A.A.; Tipton, K.D.; Schoenfeld, B.J. Age-related muscle anabolic resistance: Inevitable or preventable? Nutr. Rev. 2023, 81, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.N.; Smeuninx, B.; Morgan, P.T.; Breen, L. Nutritional strategies to offset disuse-induced skeletal muscle atrophy and anabolic resistance in older adults: From whole-foods to isolated ingredients. Nutrients 2020, 12, 1533. [Google Scholar] [CrossRef]
- Deane, C.S.; Cox, J.; Atherton, P.J. Critical variables regulating age-related anabolic responses to protein nutrition in skeletal muscle. Front. Nutr. 2024, 11, 1419229. [Google Scholar] [CrossRef]
- Lazarus, N.R.; Harridge, S.D. Inherent ageing in humans: The case for studying master athletes. Scand. J. Med. Sci. Sports 2007, 17, 461–463. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Suh, S.-H.; Lee, I.-K.; Wolfe, R.R. Applications of stable, nonradioactive isotope tracers in in vivo human metabolic research. Exp. Mol. Med. 2016, 48, e203. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Brook, M.S.; Smith, K.; Atherton, P.J. Stable isotope tracers and exercise physiology: Past, present and future. J. Physiol. 2017, 595, 2873–2882. [Google Scholar] [CrossRef]
- Biolo, G.; Fleming, R.Y.; Maggi, S.P.; Wolfe, R.R. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 1995, 268, E75–E84. [Google Scholar] [CrossRef]
- Fujita, S.; Rasmussen, B.B.; Bell, J.A.; Cadenas, J.G.; Volpi, E. Basal muscle intracellular amino acid kinetics in women and men. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E77–E83. [Google Scholar] [CrossRef]
- Atherton, P.J.; Phillips, B.E.; Wilkinson, D.J. Chapter Four—Exercise and Regulation of Protein Metabolism. In Progress in Molecular Biology and Translational Science; Bouchard, C., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 135, pp. 75–98. [Google Scholar]
- Chinkes, D.L.; Rosenblatt, J.; Wolfe, R.R. Assessment of the mathematical issues involved in measuring the fractional synthesis rate of protein using the flooding dose technique. Clin. Sci. 1993, 84, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.F.; Reid, J.J.; Price, J.C.; Lin, H.L.; Atherton, P.J.; Smith, K. CORP: The use of deuterated water for the measurement of protein synthesis. J. Appl. Physiol. 2020, 128, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Cegielski, J.; Wilkinson, D.J.; Brook, M.S.; Boereboom, C.; Phillips, B.E.; Gladman, J.F.R.; Smith, K.; Atherton, P.J. Combined in vivo muscle mass, muscle protein synthesis and muscle protein breakdown measurement: A ‘Combined Oral Stable Isotope Assessment of Muscle (COSIAM)’ approach. GeroScience 2021, 43, 2653–2665. [Google Scholar] [CrossRef] [PubMed]
- Goul, C.; Peruzzo, R.; Zoncu, R. The molecular basis of nutrient sensing and signalling by mTORC1 in metabolism regulation and disease. Nat. Rev. Mol. Cell Biol. 2023, 24, 857–875. [Google Scholar] [CrossRef]
- Wackerhage, H.; Schoenfeld, B.J.; Hamilton, D.L.; Lehti, M.; Hulmi, J.J. Stimuli and sensors that initiate skeletal muscle hypertrophy following resistance exercise. J. Appl. Physiol. 2019, 126, 30–43. [Google Scholar] [CrossRef]
- Roberts, M.D.; McCarthy, J.J.; Hornberger, T.A.; Phillips, S.M.; Mackey, A.L.; Nader, G.A.; Boppart, M.D.; Kavazis, A.N.; Reidy, P.T.; Ogasawara, R.; et al. Mechanisms of mechanical overload-induced skeletal muscle hypertrophy: Current understanding and future directions. Physiol. Rev. 2023, 103, 2679–2757. [Google Scholar] [CrossRef]
- Fry, C.S.; Drummond, M.J.; Glynn, E.L.; Dickinson, J.M.; Gundermann, D.M.; Timmerman, K.L.; Walker, D.K.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet. Muscle 2011, 1, 11. [Google Scholar] [CrossRef]
- Fingar, D.C.; Salama, S.; Tsou, C.; Harlow, E.; Blenis, J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002, 16, 1472–1487. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Z.; Erdjument-Bromage, H.; Tempst, P.; Pandolfi, P.P. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 2005, 121, 179–193. [Google Scholar] [CrossRef]
- Welle, S.; Thornton, C.; Jozefowicz, R.; Statt, M. Myofibrillar protein synthesis in young and old men. Am. J. Physiol. Endocrinol. Metab. 1993, 264, E693–E698. [Google Scholar] [CrossRef]
- Yarasheski, K.E.; Zachwieja, J.J.; Bier, D.M. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am. J. Physiol. 1993, 265, E210–E214. [Google Scholar] [CrossRef] [PubMed]
- Welle, S.; Thornton, C.; Statt, M. Myofibrillar protein synthesis in young and old human subjects after three months of resistance training. Am. J. Physiol. 1995, 268, E422–E427. [Google Scholar] [CrossRef]
- Hasten, D.L.; Pak-Loduca, J.; Obert, K.A.; Yarasheski, K.E. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E620–E626. [Google Scholar] [CrossRef] [PubMed]
- Rooyackers, O.E.; Adey, D.B.; Ades, P.A.; Nair, K.S. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc. Natl. Acad. Sci. USA 1996, 93, 15364–15369. [Google Scholar] [CrossRef]
- Short, K.R.; Vittone, J.L.; Bigelow, M.L.; Proctor, D.N.; Nair, K.S. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E92–E101. [Google Scholar] [CrossRef]
- Volpi, E.; Mittendorfer, B.; Wolf, S.E.; Wolfe, R.R. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am. J. Physiol. 1999, 277, E513–E520. [Google Scholar] [CrossRef]
- Volpi, E.; Mittendorfer, B.; Rasmussen, B.B.; Wolfe, R.R. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J. Clin. Endocrinol. Metab. 2000, 85, 4481–4490. [Google Scholar] [CrossRef]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am. J. Clin. Nutr. 2005, 82, 1065–1073. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Sheffield-Moore, M.; Zhang, X.J.; Volpi, E.; Wolf, S.E.; Aarsland, A.; Ferrando, A.A.; Wolfe, R.R. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E321–E328. [Google Scholar] [CrossRef]
- Volpi, E.; Sheffield-Moore, M.; Rasmussen, B.B.; Wolfe, R.R. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 2001, 286, 1206–1212. [Google Scholar] [CrossRef]
- Yarasheski, K.E.; Welle, S.; Nair, K.S. Muscle Protein Synthesis in Younger and Older Men. JAMA 2002, 287, 317–318. [Google Scholar] [CrossRef]
- Markofski, M.M.; Dickinson, J.M.; Drummond, M.J.; Fry, C.S.; Fujita, S.; Gundermann, D.M.; Glynn, E.L.; Jennings, K.; Paddon-Jones, D.; Reidy, P.T.; et al. Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women. Exp. Gerontol. 2015, 65, 1–7. [Google Scholar] [CrossRef]
- Wall, B.T.; Gorissen, S.H.; Pennings, B.; Koopman, R.; Groen, B.B.; Verdijk, L.B.; van Loon, L.J. Aging Is Accompanied by a Blunted Muscle Protein Synthetic Response to Protein Ingestion. PLoS ONE 2015, 10, e0140903. [Google Scholar] [CrossRef]
- Symons, T.B.; Schutzler, S.E.; Cocke, T.L.; Chinkes, D.L.; Wolfe, R.R.; Paddon-Jones, D. Aging does not impair the anabolic response to a protein-rich meal. Am. J. Clin. Nutr. 2007, 86, 451–456. [Google Scholar] [CrossRef]
- Gorissen, S.H.; Burd, N.A.; Hamer, H.M.; Gijsen, A.P.; Groen, B.B.; van Loon, L.J. Carbohydrate coingestion delays dietary protein digestion and absorption but does not modulate postprandial muscle protein accretion. J. Clin. Endocrinol. Metab. 2014, 99, 2250–2258. [Google Scholar] [CrossRef]
- Kiskini, A.; Hamer, H.M.; Wall, B.T.; Groen, B.B.; de Lange, A.; Bakker, J.A.; Senden, J.M.; Verdijk, L.B.; van Loon, L.J. The muscle protein synthetic response to the combined ingestion of protein and carbohydrate is not impaired in healthy older men. Age 2013, 35, 2389–2398. [Google Scholar] [CrossRef]
- Koopman, R.; Walrand, S.; Beelen, M.; Gijsen, A.P.; Kies, A.K.; Boirie, Y.; Saris, W.H.; van Loon, L.J. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J. Nutr. 2009, 139, 1707–1713. [Google Scholar] [CrossRef]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E381–E387. [Google Scholar] [CrossRef]
- Dillon, E.L.; Casperson, S.L.; Durham, W.J.; Randolph, K.M.; Urban, R.J.; Volpi, E.; Ahmad, M.; Kinsky, M.P.; Sheffield-Moore, M. Muscle protein metabolism responds similarly to exogenous amino acids in healthy younger and older adults during NO-induced hyperemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1408–R1417. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Bethan, E.P.; Daniel, J.W.; John, P.W.; Debbie, R.; Jonathan, N.L.; Kenneth, S.; Philip, J.A. Supplementing essential amino acids with the nitric oxide precursor, l-arginine, enhances skeletal muscle perfusion without impacting anabolism in older men. Clin. Nutr. 2017, 36, 1573–1579. [Google Scholar] [CrossRef]
- Babraj, J.A.; Cuthbertson, D.J.; Smith, K.; Langberg, H.; Miller, B.; Krogsgaard, M.R.; Kjaer, M.; Rennie, M.J. Collagen synthesis in human musculoskeletal tissues and skin. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E864–E869. [Google Scholar] [CrossRef]
- Guillet, C.; Prod’homme, M.; Balage, M.; Gachon, P.; Giraudet, C.; Morin, L.; Grizard, J.; Boirie, Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004, 18, 1586–1587. [Google Scholar] [CrossRef]
- Chevalier, S.; Goulet, E.D.; Burgos, S.A.; Wykes, L.J.; Morais, J.A. Protein anabolic responses to a fed steady state in healthy aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 681–688. [Google Scholar] [CrossRef]
- Pennings, B.; Koopman, R.; Beelen, M.; Senden, J.M.; Saris, W.H.; van Loon, L.J. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am. J. Clin. Nutr. 2011, 93, 322–331. [Google Scholar] [CrossRef]
- Shad, B.J.; Thompson, J.L.; Breen, L. Does the muscle protein synthetic response to exercise and amino acid-based nutrition diminish with advancing age? A systematic review. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E803–E817. [Google Scholar] [CrossRef]
- Sheffield-Moore, M.; Paddon-Jones, D.; Sanford, A.P.; Rosenblatt, J.I.; Matlock, A.G.; Cree, M.G.; Wolfe, R.R. Mixed muscle and hepatic derived plasma protein metabolism is differentially regulated in older and younger men following resistance exercise. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E922–E929. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Selby, A.; Rankin, D.; Patel, R.; Atherton, P.; Hildebrandt, W.; Williams, J.; Smith, K.; Seynnes, O.; Hiscock, N.; et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J. Physiol. 2009, 587, 211–217. [Google Scholar] [CrossRef]
- Kumar, V.; Atherton, P.J.; Selby, A.; Rankin, D.; Williams, J.; Smith, K.; Hiscock, N.; Rennie, M.J. Muscle protein synthetic responses to exercise: Effects of age, volume, and intensity. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 1170–1177. [Google Scholar] [CrossRef]
- Mayhew, D.L.; Kim, J.S.; Cross, J.M.; Ferrando, A.A.; Bamman, M.M. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J. Appl. Physiol. 2009, 107, 1655–1662. [Google Scholar] [CrossRef]
- Sheffield-Moore, M.; Yeckel, C.W.; Volpi, E.; Wolf, S.E.; Morio, B.; Chinkes, D.L.; Paddon-Jones, D.; Wolfe, R.R. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E513–E522. [Google Scholar] [CrossRef]
- Brook, M.S.; Wilkinson, D.J.; Mitchell, W.K.; Lund, J.N.; Phillips, B.E.; Szewczyk, N.J.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J. Physiol. 2016, 594, 7399–7417. [Google Scholar] [CrossRef]
- Reitelseder, S.; Bülow, J.; Holm, L. Divergent Anabolic Response to Exercise in Young and Older Adult Men-Dependency on Time Frame of Measurement. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, 76, 996–999. [Google Scholar] [CrossRef]
- Atherton, P.J.; Kumar, V.; Selby, A.L.; Rankin, D.; Hildebrandt, W.; Phillips, B.E.; Williams, J.P.; Hiscock, N.; Smith, K. Enriching a protein drink with leucine augments muscle protein synthesis after resistance exercise in young and older men. Clin. Nutr. 2017, 36, 888–895. [Google Scholar] [CrossRef]
- Drummond, M.J.; Dreyer, H.C.; Pennings, B.; Fry, C.S.; Dhanani, S.; Dillon, E.L.; Sheffield-Moore, M.; Volpi, E.; Rasmussen, B.B. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J. Appl. Physiol. 2008, 104, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Durham, W.J.; Casperson, S.L.; Dillon, E.L.; Keske, M.A.; Paddon-Jones, D.; Sanford, A.P.; Hickner, R.C.; Grady, J.J.; Sheffield-Moore, M. Age-related anabolic resistance after endurance-type exercise in healthy humans. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 4117–4127. [Google Scholar] [CrossRef]
- Hermans, W.J.; Fuchs, C.J.; Nyakayiru, J.; Hendriks, F.K.; Houben, L.H.; Senden, J.M.; van Loon, L.J.; Verdijk, L.B. Acute Quark Ingestion Increases Muscle Protein Synthesis Rates at Rest with a Further Increase after Exercise in Young and Older Adult Males in a Parallel-Group Intervention Trial. J. Nutr. 2023, 153, 66–75. [Google Scholar] [CrossRef]
- Koopman, R.; Verdijk, L.; Manders, R.J.; Gijsen, A.P.; Gorselink, M.; Pijpers, E.; Wagenmakers, A.J.; van Loon, L.J. Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am. J. Clin. Nutr. 2006, 84, 623–632. [Google Scholar] [CrossRef]
- Marshall, R.N.; Morgan, P.T.; Smeuninx, B.; Quinlan, J.I.; Brook, M.S.; Atherton, P.J.; Smith, K.; Wilkinson, D.J.; Breen, L. Myofibrillar Protein Synthesis and Acute Intracellular Signaling with Elastic Band Resistance Exercise in Young and Older Men. Med. Sci. Sports Exerc. 2023, 55, 398–408. [Google Scholar] [CrossRef]
- Symons, T.B.; Sheffield-Moore, M.; Mamerow, M.M.; Wolfe, R.R.; Paddon-Jones, D. The anabolic response to resistance exercise and a protein-rich meal is not diminished by age. J. Nutr. Health Aging 2011, 15, 376–381. [Google Scholar] [CrossRef]
- Horwath, O.; Moberg, M.; Hodson, N.; Edman, S.; Johansson, M.; Andersson, E.; van Hall, G.; Rooyackers, O.; Philp, A.; Apró, W. Anabolic Sensitivity in Healthy, Lean, Older Men is Associated with Higher Expression of Amino Acid Sensors and mTORC1 Activators Compared to Young. J. Cachexia Sarcopenia Muscle 2025, 16, e13613. [Google Scholar] [CrossRef]
- Farnfield, M.M.; Breen, L.; Carey, K.A.; Garnham, A.; Cameron-Smith, D. Activation of mTOR signalling in young and old human skeletal muscle in response to combined resistance exercise and whey protein ingestion. Appl. Physiol. Nutr. Metab. 2012, 37, 21–30. [Google Scholar] [CrossRef]
- Francaux, M.; Demeulder, B.; Naslain, D.; Fortin, R.; Lutz, O.; Caty, G.; Deldicque, L. Aging Reduces the Activation of the mTORC1 Pathway after Resistance Exercise and Protein Intake in Human Skeletal Muscle: Potential Role of REDD1 and Impaired Anabolic Sensitivity. Nutrients 2016, 8, 47. [Google Scholar] [CrossRef]
- Wilkinson, K.; Koscien, C.P.; Monteyne, A.J.; Wall, B.T.; Stephens, F.B. Association of postprandial postexercise muscle protein synthesis rates with dietary leucine: A systematic review. Physiol. Rep. 2023, 11, e15775. [Google Scholar] [CrossRef]
- Rudrappa, S.S.; Wilkinson, D.J.; Greenhaff, P.L.; Smith, K.; Idris, I.; Atherton, P.J. Human Skeletal Muscle Disuse Atrophy: Effects on Muscle Protein Synthesis, Breakdown, and Insulin Resistance—A Qualitative Review. Front. Physiol. 2016, 7, 361. [Google Scholar] [CrossRef]
- Phillips, B.E.; Williams, J.P.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Physiological adaptations to resistance exercise as a function of age. JCI Insight 2017, 2, e95581. [Google Scholar] [CrossRef]
- Sun, F.; Norman, I.J.; While, A.E. Physical activity in older people: A systematic review. BMC Public Health 2013, 13, 449. [Google Scholar] [CrossRef]
- Harvey, J.A.; Chastin, S.F.; Skelton, D.A. How sedentary are older people? A systematic review of the amount of sedentary behavior. J. Aging Phys. Act. 2015, 23, 471–487. [Google Scholar] [CrossRef]
- Stuart, C.A.; Shangraw, R.E.; Peters, E.J.; Wolfe, R.R. Effect of dietary protein on bed-rest-related changes in whole-body-protein synthesis. Am. J. Clin. Nutr. 1990, 52, 509–514. [Google Scholar] [CrossRef]
- Gibson, J.N.A.; Halliday, D.; Morrison, W.L.; Stoward, P.J.; Hornsby, G.A.; Watt, P.W.; Murdoch, G.; Rennie, M.J. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin. Sci. 1987, 72, 503–509. [Google Scholar] [CrossRef]
- Glover, E.I.; Phillips, S.M.; Oates, B.R.; Tang, J.E.; Tarnopolsky, M.A.; Selby, A.; Smith, K.; Rennie, M.J. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J. Physiol. 2008, 586, 6049–6061. [Google Scholar] [CrossRef]
- Wall, B.T.; Snijders, T.; Senden, J.M.G.; Ottenbros, C.L.P.; Gijsen, A.P.; Verdijk, L.B.; van Loon, L.J.C. Disuse Impairs the Muscle Protein Synthetic Response to Protein Ingestion in Healthy Men. J. Clin. Endocrinol. Metab. 2013, 98, 4872–4881. [Google Scholar] [CrossRef]
- Kortebein, P.; Ferrando, A.; Lombeida, J.; Wolfe, R.; Evans, W.J. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007, 297, 1772–1774. [Google Scholar] [CrossRef] [PubMed]
- McGlory, C.; von Allmen, M.T.; Stokes, T.; Morton, R.W.; Hector, A.J.; Lago, B.A.; Raphenya, A.R.; Smith, B.K.; McArthur, A.G.; Steinberg, G.R. Failed recovery of glycemic control and myofibrillar protein synthesis with 2 wk of physical inactivity in overweight, prediabetic older adults. J. Gerontol. Ser. A 2018, 73, 1070–1077. [Google Scholar] [CrossRef]
- Shad, B.J.; Thompson, J.L.; Holwerda, A.M.; Stocks, B.E.N.; Elhassan, Y.S.; Philp, A.; Van Loon, L.J.C.; Wallis, G.A. One Week of Step Reduction Lowers Myofibrillar Protein Synthesis Rates in Young Men. Med. Sci. Sports Exerc. 2019, 51, 2125–2134. [Google Scholar] [CrossRef]
- Smeuninx, B.; Elhassan, Y.S.; Sapey, E.; Rushton, A.B.; Morgan, P.T.; Korzepa, M.; Belfield, A.E.; Philp, A.; Brook, M.S.; Gharahdaghi, N.; et al. A single bout of prior resistance exercise attenuates muscle atrophy and declines in myofibrillar protein synthesis during bed-rest in older men. J. Physiol. 2025, 603, 87–105. [Google Scholar] [CrossRef]
- Exel, J.; Mateus, N.; Abrantes, C.; Leite, N.; Sampaio, J. Physical activity and sedentary behavior in amateur sports: Master athletes are not free from prolonged sedentary time. Sport Sci. Health 2019, 15, 385–391. [Google Scholar] [CrossRef]
- Lazarus, N.R.; Harridge, S.D.R. Declining performance of master athletes: Silhouettes of the trajectory of healthy human ageing? J. Physiol. 2017, 595, 2941–2948. [Google Scholar] [CrossRef]
- Dirks, M.L.; Wall, B.T.; van de Valk, B.; Holloway, T.M.; Holloway, G.P.; Chabowski, A.; Goossens, G.H.; van Loon, L.J.C. One Week of Bed Rest Leads to Substantial Muscle Atrophy and Induces Whole-Body Insulin Resistance in the Absence of Skeletal Muscle Lipid Accumulation. Diabetes 2016, 65, 2862–2875. [Google Scholar] [CrossRef]
- Reidy, P.T.; Lindsay, C.C.; McKenzie, A.I.; Fry, C.S.; Supiano, M.A.; Marcus, R.L.; LaStayo, P.C.; Drummond, M.J. Aging-related effects of bed rest followed by eccentric exercise rehabilitation on skeletal muscle macrophages and insulin sensitivity. Exp. Gerontol. 2018, 107, 37–49. [Google Scholar] [CrossRef]
- Yoon, M.-S. The Role of Mammalian Target of Rapamycin (mTOR) in Insulin Signaling. Nutrients 2017, 9, 1176. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, H.; Smith, K.; Atherton, P.J.; Idris, I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: A systematic review and meta-analysis. Diabetologia 2016, 59, 44–55. [Google Scholar] [CrossRef]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef]
- Atherton, P.J.; Greenhaff, P.L.; Phillips, S.M.; Bodine, S.C.; Adams, C.M.; Lang, C.H. Control of skeletal muscle atrophy in response to disuse: Clinical/preclinical contentions and fallacies of evidence. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E594–E604. [Google Scholar] [CrossRef]
- Morais, J.A.; Jacob, K.W.; Chevalier, S. Effects of aging and insulin resistant states on protein anabolic responses in older adults. Exp. Gerontol. 2018, 108, 262–268. [Google Scholar] [CrossRef]
- Timmerman, K.L.; Lee, J.L.; Dreyer, H.C.; Dhanani, S.; Glynn, E.L.; Fry, C.S.; Drummond, M.J.; Sheffield-Moore, M.; Rasmussen, B.B.; Volpi, E. Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial-dependent vasodilation and mammalian target of rapamycin complex 1 signaling. J. Clin. Endocrinol. Metab. 2010, 95, 3848–3857. [Google Scholar] [CrossRef]
- Timmerman, K.L.; Lee, J.L.; Fujita, S.; Dhanani, S.; Dreyer, H.C.; Fry, C.S.; Drummond, M.J.; Sheffield-Moore, M.; Rasmussen, B.B.; Volpi, E. Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes 2010, 59, 2764–2771. [Google Scholar] [CrossRef]
- Fujita, S.; Glynn, E.L.; Timmerman, K.L.; Rasmussen, B.B.; Volpi, E. Supraphysiological hyperinsulinaemia is necessary to stimulate skeletal muscle protein anabolism in older adults: Evidence of a true age-related insulin resistance of muscle protein metabolism. Diabetologia 2009, 52, 1889–1898. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Simental-Mendía, L.E.; Sahebkar, A. Molecular mechanisms by which aerobic exercise induces insulin sensitivity. J. Cell. Physiol. 2019, 234, 12385–12392. [Google Scholar] [CrossRef]
- Pratley, R.E.; Hagberg, J.M.; Rogus, E.M.; Goldberg, A.P. Enhanced insulin sensitivity and lower waist-to-hip ratio in master athletes. Am. J. Physiol. 1995, 268, E484–E490. [Google Scholar] [CrossRef]
- Seals, D.R.; Hagberg, J.M.; Allen, W.K.; Hurley, B.F.; Dalsky, G.P.; Ehsani, A.A.; Holloszy, J.O. Glucose tolerance in young and older athletes and sedentary men. J. Appl. Physiol. 1984, 56, 1521–1525. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, U.R.; Couppé, C.; Karlsen, A.; Grosset, J.F.; Schjerling, P.; Mackey, A.L.; Klausen, H.H.; Magnusson, S.P.; Kjær, M. Life-long endurance exercise in humans: Circulating levels of inflammatory markers and leg muscle size. Mech. Ageing Dev. 2013, 134, 531–540. [Google Scholar] [CrossRef]
- Climstein, M.; Walsh, J.; Adams, K.; Sevene, T.; Heazlewood, T.; DeBeliso, M. Prevalence of hyperglycemia in masters athletes. PeerJ 2022, 10, e13389. [Google Scholar] [CrossRef] [PubMed]
- Bunprajun, T.; Henriksen, T.I.; Scheele, C.; Pedersen, B.K.; Green, C.J. Lifelong physical activity prevents aging-associated insulin resistance in human skeletal muscle myotubes via increased glucose transporter expression. PLoS ONE 2013, 8, e66628. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, S.; Marliss, E.B.; Morais, J.A.; Lamarche, M.; Gougeon, R. Whole-body protein anabolic response is resistant to the action of insulin in obese women. Am. J. Clin. Nutr. 2005, 82, 355–365. [Google Scholar] [CrossRef]
- Chevalier, S.; Burgos, S.A.; Morais, J.A.; Gougeon, R.; Bassil, M.; Lamarche, M.; Marliss, E.B. Protein and glucose metabolic responses to hyperinsulinemia, hyperglycemia, and hyperaminoacidemia in obese men. Obesity 2015, 23, 351–358. [Google Scholar] [CrossRef]
- Guillet, C.; Delcourt, I.; Rance, M.; Giraudet, C.; Walrand, S.; Bedu, M.; Duche, P.; Boirie, Y. Changes in basal and insulin and amino acid response of whole body and skeletal muscle proteins in obese men. J. Clin. Endocrinol. Metab. 2009, 94, 3044–3050. [Google Scholar] [CrossRef]
- Murton, A.J.; Marimuthu, K.; Mallinson, J.E.; Selby, A.L.; Smith, K.; Rennie, M.J.; Greenhaff, P.L. Obesity Appears to Be Associated With Altered Muscle Protein Synthetic and Breakdown Responses to Increased Nutrient Delivery in Older Men, but Not Reduced Muscle Mass or Contractile Function. Diabetes 2015, 64, 3160–3171. [Google Scholar] [CrossRef]
- Tran, L.; Kras, K.A.; Hoffman, N.; Ravichandran, J.; Dickinson, J.M.; D’Lugos, A.; Carroll, C.C.; Patel, S.H.; Mandarino, L.J.; Roust, L.; et al. Lower Fasted-State but Greater Increase in Muscle Protein Synthesis in Response to Elevated Plasma Amino Acids in Obesity. Obesity 2018, 26, 1179–1187. [Google Scholar] [CrossRef]
- Beals, J.W.; Sukiennik, R.A.; Nallabelli, J.; Emmons, R.S.; van Vliet, S.; Young, J.R.; Ulanov, A.V.; Li, Z.; Paluska, S.A.; De Lisio, M.; et al. Anabolic sensitivity of postprandial muscle protein synthesis to the ingestion of a protein-dense food is reduced in overweight and obese young adults12. Am. J. Clin. Nutr. 2016, 104, 1014–1022. [Google Scholar] [CrossRef]
- Kouw, I.W.K.; van Dijk, J.W.; Horstman, A.M.H.; Kramer, I.F.; Goessens, J.P.B.; van Dielen, F.M.H.; Verdijk, L.B.; van Loon, L.J.C. Basal and Postprandial Myofibrillar Protein Synthesis Rates Do Not Differ between Lean and Obese Middle-Aged Men. J. Nutr. 2019, 149, 1533–1542. [Google Scholar] [CrossRef]
- Hulston, C.J.; Woods, R.M.; Dewhurst-Trigg, R.; Parry, S.A.; Gagnon, S.; Baker, L.; James, L.J.; Markey, O.; Martin, N.R.W.; Ferguson, R.A.; et al. Resistance exercise stimulates mixed muscle protein synthesis in lean and obese young adults. Physiol. Rep. 2018, 6, e13799. [Google Scholar] [CrossRef]
- Smeuninx, B.; McKendry, J.; Wilson, D.; Martin, U.; Breen, L. Age-Related Anabolic Resistance of Myofibrillar Protein Synthesis Is Exacerbated in Obese Inactive Individuals. J. Clin. Endocrinol. Metab. 2017, 102, 3535–3545. [Google Scholar] [CrossRef]
- Moro, T.; Brightwell, C.R.; Deer, R.R.; Graber, T.G.; Galvan, E.; Fry, C.S.; Volpi, E.; Rasmussen, B.B. Muscle Protein Anabolic Resistance to Essential Amino Acids Does Not Occur in Healthy Older Adults Before or After Resistance Exercise Training. J. Nutr. 2018, 148, 900–909. [Google Scholar] [CrossRef]
- Morgan, P.T.; Smeuninx, B.; Breen, L. Exploring the Impact of Obesity on Skeletal Muscle Function in Older Age. Front. Nutr. 2020, 7, 569904. [Google Scholar] [CrossRef]
- Castillo, Í.M.P.; Argilés, J.M.; Rueda, R.; Ramírez, M.; Pedrosa, J.M.L. Skeletal muscle atrophy and dysfunction in obesity and type-2 diabetes mellitus: Myocellular mechanisms involved. Rev. Endocr. Metab. Disord. 2025, 26, 1–22. [Google Scholar] [CrossRef]
- Walsh, J.; Heazlewood, I.T.; Climstein, M. Body Mass Index in Master Athletes: Review of the Literature. J. Lifestyle Med. 2018, 8, 79–98. [Google Scholar] [CrossRef]
- Walker, S.; von Bonsdorff, M.; Cheng, S.; Häkkinen, K.; Bondarev, D.; Heinonen, A.; Korhonen, M.T. Body composition in male lifelong trained strength, sprint and endurance athletes and healthy age-matched controls. Front. Sports Act. Living 2023, 5, 1295906. [Google Scholar] [CrossRef]
- Daemen, S.; Gemmink, A.; Brouwers, B.; Meex, R.C.R.; Huntjens, P.R.; Schaart, G.; Moonen-Kornips, E.; Jörgensen, J.; Hoeks, J.; Schrauwen, P.; et al. Distinct lipid droplet characteristics and distribution unmask the apparent contradiction of the athlete’s paradox. Mol. Metab. 2018, 17, 71–81. [Google Scholar] [CrossRef]
- Puzianowska-Kuźnicka, M.; Owczarz, M.; Wieczorowska-Tobis, K.; Nadrowski, P.; Chudek, J.; Slusarczyk, P.; Skalska, A.; Jonas, M.; Franek, E.; Mossakowska, M. Interleukin-6 and C-reactive protein, successful aging, and mortality: The PolSenior study. Immun. Ageing 2016, 13, 21. [Google Scholar] [CrossRef]
- Baune, B.T.; Rothermundt, M.; Ladwig, K.H.; Meisinger, C.; Berger, K. Systemic inflammation (Interleukin 6) predicts all-cause mortality in men: Results from a 9-year follow-up of the MEMO Study. Age 2011, 33, 209–217. [Google Scholar] [CrossRef]
- Singh, T.; Newman, A.B. Inflammatory markers in population studies of aging. Ageing Res. Rev. 2011, 10, 319–329. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and ‘Garb-aging’. Trends Endocrinol. Metab. 2017, 28, 199–212. [Google Scholar] [CrossRef]
- Olivieri, F.; Prattichizzo, F.; Grillari, J.; Balistreri, C.R. Cellular Senescence and Inflammaging in Age-Related Diseases. Mediat. Inflamm 2018, 2018, 9076485. [Google Scholar] [CrossRef] [PubMed]
- Rivas, D.A.; Morris, E.P.; Haran, P.H.; Pasha, E.P.; Morais Mda, S.; Dolnikowski, G.G.; Phillips, E.M.; Fielding, R.A. Increased ceramide content and NFκB signaling may contribute to the attenuation of anabolic signaling after resistance exercise in aged males. J. Appl. Physiol. 2012, 113, 1727–1736. [Google Scholar] [CrossRef]
- Rivas, D.A.; Rice, N.P.; Ezzyat, Y.; McDonald, D.J.; Cooper, B.E.; Fielding, R.A. Sphingosine-1-phosphate analog FTY720 reverses obesity but not age-induced anabolic resistance to muscle contraction. Am. J. Physiol. Cell Physiol. 2019, 317, C502–C512. [Google Scholar] [CrossRef]
- Hyde, R.; Hajduch, E.; Powell, D.J.; Taylor, P.M.; Hundal, H.S. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 461–463. [Google Scholar] [CrossRef]
- Tardif, N.; Salles, J.; Guillet, C.; Tordjman, J.; Reggio, S.; Landrier, J.F.; Giraudet, C.; Patrac, V.; Bertrand-Michel, J.; Migne, C.; et al. Muscle ectopic fat deposition contributes to anabolic resistance in obese sarcopenic old rats through eIF2α activation. Aging Cell 2014, 13, 1001–1011. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Wåhlin-Larsson, B.; Wilkinson, D.J.; Strandberg, E.; Hosford-Donovan, A.; Atherton, P.J.; Kadi, F. Mechanistic links underlying the impact of C-reactive protein on muscle mass in elderly. Cell. Physiol. Biochem. 2018, 44, 267–278. [Google Scholar] [CrossRef]
- Toth, M.J.; Matthews, D.E.; Tracy, R.P.; Previs, M.J. Age-related differences in skeletal muscle protein synthesis: Relation to markers of immune activation. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E883–E891. [Google Scholar] [CrossRef]
- Draganidis, D.; Jamurtas, A.Z.; Chondrogianni, N.; Mastorakos, G.; Jung, T.; Grune, T.; Papadopoulos, C.; Papanikolaou, K.; Papassotiriou, I.; Papaevgeniou, N.; et al. Low-Grade Systemic Inflammation Interferes with Anabolic and Catabolic Characteristics of the Aged Human Skeletal Muscle. Oxidative Med. Cell. Longev. 2021, 2021, 8376915. [Google Scholar] [CrossRef]
- Dideriksen, K.; Reitelseder, S.; Malmgaard-Clausen, N.M.; Bechshoeft, R.; Petersen, R.K.; Mikkelsen, U.R.; Holm, L. No effect of anti-inflammatory medication on postprandial and postexercise muscle protein synthesis in elderly men with slightly elevated systemic inflammation. Exp. Gerontol. 2016, 83, 120–129. [Google Scholar] [CrossRef]
- Buffière, C.; Mariotti, F.; Savary-Auzeloux, I.; Migné, C.; Meunier, N.; Hercberg, S.; Cano, N.; Rémond, D.; Duclos, M.; Dardevet, D. Slight chronic elevation of C-reactive protein is associated with lower aerobic fitness but does not impair meal-induced stimulation of muscle protein metabolism in healthy old men. J. Physiol. 2015, 593, 1259–1272. [Google Scholar] [CrossRef]
- Allen, S.L.; Marshall, R.N.; Edwards, S.J.; Lord, J.M.; Lavery, G.G.; Breen, L. The effect of young and old ex vivo human serum on cellular protein synthesis and growth in an in vitro model of aging. Am. J. Physiol. Cell Physiol. 2021, 321, C26–C37. [Google Scholar] [CrossRef]
- Pérez-Castillo, I.M.; Rueda, R.; Bouzamondo, H.; Aparicio-Pascual, D.; Valiño-Marques, A.; López-Chicharro, J.; Segura-Ortiz, F. Does Lifelong Exercise Counteract Low-Grade Inflammation Associated with Aging? A Systematic Review and Meta-Analysis. Sports Med. 2025, 55, 675–696. [Google Scholar] [CrossRef]
- Stec, M.J.; Kelly, N.A.; Many, G.M.; Windham, S.T.; Tuggle, S.C.; Bamman, M.M. Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E652–E661. [Google Scholar] [CrossRef]
- Watson, M.D.; Cross, B.L.; Grosicki, G.J. Evidence for the Contribution of Gut Microbiota to Age-Related Anabolic Resistance. Nutrients 2021, 13, 706. [Google Scholar] [CrossRef]
- Prokopidis, K.; Chambers, E.; Ni Lochlainn, M.; Witard, O.C. Mechanisms Linking the Gut-Muscle Axis With Muscle Protein Metabolism and Anabolic Resistance: Implications for Older Adults at Risk of Sarcopenia. Front. Physiol. 2021, 12, 770455. [Google Scholar] [CrossRef]
- Geiger, C.; Needhamsen, M.; Emanuelsson, E.B.; Norrbom, J.; Steindorf, K.; Sundberg, C.J.; Reitzner, S.M.; Lindholm, M.E. DNA methylation of exercise-responsive genes differs between trained and untrained men. BMC Biol. 2024, 22, 147. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on dietary reference values for protein. EFSA J. 2012, 10, 2557. [Google Scholar] [CrossRef]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper From the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Moore, D.R. Maximizing Post-exercise Anabolism: The Case for Relative Protein Intakes. Front. Nutr. 2019, 6, 147. [Google Scholar] [CrossRef]
- Trommelen, J.; van Lieshout, G.A.; Nyakayiru, J.; Holwerda, A.M.; Smeets, J.S.; Hendriks, F.K.; van Kranenburg, J.M.; Zorenc, A.H.; Senden, J.M.; Goessens, J.P. The anabolic response to protein ingestion during recovery from exercise has no upper limit in magnitude and duration in vivo in humans. Cell Rep. Med. 2023, 4, 101324. [Google Scholar] [CrossRef]
- Macnaughton, L.S.; Wardle, S.L.; Witard, O.C.; McGlory, C.; Hamilton, D.L.; Jeromson, S.; Lawrence, C.E.; Wallis, G.A.; Tipton, K.D. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol. Rep. 2016, 4, e12893. [Google Scholar] [CrossRef]
- Park, S.; Jang, J.; Choi, M.D.; Shin, Y.-A.; Schutzler, S.; Azhar, G.; Ferrando, A.A.; Wolfe, R.R.; Kim, I.-Y. The Anabolic Response to Dietary Protein Is Not Limited by the Maximal Stimulation of Protein Synthesis in Healthy Older Adults: A Randomized Crossover Trial. Nutrients 2020, 12, 3276. [Google Scholar] [CrossRef]
- Kim, I.Y.; Schutzler, S.; Schrader, A.; Spencer, H.J.; Azhar, G.; Ferrando, A.A.; Wolfe, R.R. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E73–E80. [Google Scholar] [CrossRef]
- Doering, T.M.; Reaburn, P.R.; Borges, N.R.; Cox, G.R.; Jenkins, D.G. The Effect of Higher Than Recommended Protein Feedings Post-Exercise on Recovery Following Downhill Running in Masters Triathletes. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 76–82. [Google Scholar] [CrossRef]
- Di Girolamo, F.G.; Situlin, R.; Fiotti, N.; Tence, M.; De Colle, P.; Mearelli, F.; Minetto, M.A.; Ghigo, E.; Pagani, M.; Lucini, D.; et al. Higher protein intake is associated with improved muscle strength in elite senior athletes. Nutrition 2017, 42, 82–86. [Google Scholar] [CrossRef]
- Stanzione, J.R.; Boullata, J.I.; Bruneau, M.L., Jr.; Volpe, S.L. Association between protein intake and lean body mass in a group of Masters Athletes. J. Nutr. Sci. 2022, 11, e30. [Google Scholar] [CrossRef]
- Beshgetoor, D.; Nichols, J.F. Dietary intake and supplement use in female master cyclists and runners. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 166–172. [Google Scholar] [CrossRef]
- Wall, B.T.; Hamer, H.M.; de Lange, A.; Kiskini, A.; Groen, B.B.; Senden, J.M.; Gijsen, A.P.; Verdijk, L.B.; van Loon, L.J. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin. Nutr. 2013, 32, 412–419. [Google Scholar] [CrossRef]
- Holwerda, A.M.; Paulussen, K.J.M.; Overkamp, M.; Goessens, J.P.B.; Kramer, I.F.; Wodzig, W.; Verdijk, L.B.; de Groot, L.; van Loon, L.J.C. Leucine coingestion augments the muscle protein synthetic response to the ingestion of 15 g of protein following resistance exercise in older men. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E473–E482. [Google Scholar] [CrossRef]
- Dickinson, J.M.; Gundermann, D.M.; Walker, D.K.; Reidy, P.T.; Borack, M.S.; Drummond, M.J.; Arora, M.; Volpi, E.; Rasmussen, B.B. Leucine-enriched amino acid ingestion after resistance exercise prolongs myofibrillar protein synthesis and amino acid transporter expression in older men. J. Nutr. 2014, 144, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Ely, I.A.; Phillips, B.E.; Smith, K.; Wilkinson, D.J.; Piasecki, M.; Breen, L.; Larsen, M.S.; Atherton, P.J. A focus on leucine in the nutritional regulation of human skeletal muscle metabolism in ageing, exercise and unloading states. Clin. Nutr. 2023, 42, 1849–1865. [Google Scholar] [CrossRef]
- Claudia, A.R.; Claudia, S.; Christiane, S.; Niklas, B.; Anne, S.; Wilfried, W.A. Reduced muscular fatigue after a 12-week leucine-rich amino acid supplementation combined with moderate training in elderly: A randomised, placebo-controlled, double-blind trial. BMJ Open Sport Exerc. Med. 2017, 2, e000156. [Google Scholar] [CrossRef]
- Reaburn, P.; Doering, T.; Borges, N. Masters athletes take longer to recover from high intensity exercise than training-matched younger athletes. Does increased protein intake enhance recovery? J. Sci. Med. Sport 2019, 22, S32–S33. [Google Scholar] [CrossRef]
- Easthope, C.S.; Hausswirth, C.; Louis, J.; Lepers, R.; Vercruyssen, F.; Brisswalter, J. Effects of a trail running competition on muscular performance and efficiency in well-trained young and master athletes. Eur. J. Appl. Physiol. 2010, 110, 1107–1116. [Google Scholar] [CrossRef]
- Fell, J.; Reaburn, P.; Harrison, G.J. Altered perception and report of fatigue and recovery in veteran athletes. J. Sports Med. Phys. Fit. 2008, 48, 272–277. [Google Scholar]
- Borges, N.R.; Reaburn, P.R.; Doering, T.M.; Argus, C.K.; Driller, M.W. Age-related changes in physical and perceptual markers of recovery following high-intensity interval cycle exercise. Exp. Aging Res. 2018, 44, 338–349. [Google Scholar] [CrossRef]
- Schmidt, J.; Ferrauti, A.; Kellmann, M.; Beaudouin, F.; Pfeiffer, M.; Volk, N.R.; Wambach, J.M.; Bruder, O.; Wiewelhove, T. Recovery from eccentric squat exercise in resistance-trained young and master athletes with similar maximum strength: Combining cold water immersion and compression. Front. Physiol. 2021, 12, 665204. [Google Scholar] [CrossRef]
- Minuzzi, L.G.; Ferrauti, A.; Chupel, M.U.; Hacker, S.; Weyh, C.; Valenzuela, P.L.; Lucia, A.; Krüger, K.; Reichel, T. Acute Inflammatory Response to Eccentric Exercise in Young and Master Resistance-trained Athletes. Int. J. Sports Med. 2024, 45, 897–907. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Hossain, T.; Limb, M.C.; Phillips, B.E.; Lund, J.; Williams, J.P.; Brook, M.S.; Cegielski, J.; Philp, A.; Ashcroft, S. Impact of the calcium form of β-hydroxy-β-methylbutyrate upon human skeletal muscle protein metabolism. Clin. Nutr. 2018, 37, 2068–2075. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef]
- Wilson, J.M.; Fitschen, P.J.; Campbell, B.; Wilson, G.J.; Zanchi, N.; Taylor, L.; Wilborn, C.; Kalman, D.S.; Stout, J.R.; Hoffman, J.R. International society of sports nutrition position stand: Beta-hydroxy-beta-methylbutyrate (HMB). J. Int. Soc. Sports Nutr. 2013, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.; Mohammadi, H.; Zeraattalab-Motlagh, S.; Arab, A.; Keshavarz Mohammadian, M.; Ghoreishy, S.M.; Abbaspour Tehrani Fard, M.; Amiri Khosroshahi, R.; Djafarian, K. Nutritional interventions for exercise-induced muscle damage: An umbrella review of systematic reviews and meta-analyses of randomized trials. Nutr. Rev. 2023, 82, 639–653. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Pereira, S.L.; Hays, N.P.; Oliver, J.S.; Edens, N.K.; Evans, C.M.; Wolfe, R.R. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin. Nutr. 2013, 32, 704–712. [Google Scholar] [CrossRef]
- Louis, J.; Vercruyssen, F.; Dupuy, O.; Bernard, T. Nutrition for master athletes: From challenges to optimisation strategies. Mov. Sport Sci. Sci. Mot. 2019, 104, 45–54. [Google Scholar] [CrossRef]
- Din, U.S.U.; Brook, M.S.; Selby, A.; Quinlan, J.; Boereboom, C.; Abdulla, H.; Franchi, M.; Narici, M.V.; Phillips, B.E.; Williams, J.W.; et al. A double-blind placebo controlled trial into the impacts of HMB supplementation and exercise on free-living muscle protein synthesis, muscle mass and function, in older adults. Clin. Nutr. 2019, 38, 2071–2078. [Google Scholar] [CrossRef]
- Oktaviana, J.; Zanker, J.; Vogrin, S.; Duque, G. The Effect of β-Hydroxy-β-Methylbutyrate (HMB) on Sarcopenia and Functional Frailty in Older Persons: A Systematic Review. J. Nutr. Health Aging 2019, 23, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xia, Y.; Jiang, J.; Du, H.; Guo, X.; Liu, X.; Li, C.; Huang, G.; Niu, K. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2015, 61, 168–175. [Google Scholar] [CrossRef] [PubMed]
- López-Seoane, J.; Martinez-Ferran, M.; Romero-Morales, C.; Pareja-Galeano, H. N-3 PUFA as an ergogenic supplement modulating muscle hypertrophy and strength: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 9000–9020. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. 2011, 121, 267–278. [Google Scholar] [CrossRef]
- Brook, M.S.; Din, U.; Tarum, J.; Selby, A.; Quinlan, J.; Bass, J.J.; Gharahdaghi, N.; Boereboom, C.; Abdulla, H.; Franchi, M.V.; et al. Omega-3 supplementation during unilateral resistance exercise training in older women: A within subject and double-blind placebo-controlled trial. Clin. Nutr. ESPEN 2021, 46, 394–404. [Google Scholar] [CrossRef]
- Lalia, A.Z.; Dasari, S.; Robinson, M.M.; Abid, H.; Morse, D.M.; Klaus, K.A.; Lanza, I.R. Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging 2017, 9, 1096–1129. [Google Scholar] [CrossRef]
- Murphy, C.H.; Flanagan, E.M.; De Vito, G.; Susta, D.; Mitchelson, K.A.J.; de Marco Castro, E.; Senden, J.M.G.; Goessens, J.P.B.; Mikłosz, A.; Chabowski, A.; et al. Does supplementation with leucine-enriched protein alone and in combination with fish-oil-derived n–3 PUFA affect muscle mass, strength, physical performance, and muscle protein synthesis in well-nourished older adults? A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2021, 113, 1411–1427. [Google Scholar] [CrossRef]
- Kunz, H.E.; Michie, K.L.; Gries, K.J.; Zhang, X.; Ryan, Z.C.; Lanza, I.R. A Randomized Trial of the Effects of Dietary n3-PUFAs on Skeletal Muscle Function and Acute Exercise Response in Healthy Older Adults. Nutrients 2022, 14, 3537. [Google Scholar] [CrossRef]
- Da Boit, M.; Sibson, R.; Sivasubramaniam, S.; Meakin, J.R.; Greig, C.A.; Aspden, R.M.; Thies, F.; Jeromson, S.; Hamilton, D.L.; Speakman, J.R.; et al. Sex differences in the effect of fish-oil supplementation on the adaptive response to resistance exercise training in older people: A randomized controlled trial12. Am. J. Clin. Nutr. 2017, 105, 151–158. [Google Scholar] [CrossRef]
- Huang, Y.H.; Chiu, W.C.; Hsu, Y.P.; Lo, Y.L.; Wang, Y.H. Effects of Omega-3 Fatty Acids on Muscle Mass, Muscle Strength and Muscle Performance among the Elderly: A Meta-Analysis. Nutrients 2020, 12, 3739. [Google Scholar] [CrossRef]
- Whitehouse, A.S.; Smith, H.J.; Drake, J.L.; Tisdale, M.J. Mechanism of attenuation of skeletal muscle protein catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res. 2001, 61, 3604–3609. [Google Scholar] [PubMed]
- Guo, S.; Shaoni, G.L.L.; Stuart-Smith, W.A.; Davies, A.J.; Gifford, J.A. Dietary Intake of Masters Athletes: A Systematic Review. Nutrients 2023, 15, 4973. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Gann, J.G.; La Bounty, P.M. Intermittent Fasting Programs and Their Effects on Body Composition: Implications for Weight-Restricted Sports. Strength Cond. J. 2015, 37, 60–71. [Google Scholar] [CrossRef]
- Lessan, N.; Ali, T. Energy Metabolism and Intermittent Fasting: The Ramadan Perspective. Nutrients 2019, 11, 1192. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; Vislocky, L.M.; Carbone, J.W.; Altieri, N.; Konopelski, K.; Freake, H.C.; Anderson, J.M.; Ferrando, A.A.; Wolfe, R.R.; Rodriguez, N.R. Acute energy deprivation affects skeletal muscle protein synthesis and associated intracellular signaling proteins in physically active adults. J. Nutr. 2010, 140, 745–751. [Google Scholar] [CrossRef]
- Murphy, C.H.; McGlory, C. Fish Oil for Healthy Aging: Potential Application to Master Athletes. Sports Med. 2021, 51, 31–41. [Google Scholar] [CrossRef]
- Louis, J.; Vercruyssen, F.; Dupuy, O.; Bernard, T. Nutrition for Master Athletes: Is There a Need for Specific Recommendations? J. Aging Phys. Act. 2020, 28, 489–498. [Google Scholar] [CrossRef]
| Potential Contributors to Anabolic Resistance | Methodological Considerations in Research Studies |
|---|---|
| Physical Inactivity | Describe physical activity habits in active individuals (e.g., validated questionnaires, logs, wearables), and sport discipline, years of training history, and participation in competitions in master athletes. |
| Compromised Insulin Signaling | Consider clamp protocols to isolate the contribution of insulin resistance to muscle anabolic resistance. |
| Adiposity | Describe body composition parameters and body fat distribution using accurate methodologies (e.g., DXA, MRI). |
| Low-grade Inflammation | Consider inflammatory marker panels to evaluate systematic inflammatory profiles under resting conditions. |
| Nutritional Factor | Recommendation |
|---|---|
| Protein Intake | Target daily intake of high-quality, leucine-rich protein close to the upper limit of current recommendations (e.g., 1.6–2 g/kg/day), distributed across meals providing 0.3–0.5 g/kg (approximately 22.5–37.5 g per meal) [21]. |
| n3-PUFA | Prioritize dietary sources of EPA/DHA to meet at least established recommendations (250–500 mg/day EPA + DHA) [200]. |
| Other Bioactive Ingredients | Consider supplementation with HMB (e.g., 3 g/day), particularly in situations of episodic disuse or rehabilitation [201]. |
| Energy Intake | Avoid extended periods of restricted energy availability by maintaining adequate daily energy intake (e.g., >30 kcal/kg/LBM) [201]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Castillo, Í.M.; Rueda, R.; Pereira, S.L.; Bouzamondo, H.; López-Chicharro, J.; Segura-Ortiz, F.; Atherton, P.J. Age-Related Anabolic Resistance: Nutritional and Exercise Strategies, and Potential Relevance to Life-Long Exercisers. Nutrients 2025, 17, 3503. https://doi.org/10.3390/nu17223503
Pérez-Castillo ÍM, Rueda R, Pereira SL, Bouzamondo H, López-Chicharro J, Segura-Ortiz F, Atherton PJ. Age-Related Anabolic Resistance: Nutritional and Exercise Strategies, and Potential Relevance to Life-Long Exercisers. Nutrients. 2025; 17(22):3503. https://doi.org/10.3390/nu17223503
Chicago/Turabian StylePérez-Castillo, Íñigo M., Ricardo Rueda, Suzette L. Pereira, Hakim Bouzamondo, José López-Chicharro, Felipe Segura-Ortiz, and Philip J. Atherton. 2025. "Age-Related Anabolic Resistance: Nutritional and Exercise Strategies, and Potential Relevance to Life-Long Exercisers" Nutrients 17, no. 22: 3503. https://doi.org/10.3390/nu17223503
APA StylePérez-Castillo, Í. M., Rueda, R., Pereira, S. L., Bouzamondo, H., López-Chicharro, J., Segura-Ortiz, F., & Atherton, P. J. (2025). Age-Related Anabolic Resistance: Nutritional and Exercise Strategies, and Potential Relevance to Life-Long Exercisers. Nutrients, 17(22), 3503. https://doi.org/10.3390/nu17223503






