Neonatal Outcomes Following a Preconception Lifestyle Intervention in People at Risk of Gestational Diabetes: Secondary Findings from the BEFORE THE BEGINNING Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
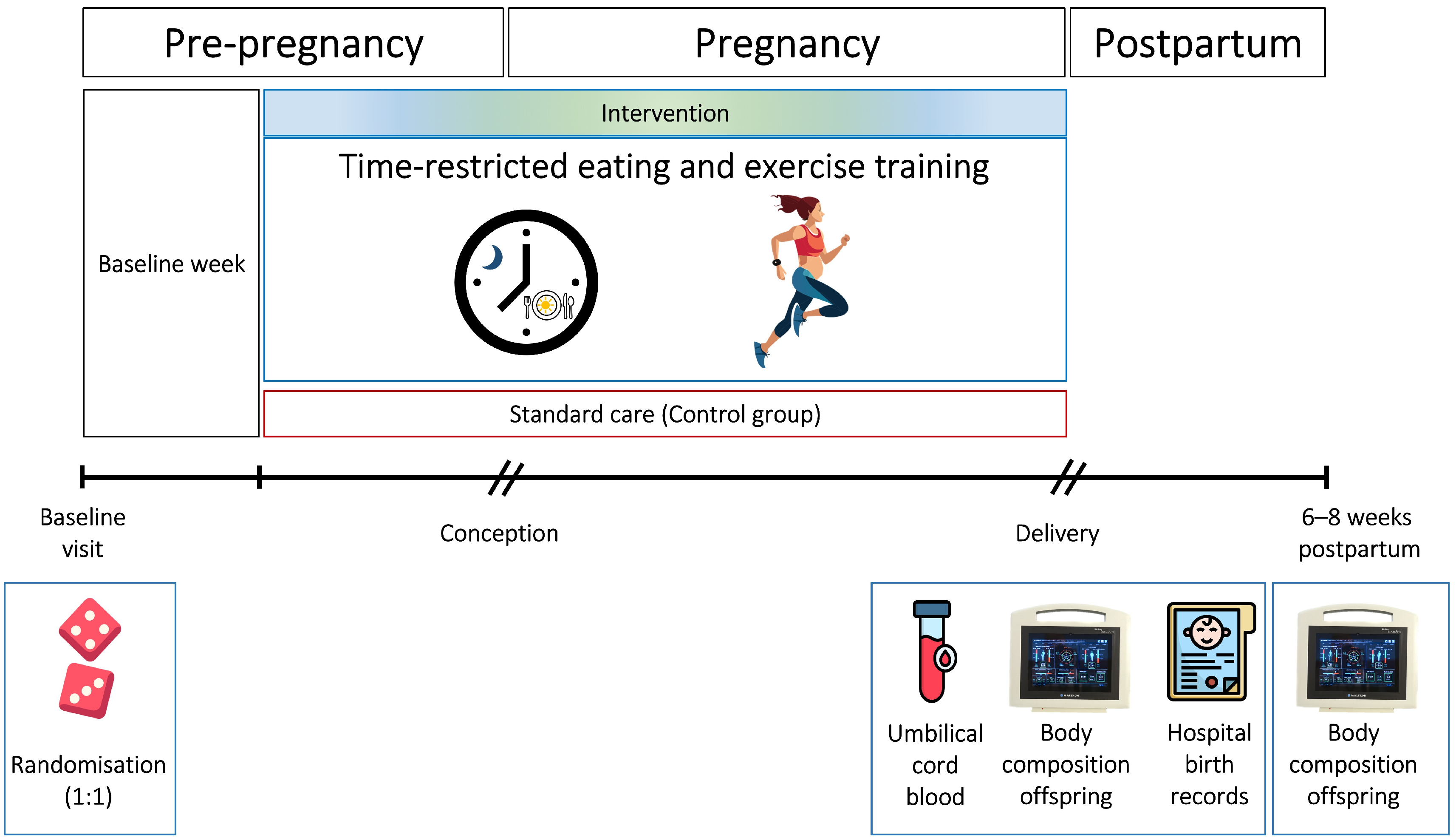
2.1. Study Design and Setting
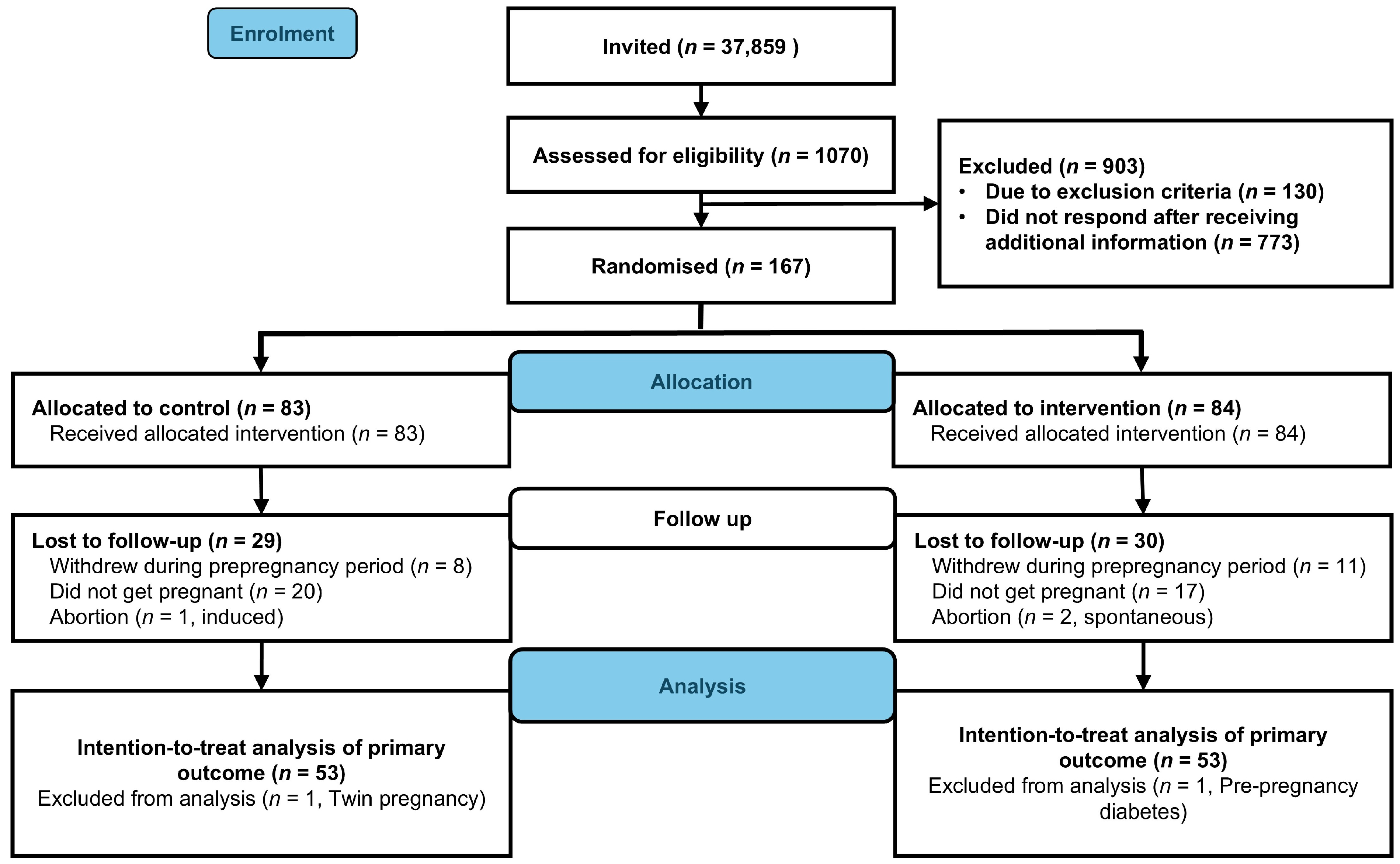
2.2. Recruitment and Participants
2.3. Randomization and Blinding
2.4. Intervention and Adherence
2.5. Experimental Procedures and Outcome Measures
2.6. Statistical Analysis
2.7. Patient and Public Involvement
3. Results
3.1. Neonatal Outcomes
3.2. Birth-Related Outcomes
3.3. Per Protocol Analysis
4. Discussion
4.1. Main Findings and Comparison with Previous Studies
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GDM | Gestational diabetes mellitus |
| BMI | Body mass index |
| PAI | Personal Activity Intelligence |
| LGA | Large for gestational age |
| BIA | Bioelectrical impedance analysis |
| SD | Standard deviation |
| CI | Confidence interval |
| HbA1c | Glycated hemoglobin |
| HDL | High-density lipoprotein cholesterol |
| LDL | Low-density lipoprotein cholesterol |
Appendix A
Appendix A.1
| Outcome | Time | Control (n = 49) | Intervention (n = 45) | Estimate | 95% CI | p |
|---|---|---|---|---|---|---|
| Weight, kg | At birth | 3.7 (0.5) | 3.6 (0.5) | |||
| 6–8 weeks | 5.3 (0.8) | 5.1 (0.8) | −0.1 | −0.4 to 0.1 | 0.337 | |
| Fat mass, kg | At birth | 0.2 (0.2) | 0.2 (0.2) | |||
| 6–8 weeks | 1.0 (0.3) | 1.0 (0.3) | −0.1 | −0.2 to 0.1 | 0.420 | |
| Fat mass, % | At birth | 6.0 (4.4) | 6.8 (5.4) | |||
| 6–8 weeks | 18.5 (5.3) | 18.6 (5.1) | −1.0 | −3.4 to 1.4 | 0.418 | |
| Fat-free mass, kg | At birth | 3.4 (0.5) | 3.3 (0.5) | |||
| 6–8 weeks | 4.3 (0.7) | 4.1 (0.6) | −0.1 | −0.3 to 0.2 | 0.583 | |
| Fat-free mass, % | At birth | 93.8 (4.2) | 93.0 (5.3) | |||
| 6–8 weeks | 81.5 (5.4) | 81.3 (5.1) | 0.9 | −1.5 to 3.3 | 0.457 | |
| Muscle mass, kg | At birth | 1.1 (0.2) | 1.1 (0.2) | |||
| 6–8 weeks | 1.5 (0.3) | 1.5 (0.3) | 0.0 | −0.1 to 0.1 | 0.944 | |
| Hydration (TBW), % | At birth | 78.2 (5.7) | 77.0 (6.7) | |||
| 6–8 weeks | 66.1 (5.6) | 65.7 (5.1) | 1.3 | −1.5 to 4.0 | 0.357 |
Appendix A.2
| Outcome | Control (n = 53) | Intervention (n = 23) | Estimate | 95% CI | p |
|---|---|---|---|---|---|
| Weight, g | 3702.5 (517.8) | 3734.3 (451.4) | 31.8 | −216.4 to 280.0 | 0.799 a |
| Length, cm | 50.1 (2.1) | 50.9 (1.9) | 0.7 | −0.3 to 1.8 | 0.158 a |
| Head circumference, cm | 36 (35, 37) | 36 (35, 37) | - | - | 0.840 b |
| Birth weight > 4 kg, n (%) | 15 (28) | 6 (26) | 0.9 | 0.4 to 2.1 | 0.843 c |
| APGAR < 7 at 5 min, n (%) | 1 (2) | 0 (0) | - | - | - |
| Preterm birth, n (%) | 1 (2) | 0 (0) | - | - | - |
| Gestational age at birth, weeks + days | 40 + 3 (39 + 2, 41 + 1) | 40 + 1 (39 + 1, 41 + 2) | - | - | 0.856 b |
| Serum glucose, mmol/L * | 5.6 (1.3) | 5.8 (1.4) | 0.2 | −0.6 to 1.0 | 0.580 a |
| Serum C peptide, nmol/L * | 0.5 (0.5, 0.7) | 0.5 (0.5, 0.6) | - | - | 0.632 b |
| Fat mass, kg ** | 0.2 (0.1, 0.3) | 0.2 (0.1, 0.3) | - | - | 0.967 b |
| Fat mass, % ** | 4.8 (2.9, 7.2) | 4.8 (2.9, 9.0) | - | - | 0.877 b |
| Fat-free mass, kg ** | 3.4 (0.5) | 3.4 (0.4) | −0.1 | −0.3 to 0.2 | 0.589 a |
| Fat-free mass, % ** | 95.2 (92.9, 97.0) | 95.2 (91.0, 97.0) | - | - | 0.948 b |
| Muscle mass, kg ** | 1.1 (0.2) | 1.2 (0.2) | −0.1 | −0.1 to 0.0 | 0.619 a |
| Hydration (TBW), % ** | 79.3 (76.2, 81.7) | 79.8 (74.2, 82.1) | - | - | 1.000 b |
Appendix A.3
| Outcome | Time | Control (n = 49) | Intervention (n = 21) | Estimate | 95% CI | p |
|---|---|---|---|---|---|---|
| Weight, kg | At birth | 3.7 (0.5) | 3.6 (0.4) | |||
| 6–8 weeks | 5.3 (0.8) | 5.2 (0.8) | −0.1 | −0.4 to 0.2 | 0.360 | |
| Fat mass, kg | At birth | 0.2 (0.2) | 0.2 (0.2) | |||
| 6–8 weeks | 1.0 (0.3) | 1.0 (0.3) | 0.0 | −0.2 to 0.1 | 0.659 | |
| Fat mass, % | At birth | 6.0 (4.4) | 6.7 (6.1) | |||
| 6–8 weeks | 18.5 (5.3) | 18.9 (4.5) | −0.5 | −3.6 to 2.6 | 0.742 | |
| Fat-free mass, kg | At birth | 3.4 (0.5) | 3.4 (0.4) | |||
| 6–8 weeks | 4.3 (0.7) | 4.2 (0.6) | −0.1 | −0.4 to 0.2 | 0.477 | |
| Fat-free mass, % | At birth | 93.8 (4.2) | 93.1 (5.9) | |||
| 6–8 weeks | 81.5 (5.4) | 81.1 (4.5) | 0.5 | −2.5 to 3.6 | 0.729 | |
| Muscle mass, kg | At birth | 1.1 (0.2) | 1.2 (0.2) | |||
| 6–8 weeks | 1.5 (0.3) | 1.5 (0.2) | −0.1 | −0.2 to 0.1 | 0.198 | |
| Hydration (TBW), % | At birth | 78.2 (5.7) | 77.3 (7.4) | |||
| 6–8 weeks | 66.1 (5.6) | 65.4 (4.4) | 0.7 | −2.7 to 4.2 | 0.678 |
Appendix A.4
| Outcome | Control (n = 53) | Intervention (n = 23) | Relative Risk | 95% CI | p |
|---|---|---|---|---|---|
| Normal vaginal delivery, n (%) | 47 (89) | 18 (78) | 0.9 | 0.7 to 1.1 | 0.292 b |
| Induced labor, n (%) | 10 (19) | 6 (26) | 1.6 | 0.7 to 3.7 | 0.346 a |
| Instrumental delivery, n (%) | 2 (4) | 2 (9) | 2.3 | 0.3 to 15.4 | 0.580 a |
| Caesarean section, n (%) | 4 (8) | 3 (13) | 1.7 | 0.4 to 7.1 | 0.668 a |
| Perineal tear *, grade 3–4, n (%) | 1 (2) | 1 (5) | - | - | - |
| Episiotomy *, n (%) | 5 (10) | 0 (0) | - | - | - |
| Shoulder dystocia, n (%) | 1 (2) | 0 (0) | - | - | - |
| Postpartum hemorrhage, n (%) | 8 (15) | 2 (9) | 0.6 | 0.1 to 2.6 | 0.714 a |
| Length of hospital stay, days | 3 (2, 4) | 3 (2, 4) | - | - | 0.358 c |
References
- World Health Organization. Obesity and Overweight; World Health Organization: Geneva, Switzerland, 2025; Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 3 September 2025).
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef] [PubMed]
- Mitanchez, D.; Ciangura, C.; Jacqueminet, S. How Can Maternal Lifestyle Interventions Modify the Effects of Gestational Diabetes in the Neonate and the Offspring? A Systematic Review of Meta-Analyses. Nutrients 2020, 12, 353. [Google Scholar] [CrossRef] [PubMed]
- Lowe, W.L.; Lowe, L.P.; Kuang, A.; Catalano, P.M.; Nodzenski, M.; Talbot, O.; Tam, W.H.; Sacks, D.A.; McCance, D.; Linder, B.; et al. Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study. Diabetologia 2019, 62, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Wendland, E.M.; Torloni, M.R.; Falavigna, M.; Trujillo, J.; Dode, M.A.; Campos, M.A.; Duncan, B.B.; Schmidt, M.I. Gestational diabetes and pregnancy outcomes—A systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth 2012, 12, 23. [Google Scholar] [CrossRef]
- Catalano, P.M.; McIntyre, H.D.; Cruickshank, J.K.; McCance, D.R.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Trimble, E.R.; Coustan, D.R.; Hadden, D.R.; et al. The Hyperglycemia and Adverse Pregnancy Outcome Study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012, 35, 780–786. [Google Scholar] [CrossRef]
- Deng, Y.; Tam, C.H.T.; Yang, A.; Shi, M.; Yuen, L.Y.; Ng, N.Y.H.; Tsang, A.Y.T.; Tsoi, K.Y.; Ozaki, R.; Li, A.M.; et al. Association of maternal overweight and gestational diabetes mellitus with offspring adiposity trajectory: From birth to early adolescence. Diabetologia 2025, 68, 2194–2204. [Google Scholar] [CrossRef]
- Armengaud, J.B.; Ma, R.C.W.; Siddeek, B.; Visser, G.H.A.; Simeoni, U. Offspring of mothers with hyperglycaemia in pregnancy: The short term and long-term impact. What is new? Diabetes Res. Clin. Pract. 2018, 145, 155–166. [Google Scholar] [CrossRef]
- Davenport, M.H.; Meah, V.L.; Ruchat, S.M.; Davies, G.A.; Skow, R.J.; Barrowman, N.; Adamo, K.B.; Poitras, V.J.; Gray, C.E.; Jaramillo Garcia, A.; et al. Impact of prenatal exercise on neonatal and childhood outcomes: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 1386–1396. [Google Scholar] [CrossRef]
- Barakat, R.; Silva-José, C.; Sánchez-Polán, M.; Zhang, D.; Lobo, P.; De Roia, G.; Montejo, R. Physical Activity during Pregnancy and Childhood Obesity: Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 3726. [Google Scholar] [CrossRef]
- Behnam, S.; Timmesfeld, N.; Arabin, B. Lifestyle Interventions to Improve Pregnancy Outcomes: A Systematic Review and Specified Meta-Analyses. Geburtshilfe Frauenheilkd. 2022, 82, 1249–1264. [Google Scholar] [CrossRef]
- Dodd, J.; Grivell, R.; Crowther, C.; Robinson, J. Antenatal interventions for overweight or obese pregnant women: A systematic review of randomised trials. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Louise, J.; Poprzeczny, A.J.; Deussen, A.R.; Vinter, C.; Tanvig, M.; Jensen, D.M.; Bogaerts, A.; Devlieger, R.; McAuliffe, F.M.; Renault, K.M.; et al. The effects of dietary and lifestyle interventions among pregnant women with overweight or obesity on early childhood outcomes: An individual participant data meta-analysis from randomised trials. BMC Med. 2021, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Thangaratinam, S.; Rogozinska, E.; Jolly, K.; Glinkowski, S.; Roseboom, T.; Tomlinson, J.W.; Kunz, R.; Mol, B.W.; Coomarasamy, A.; Khan, K.S. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: Meta-analysis of randomised evidence. BMJ 2012, 344, e2088. [Google Scholar] [CrossRef] [PubMed]
- Quinlivan, J.A.; Julania, S.; Lam, L. Antenatal Dietary Interventions in Obese Pregnant Women to Restrict Gestational Weight Gain to Institute of Medicine Recommendations: A Meta-Analysis. Obstet. Gynecol. 2011, 118, 1395. [Google Scholar] [CrossRef]
- Moholdt, T.; Hawley, J.A. Maternal Lifestyle Interventions: Targeting Preconception Health. Trends Endocrinol. Metab. 2020, 31, 561–569. [Google Scholar] [CrossRef]
- Price, S.A.L.; Sumithran, P.; Nankervis, A.J.; Permezel, M.; Prendergast, L.A.; Proietto, J. Impact of preconception weight loss on fasting glucose and pregnancy outcomes in women with obesity: A randomized trial. Obesity 2021, 29, 1445–1457. [Google Scholar] [CrossRef]
- Phelan, S.; Jelalian, E.; Coustan, D.; Caughey, A.B.; Castorino, K.; Hagobian, T.; Muñoz-Christian, K.; Schaffner, A.; Shields, L.; Heaney, C.; et al. Randomized controlled trial of prepregnancy lifestyle intervention to reduce recurrence of gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2023, 229, 158.e1–158.e14. [Google Scholar] [CrossRef]
- Rönö, K.; Stach-Lempinen, B.; Eriksson, J.G.; Pöyhönen-Alho, M.; Klemetti, M.M.; Roine, R.P.; Huvinen, E.; Andersson, S.; Laivuori, H.; Valkama, A.; et al. Prevention of gestational diabetes with a prepregnancy lifestyle intervention—Findings from a randomized controlled trial. Int. J. Women’s Health 2018, 10, 493–501. [Google Scholar] [CrossRef]
- LeBlanc, E.S.; Smith, N.X.; Vesco, K.K.; Paul, I.M.; Stevens, V.J. Weight loss prior to pregnancy and subsequent gestational weight gain: Prepare, a randomized clinical trial. Am. J. Obstet. Gynecol. 2021, 224, 99.e1–99.e14. [Google Scholar] [CrossRef]
- Che, T.; Yan, C.; Tian, D.; Zhang, X.; Liu, X.; Wu, Z. Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: A randomised controlled trial. Nutr. Metab. 2021, 18, 88. [Google Scholar] [CrossRef]
- Haganes, K.L.; Silva, C.P.; Eyjólfsdóttir, S.K.; Steen, S.; Grindberg, M.; Lydersen, S.; Hawley, J.A.; Moholdt, T. Time-restricted eating and exercise training improve HbA1c and body composition in women with overweight/obesity: A randomized controlled trial. Cell Metab. 2022, 34, 1457–1471.e4. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Ravasi, A.A.; Malandish, A.; Rosenkranz, S.K. The impact of high-intensity interval training on postprandial glucose and insulin: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2022, 186, 109815. [Google Scholar] [CrossRef] [PubMed]
- Kotarsky, C.J.; Johnson, N.R.; Mahoney, S.J.; Mitchell, S.L.; Schimek, R.L.; Stastny, S.N.; Hackney, K.J. Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiol. Rep. 2021, 9, e14868. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef]
- Anderson, J.; Pudwell, J.; McAuslan, C.; Barr, L.; Kehoe, J.; Davies, G.A. Acute fetal response to high-intensity interval training in the second and third trimesters of pregnancy. Appl. Physiol. Nutr. Metab. 2021, 46, 1552–1558. [Google Scholar] [CrossRef]
- Beetham, K.S.; Giles, C.; Noetel, M.; Clifton, V.; Jones, J.C.; Naughton, G. The effects of vigorous intensity exercise in the third trimester of pregnancy: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2019, 19, 281. [Google Scholar] [CrossRef]
- Wowdzia, J.B.; Hazell, T.J.; Davenport, M.H. Glycemic response to acute high-intensity interval versus moderate-intensity continuous exercise during pregnancy. Physiol. Rep. 2022, 10, e15454. [Google Scholar] [CrossRef]
- Sujan, A.J.; Skarstad, H.M.S.; Rosvold, G.; Fougner, S.L.; Nyrnes, S.A.; Iversen, A.-C.; Follestad, T.; Salvesen, K.Å.; Moholdt, T. Randomised controlled trial of preconception lifestyle intervention on maternal and offspring health in people with increased risk of gestational diabetes: Study protocol for the BEFORE THE BEGINNING trial. BMJ Open 2023, 13, e073572. [Google Scholar] [CrossRef]
- Sujan, M.J.; Skarstad, H.M.; Rosvold, G.; Fougner, S.L.; Follestad, T.; Salvesen, K.Å.; Moholdt, T. Time restricted eating and exercise training before and during pregnancy for people with increased risk of gestational diabetes: Single centre randomised controlled trial (BEFORE THE BEGINNING). BMJ 2025, 390, e083398. [Google Scholar] [CrossRef]
- Norwegian Directorate of Health. Offer Glucose Challenge to Pregnant Women in Weeks 24–28 with One or More Characteristics (Age, Ethnicity, Heredity, Body Mass Index and Events in Previous Pregnancies) [Internet]. Oslo: Norwegian Directorate of Health 2019. Available online: https://www.helsedirektoratet.no/retningslinjer/svangerskapsdiabetes/diagnostikk-og-tiltak-for-a-finne-uoppdaget-diabetes-og-svangerskapsdiabetes/tilby-glukosebelastning-til-gravide-i-uke-24-28-med-en-eller-flere-karakteristika-alder-etnisitet-arvelighet-kroppsmassainedeks-og-hendenser-i-tidligere-svangerskap (accessed on 24 September 2025).
- Nes, B.M.; Gutvik, C.R.; Lavie, C.J.; Nauman, J.; Wisløff, U. Personalized Activity Intelligence (PAI) for Prevention of Cardiovascular Disease and Promotion of Physical Activity. Am. J. Med. 2017, 130, 328–336. [Google Scholar] [CrossRef]
- American Academy of Pediatrics Committee on Fetus and Newborn; American College of Obstetricians and Gynecologists Committee on Obstetric Practice; Watterberg, K.L.; Aucott, S.; Benitz, W.E.; Cummings, J.J.; Eichenwald, E.C.; Goldsmith, J.; Poindexter, B.B.; Puopolo, K.; et al. The Apgar Score. Pediatrics 2015, 136, 819–822. [Google Scholar] [CrossRef]
- Hooper, R. To adjust, or not to adjust, for multiple comparisons. J. Clin. Epidemiol. 2025, 180, 111688. [Google Scholar] [CrossRef]
- Baroni, N.F.; Baldoni, N.R.; Alves, G.C.S.; Crivellenti, L.C.; Braga, G.C.; Sartorelli, D.S. Do Lifestyle Interventions in Pregnant Women with Overweight or Obesity Have an Effect on Neonatal Adiposity? A Systematic Review with Meta-Analysis. Nutrients 2021, 13, 1903. [Google Scholar] [CrossRef]
- Kampmann, U.; Suder, L.B.; Nygaard, M.; Geiker, N.R.W.; Nielsen, H.S.; Almstrup, K.; Bruun, J.M.; Magkos, F.; Ovesen, P.; Catalano, P. Prepregnancy and Gestational Interventions to Prevent Childhood Obesity. J. Clin. Endocrinol. Metab. 2024, 110, e8–e18. [Google Scholar] [CrossRef]
- Mogensen, C.S.; Zingenberg, H.; Svare, J.; Astrup, A.; Magkos, F.; Geiker, N.R.W. Gestational weight gain in women with pre-pregnancy overweight or obesity and anthropometry of infants at birth. Front. Pediatr. 2023, 11, 1142920. [Google Scholar] [CrossRef]
- Vinter, C.A.; Jensen, D.M.; Ovesen, P.; Beck-Nielsen, H.; Jørgensen, J.S. The LiP (Lifestyle in Pregnancy) study: A randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care 2011, 34, 2502–2507. [Google Scholar] [CrossRef] [PubMed]
- Dubé, M.C.; Morisset, A.S.; Tchernof, A.; Weisnagel, S.J. Cord blood C-peptide levels relate to the metabolic profile of women with and without gestational diabetes. Acta Obstet. Gynecol. Scand. 2012, 91, 1469–1473. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Fraser, L.M.; Sundernathan, T.; Deussen, A.R.; Louise, J.; Yelland, L.N.; Grivell, R.M.; Macpherson, A.; Gillman, M.W.; Robinson, J.S.; et al. The effect of an antenatal lifestyle intervention in overweight and obese women on circulating cardiometabolic and inflammatory biomarkers: Secondary analyses from the LIMIT randomised trial. BMC Med. 2017, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Hellmuth, C.; Uhl, O.; Godfrey, K.; Briley, A.; Welsh, P.; Pasupathy, D.; Seed, P.T.; Koletzko, B.; Poston, L.; et al. Cord Metabolic Profiles in Obese Pregnant Women: Insights Into Offspring Growth and Body Composition. J. Clin. Endocrinol. Metab. 2018, 103, 346–355. [Google Scholar] [CrossRef]
- Baroni, N.F.; Carvalho, M.R.; da Silva Santos, I.; Chaves, A.V.L.; de Andrade Miranda, D.E.G.; Crivellenti, L.C.; Sartorelli, D.S. Effect of a lifestyle intervention among pregnant women with overweight on neonatal adiposity: A randomized controlled clinical trial. Early Hum. Dev. 2024, 194, 106038. [Google Scholar] [CrossRef]
- Dodd, J.; Deussen, A.; Mohamad, I.; Rifas-Shiman, S.; Yelland, L.; Louise, J.; McPhee, A.; Grivell, R.; Owens, J.; Gillman, M.; et al. The effect of antenatal lifestyle advice for women who are overweight or obese on secondary measures of neonatal body composition: The LIMIT randomised trial. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Rosenn, B.; Toro-Ramos, T.; Paley, C.; Gidwani, S.; Horowitz, M.; Crane, J.; Lin, S.; Thornton, J.C.; Pi-Sunyer, X. Greater neonatal fat-free mass and similar fat mass following a randomized trial to control excess gestational weight gain. Obesity 2018, 26, 578–587. [Google Scholar] [CrossRef] [PubMed]
- van Poppel, M.N.M.; Simmons, D.; Devlieger, R.; van Assche, F.A.; Jans, G.; Galjaard, S.; Corcoy, R.; Adelantado, J.M.; Dunne, F.; Harreiter, J.; et al. A reduction in sedentary behaviour in obese women during pregnancy reduces neonatal adiposity: The DALI randomised controlled trial. Diabetologia 2019, 62, 915–925. [Google Scholar] [CrossRef]
- Van Horn, L.; Peaceman, A.; Kwasny, M.; Vincent, E.; Fought, A.; Josefson, J.; Spring, B.; Neff, L.M.; Gernhofer, N. Dietary Approaches to Stop Hypertension Diet and Activity to Limit Gestational Weight: Maternal Offspring Metabolics Family Intervention Trial, a Technology Enhanced Randomized Trial. Am. J. Prev. Med. 2018, 55, 603–614. [Google Scholar] [CrossRef]
- Thajer, A.; Vasek, M.; Schneider, S.; Kautzky-Willer, A.; Kainberger, F.; Durstberger, S.; Kranzl, A.; Horsak, B.; Greber-Platzer, S. Comparing Bioelectrical Impedance Analysis, Air Displacement Plethysmography, and Dual-Energy X-Ray Absorptiometry for Body Composition in Pediatric Obesity. Nutrients 2025, 17, 971. [Google Scholar] [CrossRef]
- Tortorella CCda, S.; Chao, B.M.P.; Rabito, E.I.; Lima, M.N.; Sarquis, A.L.F. Bioelectrical Impedance in Premature Newborns and Its Relationship with Diet Therapy in a Neonatal Intensive Care Unit. Nutrients 2024, 16, 601. [Google Scholar] [CrossRef]
- Lyons-Reid, J.; Derraik, J.G.B.; Ward, L.C.; Tint, M.T.; Kenealy, T.; Cutfield, W.S. Bioelectrical impedance analysis for assessment of body composition in infants and young children-A systematic literature review. Clin. Obes. 2021, 11, e12441. [Google Scholar] [CrossRef]
- Carberry, A.E.; Colditz, P.B.; Lingwood, B.E. Body Composition from Birth to 4.5 Months in Infants Born to Non-Obese Women. Pediatr. Res. 2010, 68, 84–88. [Google Scholar] [CrossRef]
- Butte, N.F.; Wong, W.W.; Hopkinson, J.M.; Smith, E.O.; Ellis, K.J. Infant Feeding Mode Affects Early Growth and Body Composition. Pediatrics 2000, 106, 1355–1366. [Google Scholar] [CrossRef]
- Breij, L.M.; Abrahamse-Berkeveld, M.; Acton, D.; De Lucia Rolfe, E.; Ong, K.K.; Hokken-Koelega, A.C.S. Impact of early infant growth, duration of breastfeeding and maternal factors on total body fat mass and visceral fat at 3 and 6 months of age. Ann. Nutr. Metab. 2017, 71, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Giannì, M.L.; Roggero, P.; Morlacchi, L.; Garavaglia, E.; Piemontese, P.; Mosca, F. Formula-fed infants have significantly higher fat-free mass content in their bodies than breastfed babies. Acta Paediatr. 2014, 103, e277–e281. [Google Scholar] [CrossRef]
- Amati, F.; McCann, L.; Castañeda-Gutiérrez, E.; Prior, E.; van Loo-Bouwman, C.A.; Abrahamse-Berkeveld, M.; Oliveros, E.; Ozanne, S.; Symonds, M.E.; Chang, C.-Y.; et al. Infant fat mass and later child and adolescent health outcomes: A systematic review. Arch. Dis. Child. 2024, 109, 125–129. [Google Scholar] [CrossRef]
- Viswanathan, S.; Thoene, M.; Alja’nini, Z.; Alur, P.; McNelis, K. Body Composition in Preterm Infants: Current Insights and Emerging Perspectives. Children 2025, 12, 53. [Google Scholar] [CrossRef]

| Control (n = 53) | Intervention (n = 53) | |
|---|---|---|
| Age, years | 29.5 (3.1) | 29.8 (3.4) |
| Weight, kg | 80.0 (12.8) | 80.8 (15.9) |
| Body mass index, kg/m2 | 28.5 (4.4) | 29.1 (4.9) |
| Waist circumference, cm | 93.3 (11.2) | 92.5 (12.9) |
| Fat percentage, % | 37.1 (7.1) | 36.3 (7.9) |
| Systolic blood pressure, mmHg | 120 (10) | 119 (8) |
| Diastolic blood pressure, mmHg | 79 (7) | 79 (6) |
| HbA1c, mmol/mol | 34.1 (2.8) | 34.3 (2.8) |
| Fasting glucose, mmol/L | 5.0 (0.4) | 5.0 (0.4) |
| Total cholesterol, mmol/L | 4.5 (0.8) | 4.6 (0.8) |
| HDL cholesterol, mmol/L | 1.4 (0.3) | 1.4 (0.3) |
| LDL cholesterol, mmol/L | 3.0 (0.8) | 3.0 (0.8) |
| Triglycerides, mmol/L | 1.0 (0.5) | 1.0 (0.6) |
| Parity, total number of births, n (%) | ||
| 0 | 27 (51) | 27 (51) |
| 1 | 20 (38) | 23 (43) |
| 2 | 6 (11) | 3 (6) |
| Education level, n (%) | ||
| Completed compulsory schooling and upper secondary school | 5 (9) | 10 (19) |
| Completed university education, less than 4 years | 18 (34) | 14 (26) |
| Completed university education, 4 years or more | 30 (57) | 29 (55) |
| Ethnic origin, n (%) | ||
| Europe | 47 (89) | 47 (89) |
| Africa and Middle East | 2 (4) | 0 (0) |
| Asia | 3 (6) | 4 (8) |
| North America | 0 (0) | 1 (2) |
| Latin America | 1 (2) | 1 (2) |
| Reason for inclusion *, n (%) | ||
| Body mass index ≥ 25 kg/m2 | 45 (85) | 44 (83) |
| GDM in a previous pregnancy | 1 (2) | 1 (2) |
| Family history of diabetes | 14 (26) | 15 (28) |
| Previous newborn > 4.5 kg | 0 (0) | 1 (2) |
| Outcome | Control (n = 53) | Intervention (n = 53) | Estimate | 95% CI | p |
|---|---|---|---|---|---|
| Weight, g | 3702.5 (517.8) | 3543.3 (602.7) | −159.3 | −375.7 to 57.2 | 0.148 a |
| Length, cm | 50 (49, 51) | 50 (49, 52) | - | - | 0.930 b |
| Head circumference, cm | 36.0 (35, 37) | 35 (35, 37) | - | - | 0.302 b |
| Birth weight > 4 kg, n (%) | 15 (28) | 11 (21) | 0.7 | 0.4 to 1.4 | 0.367 c |
| APGAR < 7 at 5 min, n (%) | 1 (2) | 0 (0) | - | - | - |
| Preterm birth, n (%) | 1 (2) | 2 (4) | - | - | - |
| Gestational age at birth, weeks + days | 40 + 3 (39 + 2, 41 + 1) | 40 + 0 (39 + 0, 41 + 0) | - | - | 0.319 b |
| Serum glucose, mmol/L * | 5.6 (1.3) | 5.6 (1.3) | 0.0 | −0.6 to 0.6 | 0.997 a |
| Serum insulin C-peptide, nmol/L * | 0.5 (0.5, 0.7) | 0.5 (0.5, 0.6) | - | - | 0.367 b |
| Fat mass, kg ** | 0.2 (0.1, 0.3) | 0.2 (0.1, 0.3) | - | - | 0.690 b |
| Fat mass, % ** | 4.8 (2.9, 7.2) | 5.5 (3.6, 8.0) | - | - | 0.622 b |
| Fat-free mass, kg ** | 3.5 (1.5) | 3.3 (1.5) | −0.1 | −0.3 to 0.1 | 0.218 a |
| Fat-free mass, % ** | 95.2 (92.9, 97.0) | 94.5 (92.0, 96.4) | - | - | 0.605 b |
| Muscle mass, kg ** | 1.1 (0.2) | 1.1 (0.2) | −0.1 | −0.1 to 0.0 | 0.196 a |
| Hydration (TBW), % ** | 79.6 (76.3, 81.8) | 78.6 (75.6, 81.2) | - | - | 0.449 b |
| Outcome | Control (n = 53) | Intervention (n = 53) | Relative Risk | 95% CI | p |
|---|---|---|---|---|---|
| Normal vaginal delivery, n (%) | 47 (89) | 42 (79) | 0.9 | 0.8 to 1.1 | 0.186 b |
| Induced labor, n (%) | 10 (19) | 11 (21) | 1.1 | 0.5 to 2.4 | 0.807 b |
| Instrumental delivery *, n (%) | 2 (4) | 2 (4) | - | - | - |
| Caesarean section, n (%) | 4 (8) | 9 (17) | 2.3 | 0.7 to 6.9 | 0.139 b |
| Perineal tear *, grade 3–4, n (%) | 1 (2) | 1 (2) | - | - | - |
| Episiotomy *, n (%) | 5 (10) | 2 (5) | 0.4 | 0.1 to 2.2 | 0.440 a |
| Shoulder dystocia, n (%) | 1 (2) | 0 (0) | - | - | - |
| Postpartum hemorrhage, n (%) | 8 (15) | 6 (11) | 0.8 | 0.3 to 2.0 | 0.566 a |
| Length of hospital stay, days | 3 (2, 4) | 3 (2, 4) | - | - | 0.247 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sujan, M.A.J.; Skarstad, H.; Rosvold, G.; Fougner, S.L.; Follestad, T.; Nyrnes, S.A.; Salvesen, K.; Moholdt, T. Neonatal Outcomes Following a Preconception Lifestyle Intervention in People at Risk of Gestational Diabetes: Secondary Findings from the BEFORE THE BEGINNING Randomized Controlled Trial. Nutrients 2025, 17, 3492. https://doi.org/10.3390/nu17213492
Sujan MAJ, Skarstad H, Rosvold G, Fougner SL, Follestad T, Nyrnes SA, Salvesen K, Moholdt T. Neonatal Outcomes Following a Preconception Lifestyle Intervention in People at Risk of Gestational Diabetes: Secondary Findings from the BEFORE THE BEGINNING Randomized Controlled Trial. Nutrients. 2025; 17(21):3492. https://doi.org/10.3390/nu17213492
Chicago/Turabian StyleSujan, Md Abu Jafar, Hanna Skarstad, Guro Rosvold, Stine Lyngvi Fougner, Turid Follestad, Siri Ann Nyrnes, Kjell Salvesen, and Trine Moholdt. 2025. "Neonatal Outcomes Following a Preconception Lifestyle Intervention in People at Risk of Gestational Diabetes: Secondary Findings from the BEFORE THE BEGINNING Randomized Controlled Trial" Nutrients 17, no. 21: 3492. https://doi.org/10.3390/nu17213492
APA StyleSujan, M. A. J., Skarstad, H., Rosvold, G., Fougner, S. L., Follestad, T., Nyrnes, S. A., Salvesen, K., & Moholdt, T. (2025). Neonatal Outcomes Following a Preconception Lifestyle Intervention in People at Risk of Gestational Diabetes: Secondary Findings from the BEFORE THE BEGINNING Randomized Controlled Trial. Nutrients, 17(21), 3492. https://doi.org/10.3390/nu17213492







