Abstract
Objective: Postprandial hyperglycemia is a major risk factor for type 2 diabetes and cardiovascular disease. Inhibition of α-amylase and α-glucosidase can attenuate postprandial glycemic response (PPGR). This study aimed to investigate the inhibitory effects of mulberry leaf and corn silk on these enzymes in vitro and their impact on postprandial glucose (PG) levels in prediabetic individuals using milk-based matrices. Research Design and Methods: In vitro, enzyme inhibition was assessed using the DNS method (α-amylase) and pNPG method (α-glucosidase). A randomized crossover trial was conducted in 11 prediabetic individuals with four interventions: pure milk; lactose-hydrolyzed milk; lactose-hydrolyzed milk with mulberry leaf, corn silk, and resistant dextrin; and GOS milk with mulberry leaf and corn silk. PPGR was assessed by area under the glucose curve, 1 and 2 h PG, maximum PG, and 2 h glucose excursion. Paired Wilcoxon signed-rank tests were used for comparisons. Results: Mulberry leaf and corn silk extracts inhibited both enzymes dose-dependently, with synergistic effects. No significant differences in PPGR indices were observed across interventions in the overall prediabetic individuals. However, in the overweight subgroup, the combination of GOS milk supplemented with mulberry leaf and corn silk significantly reduced 1 h PG (median difference [P25, P75]: −0.84 mmol/L [−1.05, −0.49]), maximum PG (−0.54 mmol/L [−0.75, −0.25]), and glucose excursion (−0.62 mmol/L [−0.75, −0.24]) compared to pure milk. Conclusions: Mulberry leaf and corn silk extracts inhibit α-amylase and α-glucosidase in vitro and may attenuate postprandial glucose excursions in overweight prediabetic individuals when delivered in a GOS milk matrix.
1. Introduction
Postprandial hyperglycemic response is not only a hallmark of prediabetes and type 2 diabetes but also constitutes an independent risk factor distinct from fasting blood glucose (FBG) and HbA1c. It demonstrates significant associations with type 2 diabetes progression, incident cardiovascular disease (CVD), and elevated all-cause mortality risk [1]. The escalating burden of prediabetes, a precursor to diabetes, underscores the urgency of addressing postprandial glucose management. According to the International Diabetes Federation 2021 report, the prevalence of impaired glucose tolerance and impaired fasting glucose was 9.1% and 5.8%, respectively. It is expected that these numbers will increase to 10% and 6.5% by 2045, respectively [2]. Around 38.1% of the population in China is in the prediabetes stage, and these people have a significantly higher risk of developing diabetes and its complications [3]. This high prevalence not only poses a significant threat to public health but also forecasts a substantial future economic burden on the healthcare system. A pivotal strategy for controlling postprandial hyperglycemia involves inhibiting the activity of enzymes (α-amylase and α-glucosidase) or modulating glucose transport [4,5]. Conventional pharmaceutical interventions for postprandial hyperglycemia are frequently associated with gastrointestinal adverse effects, including flatulence, diarrhea, and abdominal pain [6]. In contrast, medicinal food homologous substances—by leveraging their natural bioactive constituents (e.g., alkaloids, polysaccharides, polyphenols)—demonstrate clinically relevant glucose control with superior safety profiles in clinical investigations. Mulberry leaf, the dried foliage of Moraceae plants and a canonical “medicinal and edible” herb in China, may regulate postprandial glucose through two mechanisms: (1) direct inhibition of α-amylase and α-glucosidase activity via flavonoids that competitively block substrate binding and catalytic processes through hydrogen bonding and hydrophobic interactions at the enzymes’ active sites, thereby retarding carbohydrate hydrolysis; and (2) downregulation of mRNA expression for intestinal glucose transporters sodium-glucose cotransporter 1 (SGLT1) and glucose transporter 2 (GLUT2), consequently inhibiting enterocytic glucose translocation into systemic circulation [4,7,8,9,10]. Corn silk, comprising the dried stigmas and styles of the pistillate flowers from Zea mays L. (Poaceae family), is recognized as a medicinal food homologous substance [11]. In vitro studies have confirmed its inhibitory effects on α-amylase and α-glucosidase activity, wherein corn silk flavonoids competitively occupy the enzymes’ active sites through hydrogen bonding and π-π interactions, sterically hindering substrate binding to α-glucosidase and consequently retarding carbohydrate hydrolysis [12,13]. Detailed information on the pharmacological profiles of mulberry leaf and corn silk extracts—including their key phytochemical components, molecular structures, molecular formulas, and documented metabolic health benefits—is summarized in Supplementary Table S1.
Compared to individual bioactive compounds, combinatorial formulations leverage synergistic effects to enhance functional potency, reduce dosage requirements, and mitigate toxicity risks. Building upon the complementary advantages of mulberry leaf and corn silk extracts, this study aimed to investigate their in vitro synergistic inhibition of α-amylase and α-glucosidase. Milk, as a pivotal nutritional vehicle in human dietary systems, offers high biocompatibility and frequent consumption patterns, making it an ideal delivery matrix for homologous phytochemicals in medicinal foods [14]. Nevertheless, China’s current dairy intake (42.6 kg/capita/year) falls significantly below recommended levels (112.8 kg/year), attaining only 37.8% of dietary guidelines [15]. This gap may correlate with the high prevalence of lactase deficiency [16]. Affected individuals typically experience abdominal bloating, diarrhea, and other gastrointestinal distress symptoms following conventional milk consumption, directly suppressing dairy intake. To address this, the lactose-free dairy market continues expanding [17], with two technologically distinct products being particularly relevant: (1) Lactose-hydrolyzed milk functional dairy product wherein β-galactosidase pre-hydrolyzes lactose into glucose and galactose [18]; and (2) GOS-enriched milk, produced via β-galactosidase-mediated transgalactosylation that catalyzes intermolecular galactosyl transfer, generating milk intrinsically fortified with prebiotic galacto-oligosaccharides (GOS) [19]. Employing a randomized crossover trial design in a prediabetic cohort, we systematically compare the postprandial glucose impact of four milk matrices: (i) lactose-hydrolyzed milk; (ii) pure milk; (iii) lactose-hydrolyzed milk supplemented with mulberry leaf and corn silk extracts and resistant dextrin; and (iv) GOS-enriched milk containing mulberry leaf and corn silk extracts, thereby providing evidence-based dietary strategies for prediabetes management.
2. Materials and Methods
2.1. In Vitro Experiments
2.1.1. Chemicals and Reagents
Porcine pancreatic α-amylase, soluble starch, sodium phosphate buffer, p-nitrophenyl-α-D-glucopyranoside (pNPG), α-glucosidase, and anhydrous sodium carbonate were purchased from Yuanye Bio-Technology Co., Ltd., Shanghai, China. DNS reagent was obtained from Solarbio Science & Technology Co., Ltd., Beijing, China. Acarbose tablets were purchased from a pharmacy. Mulberry leaf and corn silk extracts were provided by Guangdong Qingyunshan Pharmaceutical Co., Ltd., Shaoguan, China. Resistant dextrin was provided by Baolingbao Biotechnology Co., Ltd., Dezhou, China. Among them, mulberry leaf and corn silk extracts were prepared by water extraction. The quantification results of bioactive compounds (e.g., flavonoid content) in mulberry leaf and corn silk extracts are presented in Supplementary Table S2.
2.1.2. α-Amylase Inhibitory Assay
A slightly modified α-amylase inhibition rate assay was adopted [20,21]. The detailed reagent volumes and procedural steps for the Control group, Blank group, Experimental group, and Experimental blank group in this α-amylase inhibitory activity assay are presented in Supplementary Table S3. Briefly, 50 μL of mulberry leaf or corn silk extracts at different concentrations were added to 150 μL of soluble starch solution. The reaction was initiated by adding 50 μL of α-amylase (10.4 U/mL in 0.1 M sodium phosphate buffer, pH 6.9) to the mixture, followed by incubation at 37 °C. After 30 min, the reaction was terminated by adding 20 μL of 2 M NaOH solution. Subsequently, 20 μL of DNS reagent was added to the reaction mixture, and the mixture was subjected to a boiling water bath for 20 min. The absorbance at a wavelength of 540 nm was measured using a microplate reader. The α-amylase inhibition experiment was performed according to the group design and operational details specified in Supplementary Table S3, and the α-amylase inhibition rate was calculated using the following Equation (1). First, background interference (arising from the intrinsic reaction system and sample color) is subtracted from the Experimental group, leaving absorbance attributable solely to α-amylase–catalyzed starch hydrolysis in the presence of the sample. Likewise, background interference is removed from the Control group, yielding absorbance corresponding to α-amylase–mediated hydrolysis without inhibition. The ratio of these values reflects the residual α-amylase activity in the Experimental group relative to the Control. Subtracting this ratio from 1 and multiplying by 100% gives the percentage inhibition of α-amylase activity by the sample.
In Equation (1), ODA is the absorbance value of the Experimental group; ODa is the absorbance value of the Experimental blank group; ODB is the absorbance value of the Control group; and ODb is the absorbance value of the Blank group. We explicitly defined the composition of two blank groups: the Experimental blank group (ODa) does not contain α-amylase/α-glucosidase but contains plant extracts; the Blank group (ODb) does not contain plant extracts and α-amylase/α-glucosidase. To ensure volume consistency in each reaction system, the volume of the missing plant extracts or enzymes was replaced with an equal volume of buffer. The intrinsic color of plant extracts (e.g., flavonoids, polyphenols) may cause non-specific absorption at 540/405 nm. Subtracting the blank absorbance ensures that the measured values exclusively reflect the hydrolysis of starch by α-amylase/α-glucosidase, eliminates background interference, and thereby ensures the accuracy of inhibition rate calculations.
2.1.3. α-Glucosidase Inhibitory Assay
A slightly modified α-glucosidase inhibition rate assay was used [22,23]. α-glucosidase (2 U/mL), the substrate p-nitrophenyl-α-D-glucopyranoside (pNPG, 8 mM), and mulberry leaf or corn silk extract solutions were all prepared using PBS (pH 6.8, 0.1 mol/L). 80 μL of sample solutions at different concentrations and 20 μL of α-glucosidase solution were mixed and incubated at 37 °C for 10 min, after which 40 μL of pNPG was added to initiate the reaction. Following a 30 min reaction at 37 °C, 60 μL of 0.1 M Na2CO3 solution was immediately added to terminate the reaction, and the absorbance at a wavelength of 405 nm was measured using a microplate reader. The α-glucosidase inhibition experiment was conducted with the experimental design the same as that of the α-amylase inhibition experiment and according to Supplementary Table S4, and the α-glucosidase inhibition rate was calculated using Equation (1).
2.1.4. Inhibitory Effect of Mulberry Leaf and Corn Silk Combinations on the Two Enzymes
Using the methods described in detail in the previous two sections, the synergistic effects of the combined extract of mulberry leaf and corn silk on α-amylase and α-glucosidase were studied. Briefly, mulberry leaf and corn silk extracts were combined at varying mass ratios, enzymatic reactions were initiated with substrate addition, inhibitory activities were quantified via the DNS method for α-amylase and the pNPG assay for α-glucosidase, and combination indices (CI) were subsequently calculated according to Chou’s method [24], and the formula for calculating CI values is as follows (Equation (2)).
In Equation (2), (D)1 is the actual dose of mulberry leaf in combination; (D)2 is the actual dose of corn silk in combination; (Dx)1 is the concentration of mulberry leaf alone for target effect (when combined with corn silk); and (Dx)2 is the concentration of corn silk alone for target effect (when combined with mulberry leaf). The values of (Dx)1 and (Dx)2 can be calculated using the concentration–enzyme inhibition rate fitting curve equations in Supplementary Figure S1.
2.2. In Vivo Experiments
2.2.1. Research Design
Extracts of mulberry leaf and corn silk, along with resistant dextrin, were added to lactose-hydrolyzed milk and galactooligosaccharide (GOS)-fortified milk. A randomized crossover trial was conducted to compare the effects of four different samples (detailed in Supplementary Table S5, which presents their content and production methods) on postprandial glucose levels in individuals with prediabetes. Each participant attended a total of four food trial sessions, with the order of test samples randomized and a 7-day interval between trials. Each session involved acute intake of one test sample and 2 h postprandial glucose monitoring. This aligns with the randomized crossover trial design for evaluating acute postprandial glycemic responses, matching the study’s goal of assessing immediate glucose effects [25]. The trial was carried out at Peking Union Medical College Hospital in Beijing, China, from 30 July 2024, to 11 January 2025. This research protocol was approved by the Institutional Review Board of the Ethics Committee of China Agricultural University (CAUHR-20231206, registered on 15 December 2023) and the Ethics Committee of Peking Union Medical College Hospital (I-24PJ0448, registered on 29 February 2024), and was registered with the Chinese Clinical Trial Registry (ChiCTR2400083330). All participants signed informed consent forms.
2.2.2. Participant Eligibility Criteria
Adults aged 18 years and above who met the diagnostic criteria for prediabetes and had not received antihyperglycemic medication treatment were included. Participants with food allergies or severe lactose intolerance (defined as persistent gastrointestinal symptoms after consuming less than 240 mL of milk) were excluded [26]. Impaired fasting blood glucose and impaired glucose tolerance are collectively referred to as prediabetes. Specifically, impaired fasting blood glucose is defined as a fasting blood glucose level of 5.6–6.9 mmol/L; impaired glucose tolerance is defined as a 2 h postprandial glucose (2 h PG) level of 7.8–11.0 mmol/L; or prediabetes can also be defined as a glycosylated hemoglobin level of 5.7–6.4% [27,28].
2.2.3. Interventions
After an overnight fast (10–12 h), the subjects participated in the food trial. The four intervention samples were as follows: (1) lactose-hydrolyzed milk group: one pack of whole-wheat bread (50 g of carbohydrates) and 220 mL of lactose-hydrolyzed milk; (2) pure milk group: one pack of whole-wheat bread (50 g of carbohydrates) and 220 mL of pure milk; (3) mulberry leaf + corn silk + resistant dextrin + lactose-hydrolyzed milk group: one pack of whole-wheat bread (50 g of carbohydrates) and 220 mL of lactose-hydrolyzed milk supplemented with mulberry leaf, corn silk extracts, and resistant dextrin; (4) mulberry leaf + corn silk + GOS milk group: one pack of whole-wheat bread (50 g of carbohydrates) and 220 mL of GOS milk supplemented with mulberry leaf and corn silk extracts. All four samples were sterilized by Ultra-High-Temperature Instantaneous Sterilization (UHT). The order of the four intervention samples for each subject, along with the allocation of their serial numbers, was determined by the study designer using random numbers generated in Excel. All intervention samples were uniformly packaged, with serial numbers printed on the outer packaging; the serial numbers (corresponding to each assigned sample) were enclosed in separate sealed opaque envelopes. On the intervention day, participants received the envelopes, and throughout the intervention, both participants and implementers were unaware of the specific sample identity, ensuring a double-blind design. Statistical analysis was performed by researchers who remained blinded. During the trial (2 h), subjects could only consume the provided intervention samples and were not allowed to eat any other food.
2.2.4. Measurement
Data on sociodemographic factors (age, gender, and education level), lifestyle factors (body mass index [BMI], smoking status, and drinking status), and family history of diabetes were collected via questionnaires. BMI was further categorized into four groups: underweight (<18.5 kg/m2), normal weight (18.5–24.0 kg/m2), overweight (24.0–28.0 kg/m2), and obese (≥28.0 kg/m2) [29]. During the follow-up period, venous blood samples were collected from the subjects by medical staff at fasting (t = 0 min) and 30, 60, 90, and 120 min after meal intake, and glucose levels at different time points were measured by a testing company. Consequently, metrics related to postprandial glycemic response (PPGR) were obtained. The primary outcome of the study was the area under the glucose curve (AUC) within 2 h after intervention. Secondary outcomes included 1 h postprandial glucose (1 h PG), 2 h PG, maximum glucose, and maximum glucose excursion from baseline.
2.2.5. Sample Size Calculation
The sample size calculation was based on a randomized crossover trial, with the area under the glucose curve as the primary outcome measure [30]. Using the nonparametric Wilcoxon signed-rank test to analyze the intra-group differences in the primary outcome variable, it was determined that a 20% difference in the mean postprandial glucose, measured by AUC, with α = 0.05 and β = 0.2, and accounting for a 10% dropout rate, would require a total of 13 participants.
2.3. Statistical Analysis
All in vitro experiments were performed in three independent replicates, and the results are expressed as the mean ± standard deviation (SD). For the human trial part, the paired Wilcoxon signed-rank test was used to compare differences in AUC, 1 h PG, 2 h PG, maximum glucose, and maximum glucose excursion from baseline among different intervention samples. Analyses were conducted using SPSS (version 27.0). GraphPad Prism 9 was used to plot. p-values < 0.05 were considered statistically significant.
3. Results
3.1. Inhibitory Activity of Mulberry Leaf Extracts on α-Amylase and α-Glucosidase
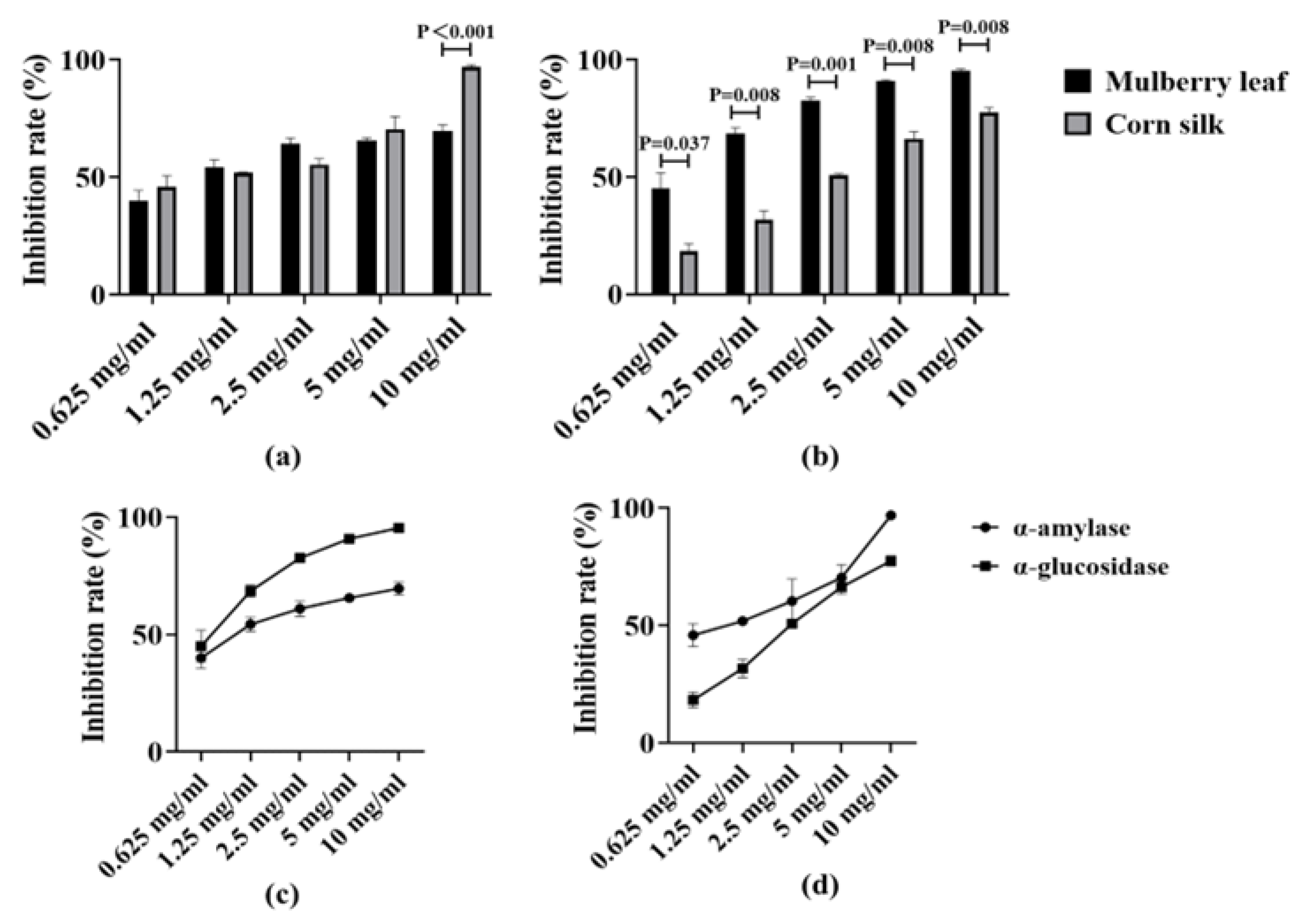
Figure 1a,b respectively compare the inhibition rates of mulberry leaf and corn silk at different concentrations on α-amylase and α-glucosidase, and there are significant differences in inhibition rates between them at most concentrations (p < 0.05). The experimental results are shown in Figure 1c. The acarbose group at a concentration of 0.625 mg/mL was used as the positive control, which exhibited high inhibitory rates against both α-amylase and α-glucosidase (63% and 99.1% respectively). Different concentrations of mulberry leaf (0.625–10 mg/mL) exerted distinct inhibitory effects on the two enzymes. For α-amylase, the inhibition rates of mulberry leaf across various concentrations were relatively stable, mostly around 60%. For α-glucosidase, the inhibition rate varied with changes in the concentration of mulberry leaf. Overall, at most concentrations, its inhibition rate was higher than that against α-amylase, and could reach 70–90% at certain concentrations (e.g., 5 mg/mL, 10 mg/mL, etc.). At high concentrations (5–10 mg/mL), its inhibitory rate on the enzyme is comparable to that of acarbose.
Figure 1.
Inhibition rates of extracts at different concentrations on α-amylase and α-glucosidase. (a) Comparison of the inhibition rates of mulberry leaf and corn silk on α-amylase at the same concentration; (b) Comparison of the inhibition rates of mulberry leaf and corn silk on α-glucosidase at the same concentration; (c) Line chart of inhibition rates of mulberry leaf extracts at different concentrations on α-amylase and α-glucosidase; (d) Line chart of inhibition rates of corn silk extracts at different concentrations on α-amylase and α-glucosidase.
3.2. Inhibitory Activity of Corn Silk Extracts on α-Amylase and α-Glucosidase
The experimental results are shown in Figure 1d. For α-amylase, the inhibitory rate of corn silk exhibits an upward trend with increasing concentration, reaching approximately 50% at low concentrations (0.625–1.25 mg/mL), rising to close to 60% at medium concentrations, and significantly increasing at high concentrations. For α-glucosidase, its inhibitory rate also increases with the rise in concentration, being relatively low at low concentrations with a gentle increasing trend, and exceeding 60% and 80% respectively at high concentrations (5–10 mg/mL). Among them, the inhibitory rate of corn silk on α-amylase at high concentrations exceeds that of the positive control acarbose, and the inhibitory rate on α-glucosidase is also close to the level of acarbose.
3.3. Inhibitory Effects of Mulberry Leaf and Corn Silk Extracts Combination on α-Amylase and α-Glucosidase
The inhibitory effects of the mixture on α-amylase and α-glucosidase were evaluated by mixing mulberry leaf and corn silk extracts at different concentrations. Nine combinations were obtained by mixing the approximate values of IC25 (0.38 mg/mL), IC50 (1.13 mg/mL), and IC75 (14.06 mg/mL) of mulberry leaf extracts for α-amylase inhibition with the approximate values of IC25 (0.27 mg/mL), IC50 (1.06 mg/mL), and IC75 (5.88 mg/mL) of corn silk extracts for α-amylase inhibition, respectively. Another nine combinations were obtained by mixing the approximate values of IC25 (0.29 mg/mL), IC50 (0.71 mg/mL), and IC75 (1.72 mg/mL) of mulberry leaf extracts for α-glucosidase inhibition with the approximate values of IC25 (0.87 mg/mL), IC50 (2.61 mg/mL), and IC75 (7.83 mg/mL) of corn silk extracts for α-glucosidase inhibition, respectively. The above values can be calculated using the concentration–enzyme inhibition rate fitting curve equations in Supplementary Figure S1. Then, the combination index (CI) was generated based on the in vitro experimental results (Table 1). According to the research on drug synergism, CI < 1, CI = 1, and CI > 1 indicate synergism, additive effect, and antagonism, respectively [24].

Table 1.
Combination study of mulberry leaf and corn silk on the inhibition of the two enzymes.
When mulberry leaf and corn silk extracts are used in combination, they exhibit a synergistic inhibitory effect on α-amylase, with a CI value < 1. Meanwhile, their combined use also shows a synergistic effect on the inhibition of α-glucosidase, with a CI value < 1. This synergistic effect can reduce the concentration of the extracts used while maintaining or even increasing the enzyme inhibition rate, thereby reducing the release of glucose into the bloodstream and alleviating postprandial glucose.
3.4. Impact of Milk Supplemented with Mulberry Leaf, Corn Silk Extracts, and Resistant Dextrin on Postprandial Glucose
A total of 13 prediabetic individuals were enrolled in the study, among whom 11 (84.6%) completed the randomized crossover trial with four samples (Supplementary Figure S2). The mean age of participants was 54 ± 9 years, with ten females, ten having senior high school or higher education, and eight having overweight or obesity (Table 2).

Table 2.
Characteristics of subjects at baseline.
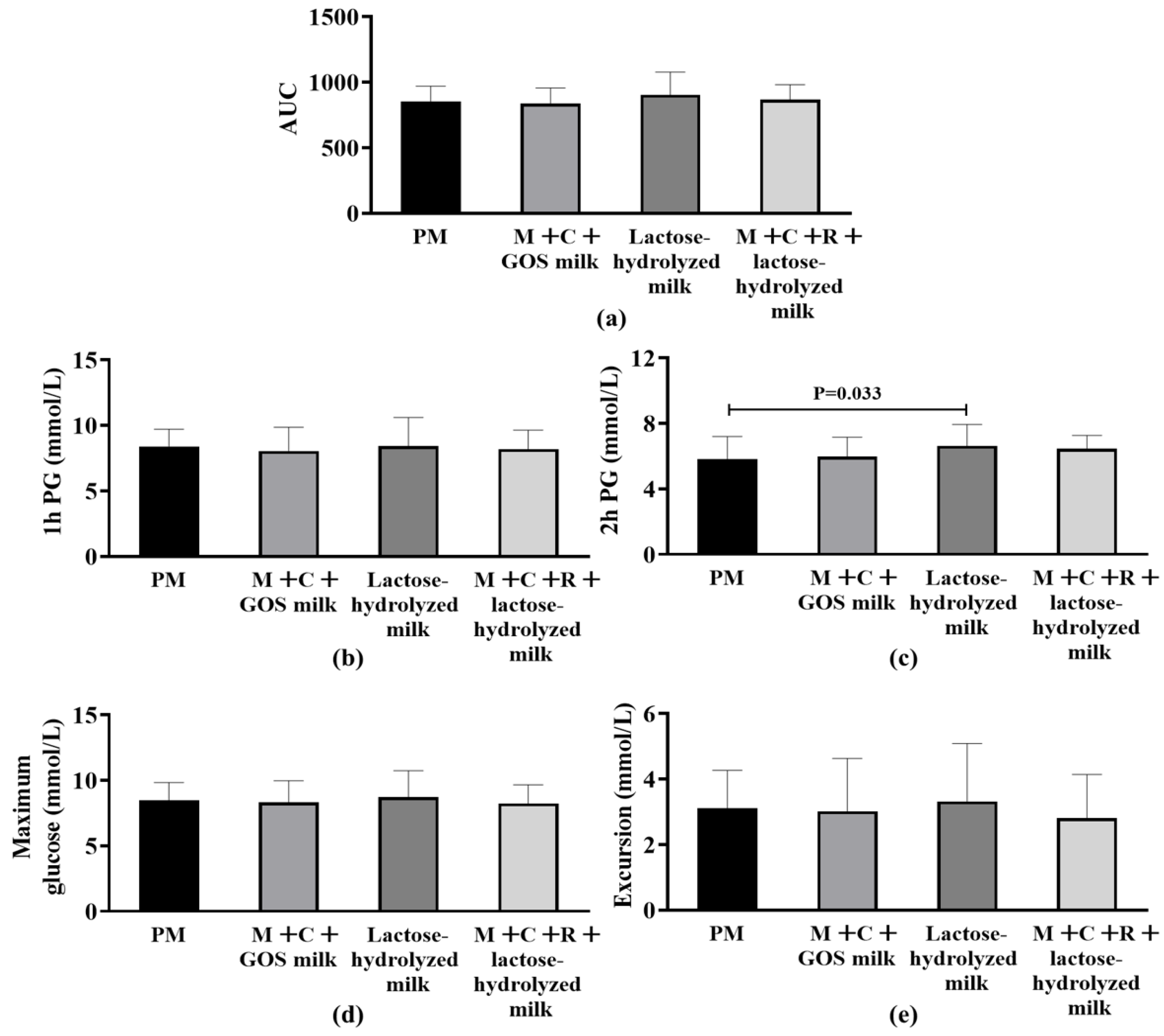
Figure 2a shows the effects of different interventions on AUC after the subjects consumed 50 g of carbohydrates. The results showed no statistically significant difference in the AUC index among the four groups (p > 0.05). Figure 2b–e presents and compares the differences in secondary outcomes among different intervention groups. No statistically significant differences were found among the groups in terms of postprandial maximum glucose, maximum glucose excursion from baseline, and 1 h PG (p > 0.05). Notably, the results of 2 h PG showed that the pure milk group was significantly lower than the lactose-hydrolyzed milk group (Median of difference [P25, P75]: −0.86 mmol/L [−1.39, −0.42], p = 0.033).
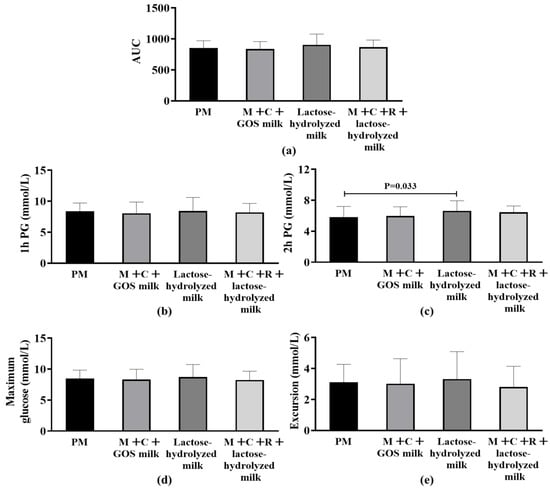
Figure 2.
Differences in postprandial glycemic response (PPGR) among four interventions. (a) Area Under the Curve (AUC) of postprandial glucose. (b) 1 h postprandial glucose (1 h PG). (c) 2 h postprandial glucose (2 h PG); statistical significance (p = 0.033) is indicated between groups. (d) Maximum postprandial glucose concentration. (e) Glucose excursion (range of postprandial glucose change). Abbreviation: AUC, Area Under the Curve; PM, pure milk. M + C + GOS milk, mulberry leaf + corn silk + GOS milk; M + C + R + lactose-hydrolyzed milk, mulberry leaf + corn silk + resistant dextrin + lactose-hydrolyzed milk; PG, postprandial glucose.
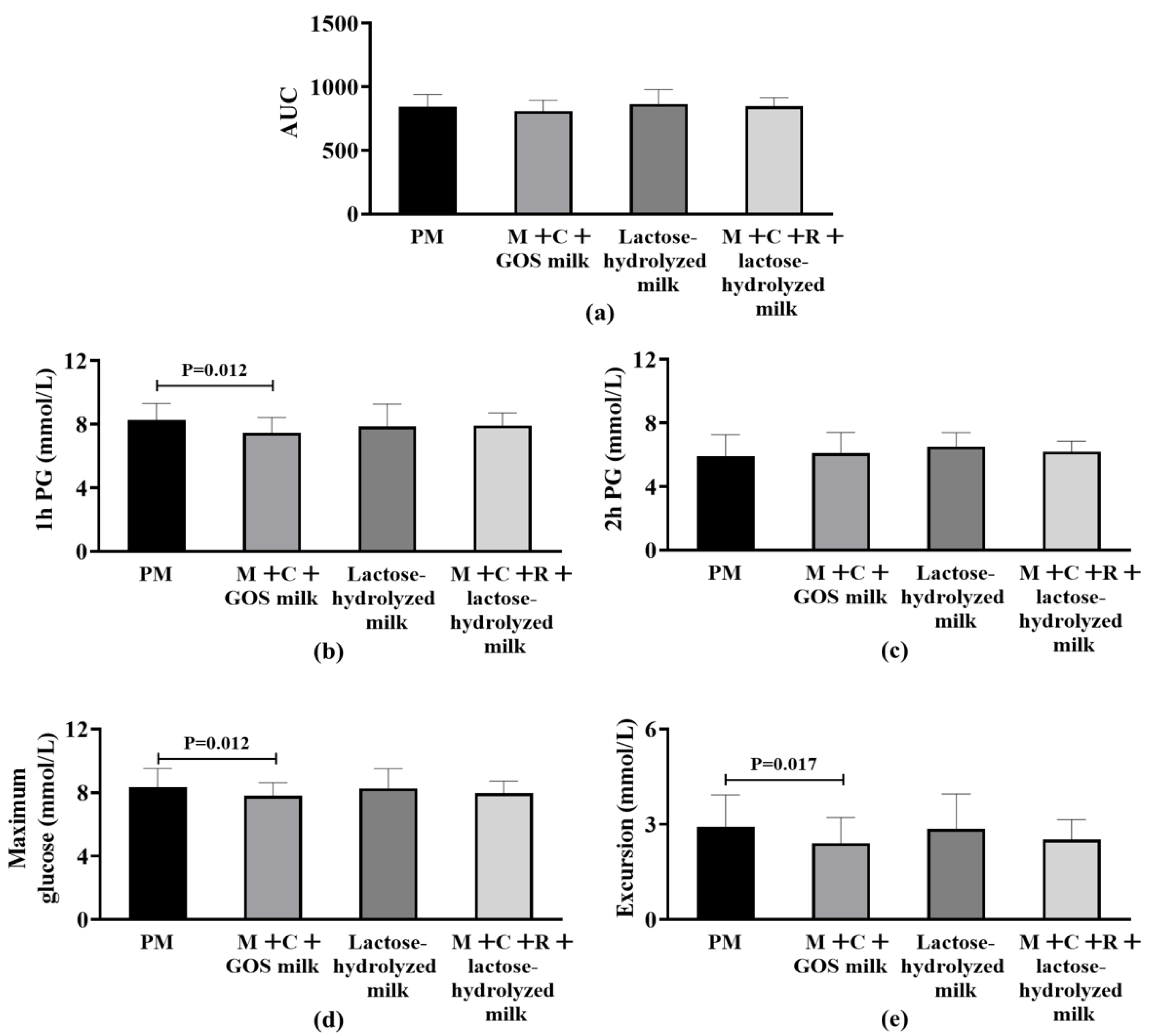
Among participants being overweight (BMI ≥ 24 kg/m2), no statistically significant differences were observed in AUC among the four groups (p > 0.05) (Figure 3a). Figure 3b–e shows the differences in secondary outcome indicators among the four intervention groups. The 1 h PG in the mulberry leaf + corn silk + GOS milk group was significantly lower than that in the pure milk group (−0.84 mmol/L [−1.05, −0.49], p = 0.012), representing a 4.2% reduction compared to pure milk alone. For the indicator of 2 h PG, no significant difference was found among the groups after statistical testing. Regarding the postprandial maximum glucose level, the mulberry leaf + corn silk + GOS milk group (BMI ≥ 24 kg/m2) demonstrated significantly lower values than the pure milk group (BMI ≥ 24 kg/m2) (−0.54 mmol/L [−0.75, −0.25], p = 0.012), corresponding to a 1.8% reduction compared to pure milk alone. No significant statistical difference was observed between other groups. For maximum glucose excursion from baseline, the mulberry leaf + corn silk + GOS milk group demonstrated significantly lower values than the pure milk group (BMI ≥ 24 kg/m2) (−0.62 mmol/L [−0.75, −0.24], p = 0.017), representing a 2.9% reduction compared to pure milk alone, and no statistically significant difference was found in this indicator among other sample groups.
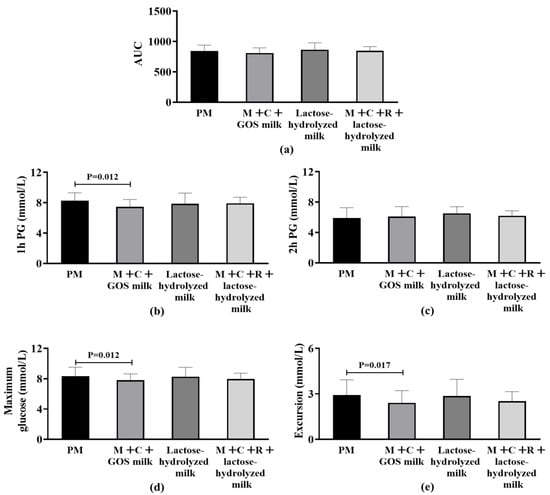
Figure 3.
Differences in postprandial glycemic response (PPGR) among overweight subjects (BMI ≥ 24 kg/m2) after four intervention. (a) Area Under the Curve (AUC) of postprandial glucose. (b) 1 h postprandial glucose (1 h PG); statistical significance (p = 0.012) is indicated between groups. (c) 2 h postprandial glucose (2 h PG). (d) Maximum postprandial glucose concentration; statistical significance (p = 0.012) is indicated between groups. (e) Glucose excursion (range of postprandial glucose change); statistical significance (p = 0.017) is indicated between groups. Abbreviation: AUC, Area Under the Curve; PM, pure milk; M + C + GOS milk, mulberry leaf + corn silk + GOS milk; M + C + R + lactose-hydrolyzed milk, mulberry leaf + corn silk + resistant dextrin + lactose-hydrolyzed milk; PG, postprandial glucose.
4. Discussion
In vitro experiments, both mulberry leaf and corn silk extracts have dose-dependent associations with inhibitory rates against α-amylase and α-glucosidase, with a synergistic inhibitory effect. No significant differences in PPGR indices were observed across interventions in the overall prediabetic individuals, except that the 2 h PG in the pure milk group was significantly lower than that in the lactose-hydrolyzed milk group. Among the overweight population, the mulberry leaf + corn silk + GOS milk group, but not other groups, had lower levels of 1 h PG, maximum glucose, and maximum glucose excursion compared to those of pure milk. This implies that mulberry leaf + corn silk + GOS milk may have certain advantages in regulating these blood glucose-related indicators in the overweight population.
As the concentration of mulberry leaf and corn silk extracts increased, their inhibitory rates against the two enzymes gradually approached and even reached the inhibitory level of acarbose at 0.625 mg/mL to a certain extent. These unmodified plant extracts exhibit substantial enzyme inhibitory activity approaching that of conventional drugs, offering valuable insights due to their natural origin and safety profile. Our results were similar to previous studies [31,32]. Han and Gong et al. demonstrated in in vitro enzyme inhibition assays that both mulberry leaf and corn silk extracts exhibited dose-dependent increases in inhibitory rates against α-amylase and α-glucosidase, with efficacy approaching that of the positive control acarbose. Both mulberry leaf and corn silk can bind to the active sites of α-amylase/α-glucosidase via their flavonoids, exerting competitive inhibition on carbohydrate hydrolysis. Mulberry leaf have a stronger inhibitory effect on α-glucosidase, while corn silk is more potent in inhibiting α-amylase [10,12,13]. It is reasonable that their combination may produce a synergistic inhibitory effect through target complementarity. In this study, we did find that the mulberry leaf and corn silk have a synergistic inhibitory effect on the activities of α-amylase and α-glucosidase.
Although there was no significant difference in the PPGR index between different intervention measures in the overall prediabetic individuals, among those with overweight, the combination of GOS milk supplemented with mulberry leaf and corn silk significantly reduced 1 h PG, maximum PG, and 2 h maximum glucose excursion compared with pure milk. This may be explained by insulin sensitivity. Individuals with overweight (BMI ≥ 24 kg/m2) are often accompanied by decreased insulin sensitivity, an increased risk of insulin resistance, and weakened ability to regulate postprandial glucose on their own (such as delayed insulin secretion and reduced glucose uptake by peripheral tissues) [33]. In contrast, individuals with normal weight (BMI < 24 kg/m2) have better insulin sensitivity. After postprandial glucose rises, it can be rapidly regulated by insulin, leaving limited additional “hypoglycemic space” for intervention measures. Therefore, the difference between the other group and the pure milk group is difficult to detect among those of normal weight. Meanwhile, compared with people of normal weight, overweight individuals have reduced gut microbiota diversity and an increased Firmicutes/Bacteroidetes ratio. As a prebiotic, GOS can promote the growth of beneficial bacteria (such as Bifidobacteria and Lactobacilli) and be rapidly fermented by Bifidobacteria in the gut to produce short-chain fatty acids (SCFAs) like butyrate. SCFAs stimulate the secretion of Glucagon-like Peptide-1 (GLP-1), thereby indirectly improving insulin sensitivity [34,35].
In lactose-hydrolyzed milk, resistant dextrin was also added. However, different from the mechanism of action of mulberry leaf and corn silk, it does not directly affect enzyme activity. Instead, relying on its special molecular structure and physical properties, it increases the viscosity of chyme to form a physical barrier, thereby hindering contact between enzymes and carbohydrates and reducing postprandial glucose levels [36]. Therefore, in the in vitro enzyme inhibition assay, we did not investigate its effect.
The strengths of this study include investigating the effect of mulberry leaf and corn silk on postprandial glucose from the perspective of an in vitro enzyme inhibition experiment and an in vivo human clinical trial. However, this study also has some limitations. First, the relatively small sample size may compromise the statistical power of results and their generalizability to the broader population; notably, the subgroup analysis for overweight participants was not pre-specified, with an even smaller subgroup sample size (reducing conclusion stability) and no significant differences in the primary outcome (AUC) across groups. Second, only postprandial glucose was measured, lacking data on mechanistic biomarkers such as insulin and GLP-1, which hinders in-depth elaboration of specific glucose-regulating mechanisms. Third, the short intervention duration prevents assessing sustained effects of long-term consumption of such milk on blood glucose and metabolic indicators in prediabetic individuals. Lastly, the study only recruited prediabetic participants, so results should be cautiously generalized to other populations. In the future, it is necessary to verify our findings among studies with larger samples and subgroup designs.
5. Conclusions
This study systematically evaluated the effects of mulberry leaf and corn silk extracts, as well as their combinations, on α-amylase and α-glucosidase activities, and postprandial glucose regulation, using both in vitro enzyme inhibition assays and an in vivo human intervention trial. In vitro, mulberry leaf and corn silk extracts demonstrated dose-dependent inhibition of α-amylase and α-glucosidase, supporting their potential as natural enzyme inhibitors with favorable safety profiles. Notably, specific combinations of the two extracts exhibited synergistic effects, enhancing inhibitory activity and suggesting optimized ratios for functional applications. In the human trial, no significant differences in PPGR were observed across all interventions in the overall prediabetic participants. However, in the overweight subgroup (BMI ≥ 24 kg/m2), the combination of mulberry leaf, corn silk, and GOS milk significantly improved PPGR compared to pure milk. Notably, the administered doses of mulberry leaf extract and corn silk extract in this short-term intervention were below their respective maximum recommended daily intake levels; as these substances are classified as both food and medicine, they are generally regarded as safe, and no adverse events were reported by participants following sample consumption. These findings suggest that this functional milk formulation may improve postprandial glucose control in overweight individuals with prediabetes, providing a basis for incorporating natural extracts into personalized nutritional strategies for glycemic management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17213438/s1; Supplementary Table S1. Pharmacological properties table of mulberry leaf and corn silk extracts; Supplementary Table S2. The content of active ingredients in substances of homology of food and medicine; Supplementary Table S3. In vitro α-amylase inhibitory activity assay; Supplementary Table S4. In vitro α-glucosidase inhibitory activity assay; Supplementary Figure S1. The concentration-enzyme inhibition fitting curve of mulberry leaf and corn silk; Supplementary Table S5. Sample information; Supplementary Figure S2. Flow chart.
Author Contributions
J.G. designed the research. Y.W., Y.L. and Q.Z. conducted the study, recruited subjects, and collected the data. J.H. and X.N. randomized the order of sample interventions. Y.S. conducted data analyses. Y.S. and X.N. drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors contributed to the interpretation of the data, revision of drafts, and approval of the final manuscript. J.G. is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by 2021-National Center of Technology Innovation for Dairy-5.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of China Agricultural University Ethics Committee (CAUHR-20231206, registered on 15 December 2023) and the Ethics Committee of Peking Union Medical College Hospital (I-24PJ0448, registered on 29 February 2024). This study has also been registered on the Chinese Clinical Trial Registry (ChiCTR2400083330).
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
Data are contained within the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors sincerely thank the participants of this trial. The research described in this manuscript has not been presented or published as an abstract at a meeting or in the proceedings of the meeting, nor has it been posted on a preprint server.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- Wu, Y.; Ehlert, B.; Metwally, A.A.; Perelman, D.; Park, H.; Brooks, A.W.; Abbasi, F.; Michael, B.; Celli, A.; Bejikian, C.; et al. Individual Variations in Glycemic Responses to Carbohydrates and Underlying Metabolic Physiology. Nat. Med. 2025, 31, 2232–2243. [Google Scholar] [CrossRef]
- Rooney, M.R.; Fang, M.; Ogurtsova, K.; Ozkan, B.; Echouffo-Tcheugui, J.B.; Boyko, E.J.; Magliano, D.J.; Selvin, E. Global Prevalence of Prediabetes. Diabetes Care 2023, 46, 1388–1394. [Google Scholar] [CrossRef]
- WenJun, T.; Yimeng, X.; Ding, N. The Prevalence and Treatment of Diabetes in China From 2013 to 2018. JAMA 2022, 327, 1706. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, C.; Liu, F.; Hu, T.; Shen, W.; Li, E.; Liao, S.; Zou, Y. Mulberry Leaf Polyphenols Attenuated Postprandial Glucose Absorption via Inhibition of Disaccharidases Activity and Glucose Transport in Caco-2 Cells. Food Funct. 2020, 11, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Tolmie, M.; Bester, M.J.; Apostolides, Z. Inhibition of A-glucosidase and A-amylase by Herbal Compounds for the Treatment of Type 2 Diabetes: A Validation of In Silico Reverse Docking with In Vitro Enzyme Assays. J. Diabetes 2021, 13, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Tshiyoyo, K.S.; Rabbad, A.; Yusuf, A.A.; Malgas, S. Combination of Citrus Peel-Derived Essential Oils with Acarbose to Inhibit Amylolytic Enzymes—A Potential Type II Diabetes Treatment Approach. Int. J. Biol. Macromol. 2025, 306, 141504. [Google Scholar] [CrossRef]
- Chanida, H.; Jun, K. Alpha-glucosidase inhibitory effect of mulberry (Morus alba) leaves on Caco-2. Fitoterapia 2006, 77, 568–573. [Google Scholar]
- Wood, I.S.; Trayhurn, P. Glucose Transporters (GLUT and SGLT): Expanded Families of Sugar Transport Proteins. Br. J. Nutr. 2003, 89, 3–9. [Google Scholar] [CrossRef]
- Peng, T.; Jiang, D.; Chen, Y.; Wang, Y.; Lai, F.; Chen, Z.; Huang, L. Optimization of Decolorization Process of Mulberry Leaf Extract by Response Surface Methodology and Its Inhibitory Activity on α-Glucosidase. Food Ferment. Sci. Technol. 2024, 60, 62–69. [Google Scholar]
- Wang, Z.; Zhang, L.; Wang, M.; Ding, Z.; Ren, D.; Duan, S. Discovery of Vitexin as a Novel α-Glucosidase Inhibitors in Mulberry (Morus alba L.) by Untargeted Metabolomics Combined with Molecular Docking: A Comprehensive Study from Mechanism to Synergy Effects. eFood 2024, 5, e144. [Google Scholar] [CrossRef]
- He, Z.; Wu, X.; Xiang, Z.; Bai, G.; Wang, Y.; Li, S.; Du, Z.; Dai, X. Research Progress on Bioactive Components, Efficacy and Extraction Methods of Maize Silk. J. North. Agric. 2023, 51, 96–104. [Google Scholar]
- Wang, K.-J.; Zhao, J.-L. Corn Silk (Zea mays L.), a Source of Natural Antioxidants with α-Amylase, α-Glucosidase, Advanced Glycation and Diabetic Nephropathy Inhibitory Activities. Biomed. Pharmacother. 2019, 110, 510–517. [Google Scholar] [CrossRef]
- Landeros-Martínez, L.-L.; Campos-Almazán, M.I.; Sánchez-Bojorge, N.-A.; Flores, R.; Palomares-Báez, J.P.; Rodríguez-Valdez, L.M. Theoretical Studies for the Discovery of Potential Sucrase-Isomaltase Inhibitors from Maize Silk Phytochemicals: An Approach to Treatment of Type 2 Diabetes. Molecules 2023, 28, 6778. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, S.; Body, J.-J.; Bruyère, O.; Bergmann, P.; Brandi, M.L.; Cooper, C.; Devogelaer, J.-P.; Gielen, E.; Goemaere, S.; Kaufman, J.-M.; et al. Effects of Dairy Products Consumption on Health: Benefits and Beliefs—A Commentary from the Belgian Bone Club and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases. Calcif. Tissue Int. 2016, 98, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.; Liu, Y.; Wang, J.; Li, S.; Mao, X.; Zhou, Z.; Xing, H.; Yan, Q.; Zhao, W.; Zhang, F.; et al. Research Report on the High-quality Development Strategy of China’s Dairy Industry. China Dairy Cattle. 2023, 11, 1–15. [Google Scholar]
- Zhang, F.; Debras, C.; Matta, J.; Wang, D. Consumption of a milk low in lactose high in intrinsic fiber is associated with improved nutrient intake adequacies in Chinese adults: A diet modelling study. Proc. Nutr. Soc. 2024, 83, E375. [Google Scholar] [CrossRef]
- Schulz, P.; Rizvi, S.S.H. Hydrolysis of Lactose in Milk: Current Status and Future Products. Food Rev. Int. 2023, 39, 2875–2894. [Google Scholar] [CrossRef]
- Sharp, E.; D’Cunha, N.M.; Ranadheera, C.S.; Vasiljevic, T.; Panagiotakos, D.B.; Naumovski, N. Effects of Lactose-Free and Low-Lactose Dairy on Symptoms of Gastrointestinal Health: A Systematic Review. Int. Dairy J. 2021, 114, 104936. [Google Scholar] [CrossRef]
- Liburdi, K.; Esti, M. Galacto-Oligosaccharide (GOS) Synthesis during Enzymatic Lactose-Free Milk Production: State of the Art and Emerging Opportunities. Beverages 2022, 8, 21. [Google Scholar] [CrossRef]
- Dehghan, H.; Salehi, P.; Amiri, M.S. Bioassay-Guided Purification of α-Amylase, α-Glucosidase Inhibitors and DPPH Radical Scavengers from Roots of Rheum turkestanicum. Ind. Crops Prod. 2018, 117, 303–309. [Google Scholar] [CrossRef]
- Akkarachiyasit, S.; Yibchok-Anun, S.; Wacharasindhu, S.; Adisakwattana, S. In Vitro Inhibitory Effects of Cyandin-3-rutinoside on Pancreatic α-Amylase and Its Combined Effect with Acarbose. Molecules 2011, 16, 2075–2083. [Google Scholar] [CrossRef]
- Tao, Q.; Li, J.; Cao, W.; Chen, N. Inhibitory Mechanism and Stability of Peptide Tyr-Pro-Ile-Trp (YPIW) on α-Glucosidase. Food Sci. 2025, 46, 43–50. [Google Scholar]
- Zheng, Y.; Tian, J.; Yang, W.; Chen, S.; Liu, D.; Fang, H.; Zhang, H.; Ye, X. Inhibition mechanism of ferulic acid against α-amylase and α-glucosidase. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef]
- Chou, T.-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Verboven, K.; Van Ryckeghem, L.; Schweiggert, R.; B-Steingass, C.; Gojevic, T.; H-S Ruxton, C.; Hansen, D. Acute glycaemic response of orange juice consumption with breakfast in individuals with type 2 diabetes: A randomized cross-over trial. Nutr. Diabetes 2025, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Suarez, F.L.; Savaiano, D.A.; Levitt, M.D. A Comparison of Symptoms after the Consumption of Milk or Lactose-Hydrolyzed Milk by People with Self-Reported Severe Lactose Intolerance. N. Engl. J. Med. 1995, 333, 1–4. [Google Scholar] [CrossRef]
- Zhu, D.; Chinese Diabetes Society. Guideline for the Prevention and Treatment of Type 2 Diabetes Mellitus in China (2020 Edition) (Part 1). Chin. J. Pract. Intern. Med. 2021, 41, 668–6925. [Google Scholar]
- American Diabetes Association Professional Practice Committee. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2023, 47 (Suppl. 1), S20–S42. [Google Scholar] [CrossRef]
- WS/T 428-2013; Determination of Adult Body Weight. China Standards Press: Beijing, China, 2013.
- Åberg, S.; Mann, J.; Neumann, S.; Ross, A.B.; Reynolds, A.N. Whole-Grain Processing and Glycemic Control in Type 2 Diabetes: A Randomized Crossover Trial. Diabetes Care 2020, 43, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zheng, D.; Zhang, X.; Du, B.; Sun, Q. Analysis of Chemical Composition and Biological Activity of Mulberry Leaf Polysaccharides in Cold Regions. Food Sci. 2024, 45, 59–66. [Google Scholar]
- Gong, C.; Li, Y.; Lian, Y.; Zheng, C.; Sun, C.; Wang, T. Study on Isolation, Purification, Structural Characterization and Hypoglycemic Activity of Maize Silk Polysaccharides. Food Ferment. Ind. 2025; 1–13. [Google Scholar] [CrossRef]
- Tracey, M.; Gregory, M.; Fahim, A.; Cindy, L.; Gerald, R. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metab. Clin. Exp. 2004, 53, 495–499. [Google Scholar]
- Augustynowicz, G.; Lasocka, M.; Paweł Szyller, H.; Dziedziak, M.; Mytych, A.; Braksator, J.; Pytrus, T. The Role of Gut Microbiota in the Development and Treatment of Obesity and Overweight: A Literature Review. J. Clin. Med. 2025, 14, 4933. [Google Scholar] [CrossRef] [PubMed]
- Lange, O.; Proczko-Stepaniak, M.; Mika, A. Short-Chain Fatty Acids-A Product of the Microbiome and Its Participation in Two-Way Communication on the Microbiome-Host Mammal Line. Curr. Obes. Rep. 2023, 12, 108–126. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Wang, J.; Kang, T.; Xu, F.; Ma, A. Effects of Resistant Starch on Glycaemic Control: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2021, 125, 1260–1269. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).