Berries derived Polyphenols and Bone Health: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
- -
- Population: in human studies are included premenopausal women, twin pairs women and postmenopausal women (with or without osteoporosis/osteopenia…), in vivo studies involve OVX female rats to simulated age related bone loss, 20 days olds Sprague-Dawley rats to study early bone growth and young and old female C57BL/6J mice. In vitro studies include human bone marrow cells (pre-osteoclastic, CD34+, CD133), mouse bone marrow macrophages (BMMs), RAW264.7 cell line (a model for osteoclast studies), stromal cells from human exfoliated deciduous teeth (SHED) and MC3T3-E1 osteoblast.
- -
- Intervention/Exposure: The studied exposures take into account the consumption of berries or the supplementation of specific polyphenols/berries extracts such as: Blueberry (blueberry extract, freeze dried blueberry powder, phenolic acids derived from blueberries), raspberry (whole fruit and specific fraction of rubus tozawae RL-Hex-NF3), blackcurrant (blackcurrant extract, powder and specific anthocyanins), blackberry (blackberries rich in cyanidin 3-O-β-D-glucoside and blackberries anthocyanins), cranberry (extract and A-type cranberry proanthocyanidins). The classes of polyphenols or specific compound involved in the studies are Flavonoids (catechin, procyanidin, flavanones, anthocyanins (such as pelargonidin, cyanidin, delphinidin, peonidin, petunidin, malvidin), Phenolic Acids (hydroxycinnamic acids (caffeic, ferulic), hydroxybenzoic acids (p-hydroxybenzoic, gallic, salicylic, vanillic, ellagic), Tannins (hydrolysable and ellagic tannins, condensed tannins), Petunidin.
- -
- Comparators: used in these studies include placebo (for clinical trials), control groups (Sham, in animal models), no treatment (for clinical and animal studies), different doses, standard pharmacological treatments (for example estradiol and progesterone as a positive control in animal models) and baseline (measurements at the start of the study compared to the end).
- -
- Outcomes: In this review, ‘relevant outcomes’ were pre-specified as: PRIMARY—areal BMD (DXA at lumbar spine, total hip, femoral neck, whole-body) and circulating bone turnover biomarkers (formation: P1NP, osteocalcin; resorption: CTX); SECONDARY—bone microarchitecture (e.g., BV/TV, Tb.N, Tb.Th), bone strength, calcium balance/retention, and molecular/cellular endpoints (e.g., RANKL/OPG, osteoclast/osteoblast assays). Human clinical outcomes were interpreted separately from animal and in vitro findings; no statistical pooling across models was attempted.
2.3. Search Strategy
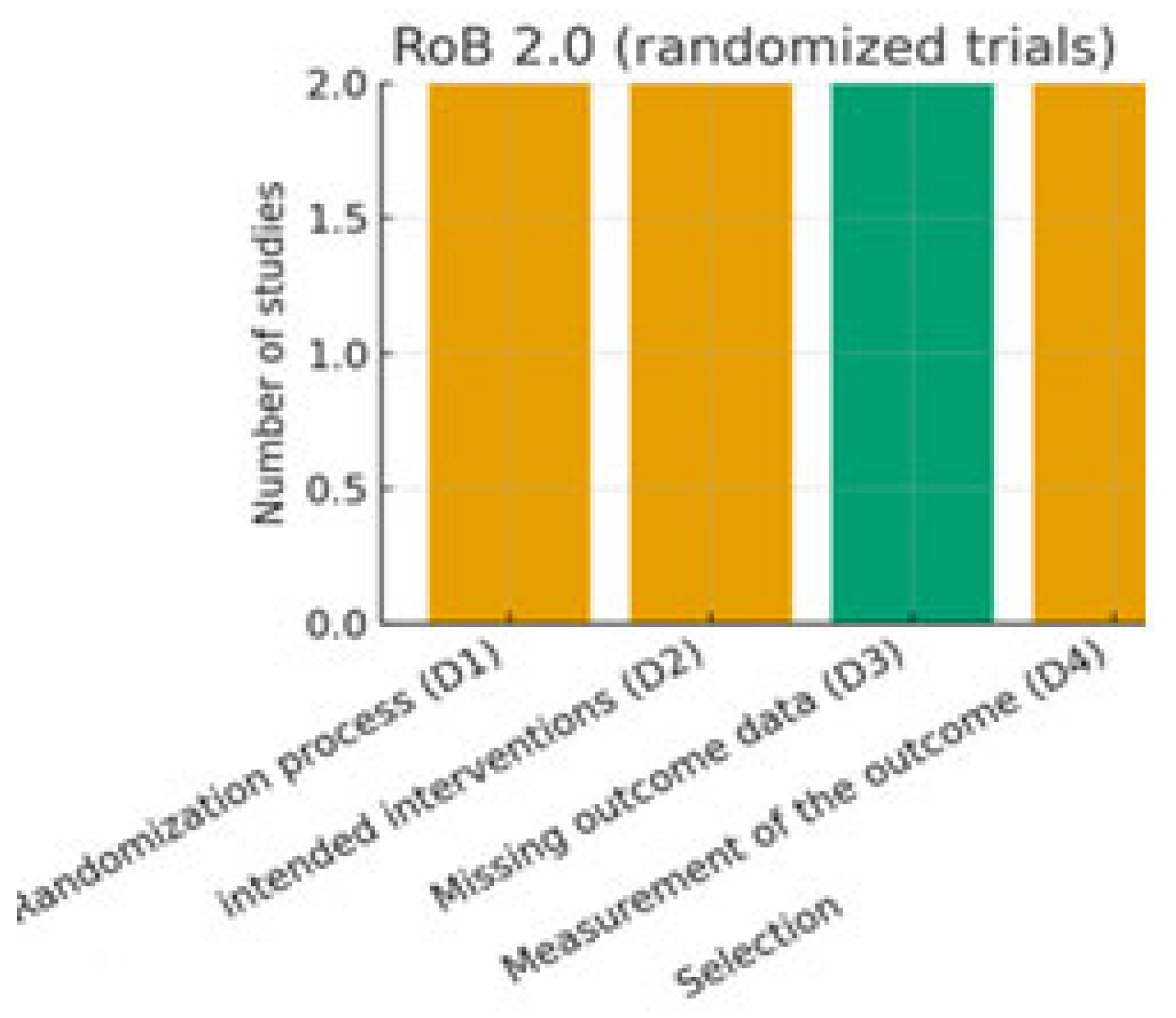
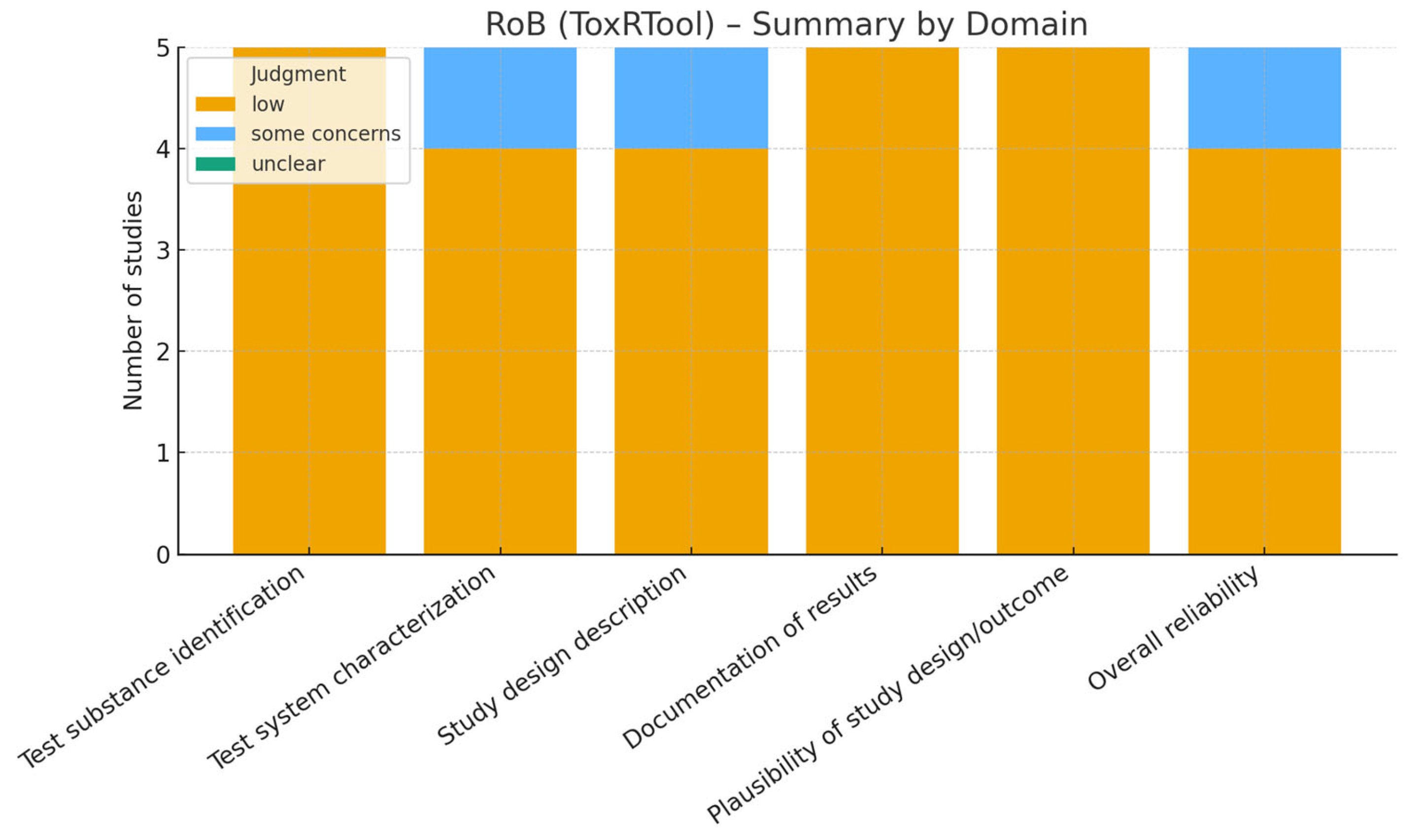
2.4. Risk of Bias Assessment
- -
- Randomized controlled trials (RCTs): assessed using the revised Cochrane Risk of Bias tool for randomized trials (RoB 2.0) [10]. This tool evaluates domains such as the randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of the reported results.
- -
- Non-randomized and observational studies: assessed using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool [11], which considers confounding, selection bias, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selective reporting.
- -
- Animal studies: assessed with the SYRCLE Risk of Bias tool [12], specifically developed for preclinical studies, which adapts the Cochrane domains to the context of animal experiments, including allocation concealment, blinding, and incomplete outcome data.
- -
- In vitro studies: since no standardized tool exists, we applied an adapted version of the ToxRTool (Toxicological data Reliability Assessment Tool), which evaluates the reliability of toxicological and mechanistic studies based on reporting quality, methodological adequacy, and plausibility of results [13]. Studies were classified as “reliable without restriction,” “reliable with restrictions,” or “not reliable.”
3. Results
3.1. Characteristics of Included Studies
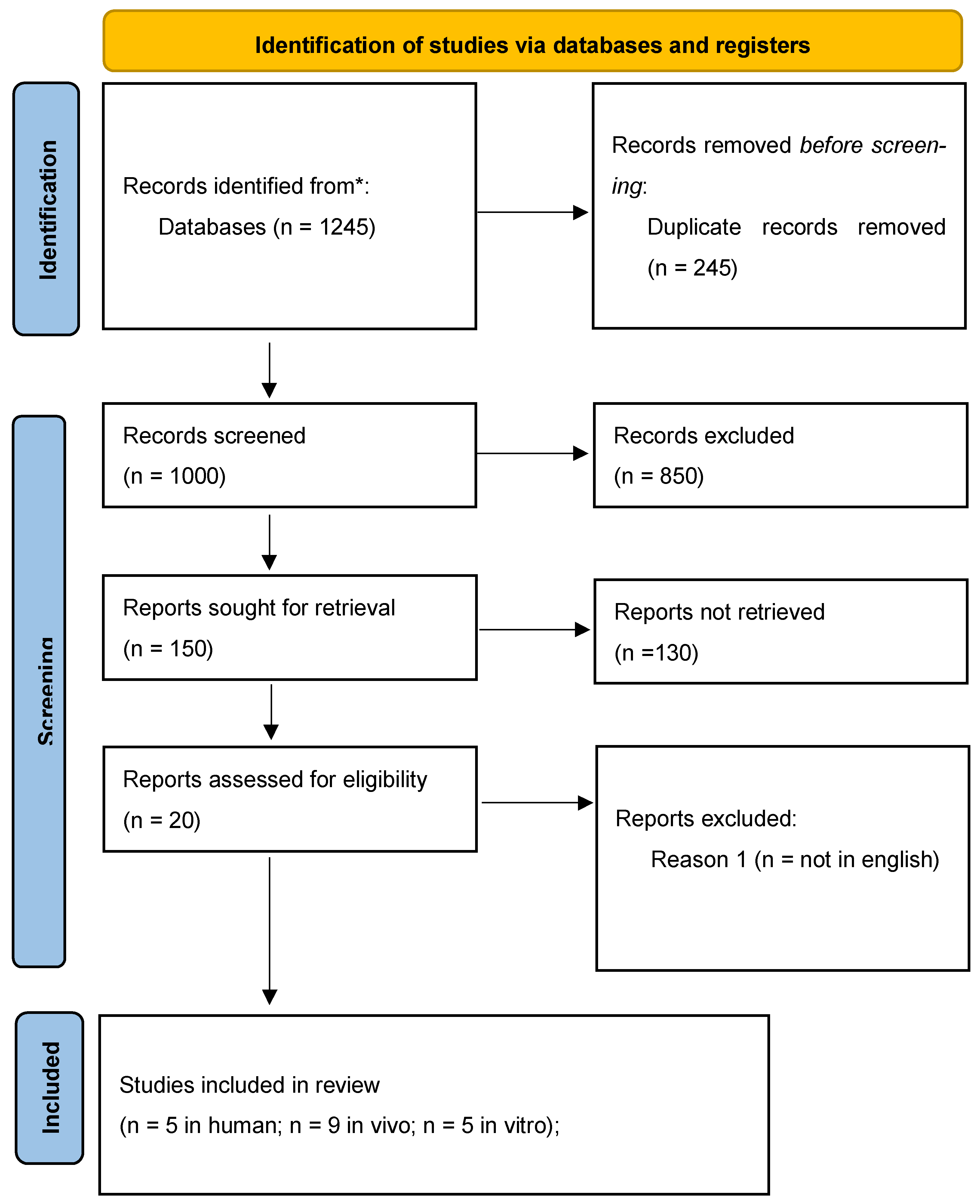
3.2. Human Studies
3.3. Animal Studies
3.4. In Vitro Studies
3.5. Risk of Bias

3.6. GRADE Assestment for Human Studies
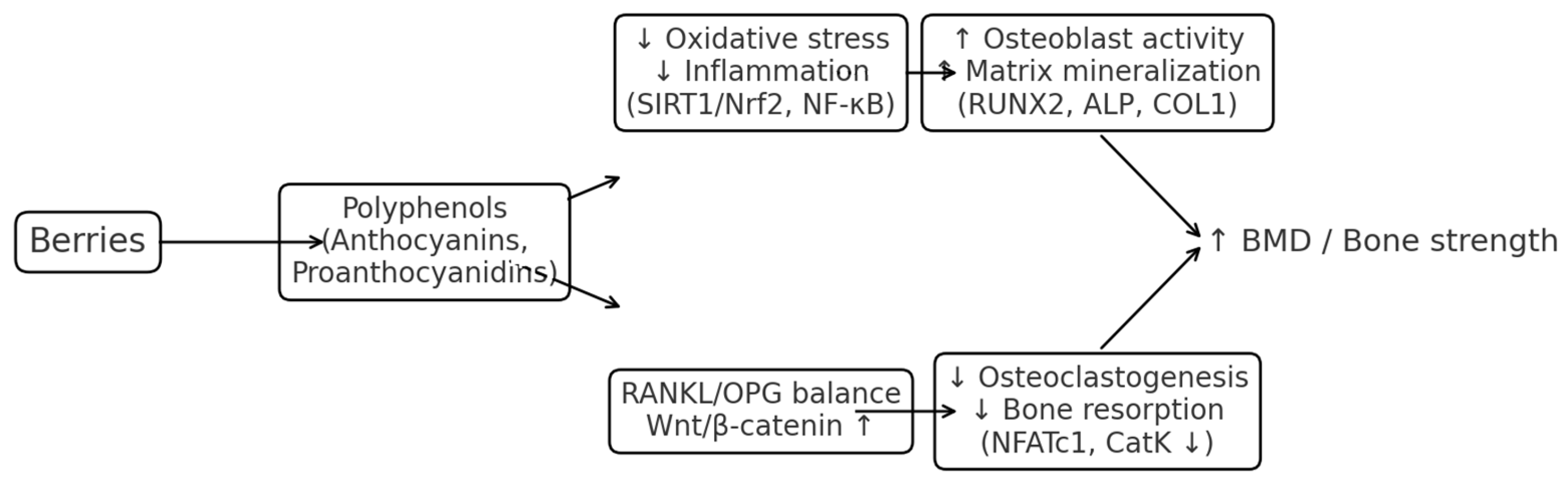
4. Discussion
4.1. Limitations
4.2. Future Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamidi, M.; Boucher, B.A.; Cheung, A.M.; Beyene, J.; Shah, P.S. Fruit and vegetable intake and bone health in women aged 45 years and over: A systematic review. Osteoporos. Int. 2011, 22, 1681–1693. [Google Scholar] [CrossRef]
- Brondani, J.E.; Comim, F.V.; Flores, L.M.; Martini, L.A.; Premaor, M.O. Fruit and vegetable intake and bones: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0217223. [Google Scholar] [CrossRef]
- Salvio, G.; Ciarloni, A.; Gianfelice, C.; Lacchè, F.; Sabatelli, S.; Giacchetti, G.; Balercia, G. The Effects of Polyphenols on Bone Metabolism in Postmenopausal Women: Systematic Review and Meta-Analysis of Randomized Control Trials. Antioxidants 2023, 12, 1830. [Google Scholar] [CrossRef]
- Feng, R.C.; Dong, Y.H.; Hong, X.L.; Su, Y.; Wu, X.V. Effects of anthocyanin-rich supplementation on cognition of the cognitively healthy middle-aged and older adults: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2023, 81, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Avantario, P.; Azzollini, D.; Buongiorno, S.; Viapiano, F.; Campanelli, M.; Ciocia, A.M.; De Leonardis, N.; et al. Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism-A Systematic Review. Nutrients 2022, 14, 3519. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Chen, J.; Chen, M.; Lin, S.; Dong, S. Protective effect and possible mechanisms of resveratrol in animal models of osteoporosis: A preclinical systematic review and meta-analysis. Phytother. Res. PTR 2023, 37, 5223–5242. [Google Scholar] [CrossRef] [PubMed]
- Shuid, A.N.; Abdul Nasir, N.A.; Ab Azis, N.; Shuid, A.N.; Razali, N.; Ahmad Hairi, H.; Mohd Miswan, M.F.; Naina Mohamed, I. A Systematic Review on the Molecular Mechanisms of Resveratrol in Protecting Against Osteoporosis. Int. J. Mol. Sci. 2025, 26, 2893. [Google Scholar] [CrossRef]
- Bellavia, D.; Caradonna, F.; Dimarco, E.; Costa, V.; Carina, V.; De Luca, A.; Raimondi, L.; Fini, M.; Gentile, C.; Giavaresi, G. Non-flavonoid polyphenols in osteoporosis: Preclinical evidence. Trends Endocrinol. Metab. TEM 2021, 32, 515–529. [Google Scholar] [CrossRef]
- Zeraattalab-Motlagh, S.; Ghoreishy, S.M.; Arab, A.; Mahmoodi, S.; Hemmati, A.; Mohammadi, H. Fruit and Vegetable Consumption and the Risk of Bone Fracture: A Grading of Recommendations, Assessment, Development, and Evaluations (GRADE)-Assessed Systematic Review and Dose-Response Meta-Analysis. JBMR Plus 2023, 7, e10840. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Schwarz, M.; Burkholder, I.; Kopp-Schneider, A.; Edler, L.; Kinsner-Ovaskainen, A.; Hartung, T.; Hoffmann, S. ToxRTool—A new tool to assess the reliability of toxicological data. ALTEX 2009, 26, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, A.C.; Aucott, L.; Reid, D.M.; Macdonald, H.M. Associations between dietary flavonoid intakes and bone health in a Scottish population. J. Bone Miner. Res. 2011, 26, 941–947. [Google Scholar] [CrossRef]
- Welch, A.; MacGregor, A.; Jennings, A.; Fairweather-Tait, S.; Spector, T.; Cassidy, A. Habitual flavonoid intakes are positively associated with bone mineral density in women. J. Bone Miner. Res. 2012, 27, 1872–1878. [Google Scholar] [CrossRef]
- Nosal, B.M.; Sakaki, J.R.; Macdonald, Z.; Mahoney, K.; Kim, K.; Madore, M.; Thornton, S.; Tran, T.D.B.; Weinstock, G.; Lee, E.C.-H.; et al. Blackcurrants reduce the risk of postmenopausal osteoporosis: A pilot double-blind, randomized, placebo-controlled clinical trial. Nutrients 2022, 14, 4971. [Google Scholar] [CrossRef]
- Hatcher, K. The Effect of Whole Red Raspberry Juice on Bone Density and Biomarkers of Bone in Postmenopausal Osteopenic Women. Doctoral Dissertation, Texas Woman’s University, Denton, TX, USA, 2017. [Google Scholar]
- Hodges, J.K.; Maiz, M.; Cao, S.; Lachcik, P.J.; Peacock, M.; McCabe, G.P.; McCabe, L.D.; Cladis, D.P.; Jackson, G.S.; Ferruzzi, M.G.; et al. Moderate consumption of freeze-dried blueberry powder increased net bone calcium retention compared with no treatment in healthy postmenopausal women: A randomized crossover trial. Am. J. Clin. Nutr. 2023, 118, 382–390. [Google Scholar] [CrossRef]
- Li, T.; Wu, S.M.; Xu, Z.Y.; Ou-Yang, S. Rabbiteye blueberry prevents osteoporosis in ovariectomized rats. J. Orthop. Surg. Res. 2014, 9, 56. [Google Scholar] [CrossRef]
- Zheng, X.; Mun, S.; Gil Lee, S.; Vance, T.M.; Hubert, P.; Koo, S.I.; Lee, S.-K.; Chun, O.K. Anthocyanin-rich blackcurrant extract attenuates ovariectomy-induced bone loss in mice. J. Med. Food 2016, 19, 390–397. [Google Scholar] [CrossRef]
- Melough, M.M.; Sun, X.; Chun, O.K. The role of AOPP in age-related bone loss and the potential benefits of berry anthocyanins. Nutrients 2017, 9, 789. [Google Scholar] [CrossRef]
- Sakaki, J.; Melough, M.; Lee, S.G.; Kalinowski, J.; Koo, S.I.; Lee, S.K.; Chun, O.K. Blackcurrant supplementation improves trabecular bone mass in young but not aged mice. Nutrients 2018, 10, 1671. [Google Scholar] [CrossRef]
- Kaume, L.; Gilbert, W.C.; Brownmiller, C.; Howard, L.R.; Devareddy, L. Cyanidin 3-O-β-D-glucoside-rich blackberries modulate hepatic gene expression, and anti-obesity effects in ovariectomized rats. J. Funct. Foods 2012, 4, 480–488. [Google Scholar] [CrossRef]
- Hong, S.; Kwon, J.; Song, S.; Park, I.; Jung, D.S.; Saruul, E.; Nho, C.W.; Kwon, H.C.; Yoo, G. Suppressive Effects of Geoje Raspberry (Rubus tozawae Nakai ex JY Yang) on Post-Menopausal Osteoporosis via Its Osteogenic Activity on Osteoblast Differentiation. Nutrients 2024, 16, 3856. [Google Scholar] [CrossRef] [PubMed]
- Devareddy, L.; Hooshmand, S.; Collins, J.K.; Lucas, E.A.; Chai, S.C.; Arjmandi, B.H. Blueberry prevents bone loss in ovariectomized rat model of postmenopausal osteoporosis. J. Nutr. Biochem. 2008, 19, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Lazarenko, O.P.; Wu, X.; Kang, J.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J. Dietary-induced serum phenolic acids promote bone growth via p38 MAPK/beta-catenin canonical WNT signaling. J. Bone Miner. Res. 2010, 25, 2399–2411. [Google Scholar] [CrossRef]
- Zhang, J.; Lazarenko, O.P.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J.; Chen, J.R. Feeding blueberry diets in early life prevent senescence of osteoblasts and bone loss in ovariectomized adult female rats. PLoS ONE 2011, 6, e24486. [Google Scholar] [CrossRef]
- Tanabe, S.; Santos, J.; La, V.D.; Howell, A.B.; Grenier, D. A-type cranberry proanthocyanidins inhibit the RANKL-dependent differentiation and function of human osteoclasts. Molecules 2011, 16, 2365–2374. [Google Scholar] [CrossRef]
- Bickford, P.C.; Tan, J.; Shytle, R.D.; Sanberg, C.D.; El-Badri, N.; Sanberg, P.R. Nutraceuticals synergistically promote proliferation of human stem cells. Stem Cells Dev. 2006, 15, 118–123. [Google Scholar] [CrossRef]
- Bauer, Y.G.; Magini, E.B.; Farias, I.V.; Neto, J.D.P.; Fongaro, G.; Reginatto, F.H.; Silva, I.T.; Cruz, A.C.C. Potential of Cranberry to Stimulate Osteogenesis: An In Vitro Study. Coatings 2024, 14, 1352. [Google Scholar] [CrossRef]
- Nagaoka, M.; Maeda, T.; Moriwaki, S.; Nomura, A.; Kato, Y.; Niida, S.; Kruger, M.C.; Suzuki, K. Petunidin, a B-ring 5′-O-Methylated Derivative of Delphinidin, Stimulates Osteoblastogenesis and Reduces sRANKL-Induced Bone Loss. Int. J. Mol. Sci. 2019, 20, 2795. [Google Scholar] [CrossRef]
| Paper | Area of Interest | Compound | Sample | Dosage/Posology | Mechanism | Main Results |
|---|---|---|---|---|---|---|
| Hardcastle et al., 2011 [14] | Bone Health (BMD) measurement at femoral neck and lumbar spine) | Flavonoids (Catechin, Procyanidin, Flavanones) | Premenopausal women (n = 2929) | Diets via FFQ analyzed for flavonoid intake | Catechin e procyanidin: associated with increased BMD; Flavanones: no effect | Catechin and procyanidin associated with increased BMD; Flavanones showed no effect |
| Welch et al., 2012 [15] | Association between habitual flavonoid intake and BMD | Flavonoids (anthocyanins, flavanones) | Women (twins) (n = 3160) | Habitual intake assessed via FFQ | Not specified (observational) | Anthocyanins associated with the highest observed BMD; high flavanone intake positively associated with hip BMD |
| Nosal et al., 2022 [16] | Postmenopausal osteoporosis | Blackcurrant powder | Randomized, double-blind, placebo-controlled clinical trial on postmenopausal women (n = 40 in the final analysis). Groups: placebo (Control, n = 13), 392 mg/day (low BC, n = 16), 784 mg/day (high BC, n = 11) | 392 mg/day and 784 mg/day for 6 months | Not specified in detail | Changing in whole body bone mineral (BMD) from baseline after 6 months and effect of different doses of blackcurrant powder on bone metabolism biomarkers and immuno-inflammatory status. |
| Hatcher, 2017 [17] | Bone health, osteoporosis, bone mineral density (BMD) | Raspberry | Postmenopausal women (placebo and raspberry groups) | Quantity not specified | Effects on bone formation and resorption biomarkers | Effects on bone mineral density of the radius, femoral, neck and total body baseline and at the end of the study |
| Hodges et al., 2023 [18] | Bone calcium balance, net bone calcium retention | Freeze-dried blueberry (BB) | Clinical study with 14 postmenopausal women | Human study: low (17.5 g/day), medium (35 g/day), and high (70 g/day) of BB powder | Measurement of urinary 41Ca:Ca ratio in women to assess net bone calcium retention. Secondary assessment of bone metabolism biomarker | increase in net bone calcium retention with low and medium doses of BB powder compared to no treatment. No significant differences in serum calcium or 25(OH)D levels between interventions. Markers of bone resorption and osteocyte activity did not show significant changes with BB consumption |
| Paper | Area of Interest | Compound | Sample | Dosage/Posology | Mechanism | Main Results |
|---|---|---|---|---|---|---|
| Li et al. (2014) [19] | Prevention of osteoporosis induced by ovariectomy | Blueberry extract | Ovariectomized female Sprague Dawley rats | 10% w/w freeze-dried blueberry powder in the diet | Inhibition of bone resorption | Blueberries inhibit bone resorption, bone loss, and reduction in bone strength in OVX rats. |
| Zheng et al. (2016) [20] | Reduction in trabecular and cortical bone loss | Blackcurrant extract | Ovariectomized female C57BL/6J mice | 1% blackcurrant extract in the diet | Attenuation of bone resorption | Blackcurrant attenuates OVX-induced bone loss, as measured by BMD and trabecular volume; reduces bone resorption activity. |
| Melough et al. (2017) [21] | Impact of berries antioxidants on ovariectomy-induced bone loss | Blueberry | Ovariectomized Sprague Dawley female rats | 5% w/w dried blueberry powder for 100 days | Prevention of BMD loss | Blueberry prevents OVX-induced loss of whole-body BMD; the blueberry-treated group has lower serum osteocalcin levels |
| Sakaki et al. (2018) [22] | Improvement of mice bone mass (age-related bone loss) | Blackcurrant extract | Young and old female C57BL/6J mice | 1% (w/w) blackcurrant extract in chow diet for four months | Improved glutathione peroxidase and catalase activity, increased trabecular bone volume, OB surface, and bone mineral content | Consumption of blackcurrant early in life prevented ageing-associated bone loss in young mice; no effect in aged mice |
| Kaume, L. K. (2012) [23] | Bone mass and microarchitectural properties | Blackberries rich in cyanidin 3-O-β-D-glucoside | Ovariectomized (OVX) rats. Groups: Sham + Control (n = 12), OVX + Control (n = 11), OVX + BB 5% (5% w/w in the diet, n = 6), OVX + BB 10% (10% w/w in the diet, n = 7 | 5% and 10% (w/w) of blackberries in the die | Not specified in detail in the abstract/introduction | Blackberries consumption improved bone mass and microarchitectural properties in the OVX rat model |
| Hong et al., 2024 [24] | Postmenopausal osteoporosis | Fraction of Rubus tozawae (RL-Hex-NF3) | 8-week-old female C57BL/6J mice with ovariectomy (OVX). Groups: SHAM (n = 8), OVX (n = 8), E + P (0.1 mg/kg β-estradiol and 1 mg/kg progesterone, n = 8), RLL (10 mg/kg RL-Hex-NF3, n = 8), RLH (40 mg/kg RL-Hex-NF3, n = 8 | 10 mg/kg and 40 mg/kg of RL-Hex-NF3 administered orally for 12 weeks | Induction of osteoblast differentiation | Oral administration of RL-Hex-NF3 restored bone density in OVX mice. Increased serum levels of osteocalcin (OCN). Improved bone microarchitecture parameters (BMD, BV/TV, BS/BV, BS/TV, Tb.Th, Tb.N). Increased type 1 collagen (COL1) content in the femur and alkaline phosphatase (ALP) activity. Increased expression of osteoblast stimulators (β-catenin, TFG β, BMP2/4) and osteoblastic markers (OPN, OSX, RUNX2, COL1). Increased mRNA expression of Alp, Runx2, and Ocn. Three active compounds were identified in RL-Hex-NF3: 3β-hydroxy-18α,19α-urs-20-en-28-oic acid (1), betulinic acid (2), and (1S,6R,7S)-muurola-4,10(14)-dien-15-ol (3) |
| Devareddy et al., 2008 [25] | Effect of blueberry on bone loss in ovariectomized rat model of postmenopausal osteoporosis | Blueberry | 3-month-old female Sprague-Dawley rats with ovariectomy (Ovx). Groups: Sham (n = 10), Ovx (n = 10), Ovx + Blueberry (n = 6) | OVX + 5% blueberry for 100 days duration | Berries demonstrate a positive effect on bone metabolism due to their antioxidant power by phenolic content | OVX + 5% blueberry group increased whole body BMD and serum ALP |
| Chen et al., 2010 [26] | Effect of blueberry on bone growth, BMD, BMC, osteoblast and osteoclast numbers. | Blueberry (phenolic acids derived from blueberries) | Sprague-Dawley male/female rats; 20 days old (n = 20) | Control vs. 10% blueberry for 40 days duration | Dietary-induced serum phenolic acids promote bone growth via the p38 MAPK/β-catenin canonical WNT signaling pathway, upregulating osteoblast differentiation and osteocalcin production; decreases RANKL mRNA expression and impairs osteoclastogenesis | Increases in bone mass, BMD, Bone Mineral Content (BMC). Associated with increases in osteoblast number and decreased osteoclast number |
| Zhang et al., 2011 [27] | Effect of early life blueberry supplementation on preventing osteoblast senescence and adult bone loss | Blueberry diet | Sprague-Dawley female rats, 20 days old | 10% blueberry diet fed rats only between postnatal day 20 and postnatal day 34 | Preventing osteoblast senescence; anti-oxidative and anti-inflammatory properties of anthocyanins | Early blueberry supplementation prevented osteoblast senescence and adult bone loss. Increased trabecular bone volume, osteoblast number, bone formation rate, and osteocalcin levels |
| Paper | Area of Interest | Compound | Sample | Dosage/Posology | Mechanism | Main Results |
|---|---|---|---|---|---|---|
| Tanabe et al., 2011 [28] | Effects of cranberry extract on bone degradation | Cranberry extract (A-type cranberry proanthocyanidins) | Human bone marrow cells (pre-osteoclastic) | 10, 25, 50, 100 µg/mL for 4 days | Inhibits RANKL-dependent osteoclasts; impairs cell maturation and decreases bone resorption | Decreased rate of bone degradation by inhibiting RANKL-dependent osteoclasts. |
| Bickford et al., 2006 [29] | Effects of blueberry extract on human stem cell proliferation | Blueberry extract | Human bone marrow cells (CD34+ or CD133) | 500 ng/mL for 72 h | Promotes proliferation of human bone marrow cells (osteoblast progenitor cells) and decreases osteoclastogenesis | Increased proliferation of human bone marrow cells, decreased TRAP staining and number of RANKL-dependent osteoclasts |
| Bauer et al., 2024 [30] | Osteogenesis | Cranberry extract | Stromal cells from human exfoliated deciduous teeth (SHED) and Osteoblasts MC3T3-E1 (in vitro) | Cranberry extract at 0.2 mg/mL, 2 mg/mL, 20 mg/mL, 50 mg/mL, 100 mg/mL, 150 mg/mL, 200 mg/mL, and 250 mg/m | Evaluation of biocompatibility and stimulation of osteogenic activity | Demonstration of biocompatibility and improving |
| Zheng et al. 2016 [20] | Osteoclastogenesis, osteoblast differentiation, ovariectomy-induced bone loss | Blackcurrant anthocyanins (BCA), blackberry anthocyanins (BKA), anthocyanin-rich blackcurrant extract | Mouse bone marrow macrophages (BMMs). In vivo: Ovariectomized female C57BL/6J mic | 1 and 3 µg/mL of BCA and BKA. In vivo: 1% anthocyanin-rich blackcurrant extract in the diet for 12 weeks | Effects on osteoclast formation (TRAP assay, pit formation assay), osteoblast differentiation (alkaline phosphatase assay), bone histomorphometry analysis | Blackcurrant supplementation attenuated ovariectomy-induced bone loss in mice. BMD was higher in the OVX + BC group compared to OVX. Histological analysis showed improved trabecular bone parameters in the OVX + BC group. Osteoclast activity and differentiation were reduced by blackcurrant supplementation |
| Nagaoka et al. 2019 [31] | Suppression of osteoclast differentiation | petunidin | RAW264.7 cell line | >5 μg/ml | Significantly suppressed OCs’ differentiation, down-regulated expression of genes for c-Fos, NFATc1, matrix metalloproteinase 9 and cathepsin K | Petunidin significantly suppressed OCs’ differentiation |
| Outcome | Human Studies | Animal Studies | In Vitro Studies | Overall Direction/Certainty |
|---|---|---|---|---|
| Bone Mineral Density (BMD) | ↑ (modest, low–moderate certainty) | ↑↑ (consistent, moderate strength) | — | Positive, low–moderate certainty |
| Bone Biomarkers (OCN, ALP, P1NP) | ± (mixed) | ↑ (consistent increases) | ↑ (cell-level support) | Partially positive, low certainty |
| Osteoclast Activity | ↓ (limited) | ↓↓ (consistent inhibition) | ↓↓↓ (strong inhibition) | Strong inhibition of resorption |
| Osteoblast Differentiation | ↑ (suggestive) | ↑↑ (enhanced in OVX models) | ↑↑↑ (strong activation) | Strong stimulation of formation |
| Fracture Outcomes | Not assessed | ↓ (indirect improvement) | — | Unclear, very low certainty |
| Outcome | Population and Setting | Studies (Design) | What Was Measured | What the Studies Showed | Certainty (GRADE) | Why the Certainty Is Not Higher |
|---|---|---|---|---|---|---|
| Bone mineral density (BMD) at LS/TH/FN/whole-body | Adults, mostly postmenopausal women | 2 cohorts; 2 RCTs | DXA BMD (site-specific) | Cohorts: higher anthocyanin intake associated with greater BMD; RCTs: modest ↑(up) in whole-body BMD/↑(up) net Ca retention | Low–Moderate | Small RCTs, short duration; residual confounding in cohorts; inconsistency across sites |
| Bone turnover biomarkers (P1NP, OC, CTX, ALP) | Adults | 2 RCTs + 1 pilot | Standard serum markers | Mixed findings (some ↑(up) formation markers; others unchanged) | Low | Imprecision (small n), inconsistency, heterogeneity of products/doses |
| Fracture outcomes | Adults | None | Incident fractures | Not assessed | Very Low | No direct evidence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perna, S.; Ruina, G.F.; Acharya, A.; Mazzola, G.; Rondanelli, M.; Riso, P. Berries derived Polyphenols and Bone Health: A Systematic Review. Nutrients 2025, 17, 3440. https://doi.org/10.3390/nu17213440
Perna S, Ruina GF, Acharya A, Mazzola G, Rondanelli M, Riso P. Berries derived Polyphenols and Bone Health: A Systematic Review. Nutrients. 2025; 17(21):3440. https://doi.org/10.3390/nu17213440
Chicago/Turabian StylePerna, Simone, Giorgia F. Ruina, Asmita Acharya, Giuseppe Mazzola, Mariangela Rondanelli, and Patrizia Riso. 2025. "Berries derived Polyphenols and Bone Health: A Systematic Review" Nutrients 17, no. 21: 3440. https://doi.org/10.3390/nu17213440
APA StylePerna, S., Ruina, G. F., Acharya, A., Mazzola, G., Rondanelli, M., & Riso, P. (2025). Berries derived Polyphenols and Bone Health: A Systematic Review. Nutrients, 17(21), 3440. https://doi.org/10.3390/nu17213440







