Soy Protein Outperforms Whey Protein in Ameliorating Insulin Resistance but Not Obesity in High-Fat Diet-Induced Obese Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Diets
2.3. Food Intake, Water Consumption and Body Weight
2.4. Serum Parameter Analysis
2.5. Histopathology
2.6. TG and TC Levels in Feces and Liver Tissues
2.7. Western Blotting
2.8. Metabolomics Analysis
2.9. Statistical Analysis
3. Results
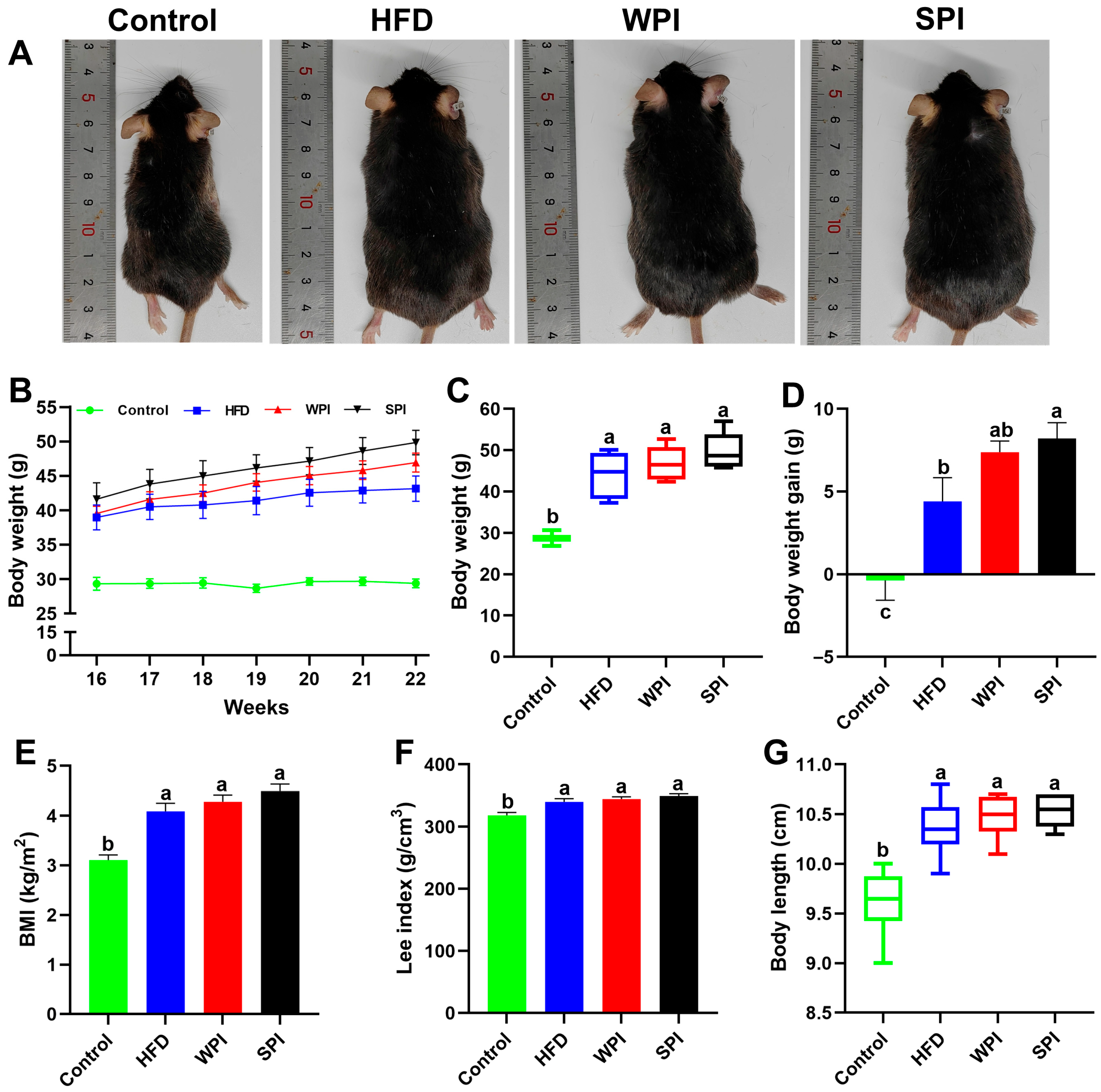
3.1. Effect of WPI and SPI on Body Weight
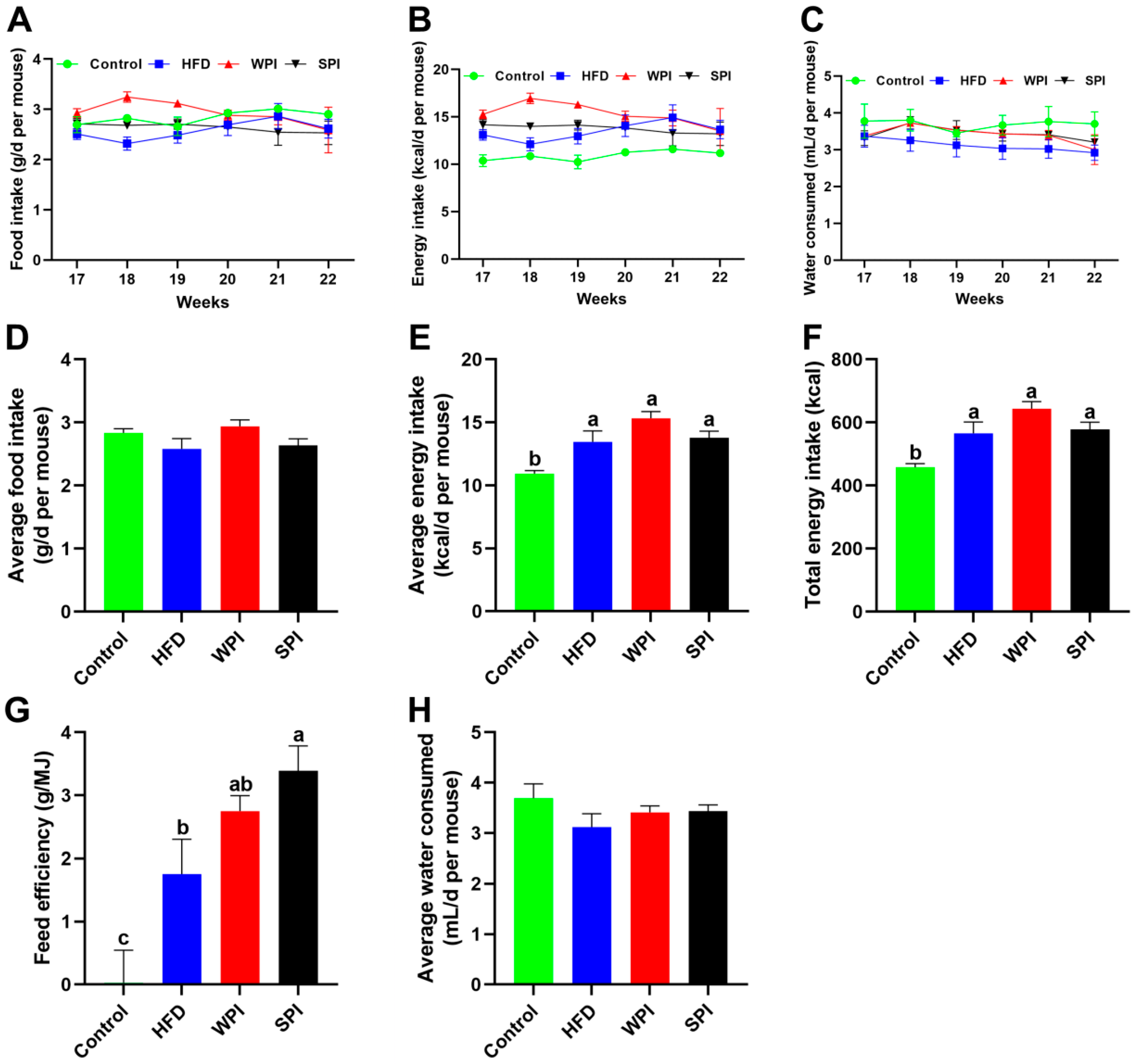
3.2. Effect of WPI and SPI on Food Intake, Energy Intake and Water Consumption
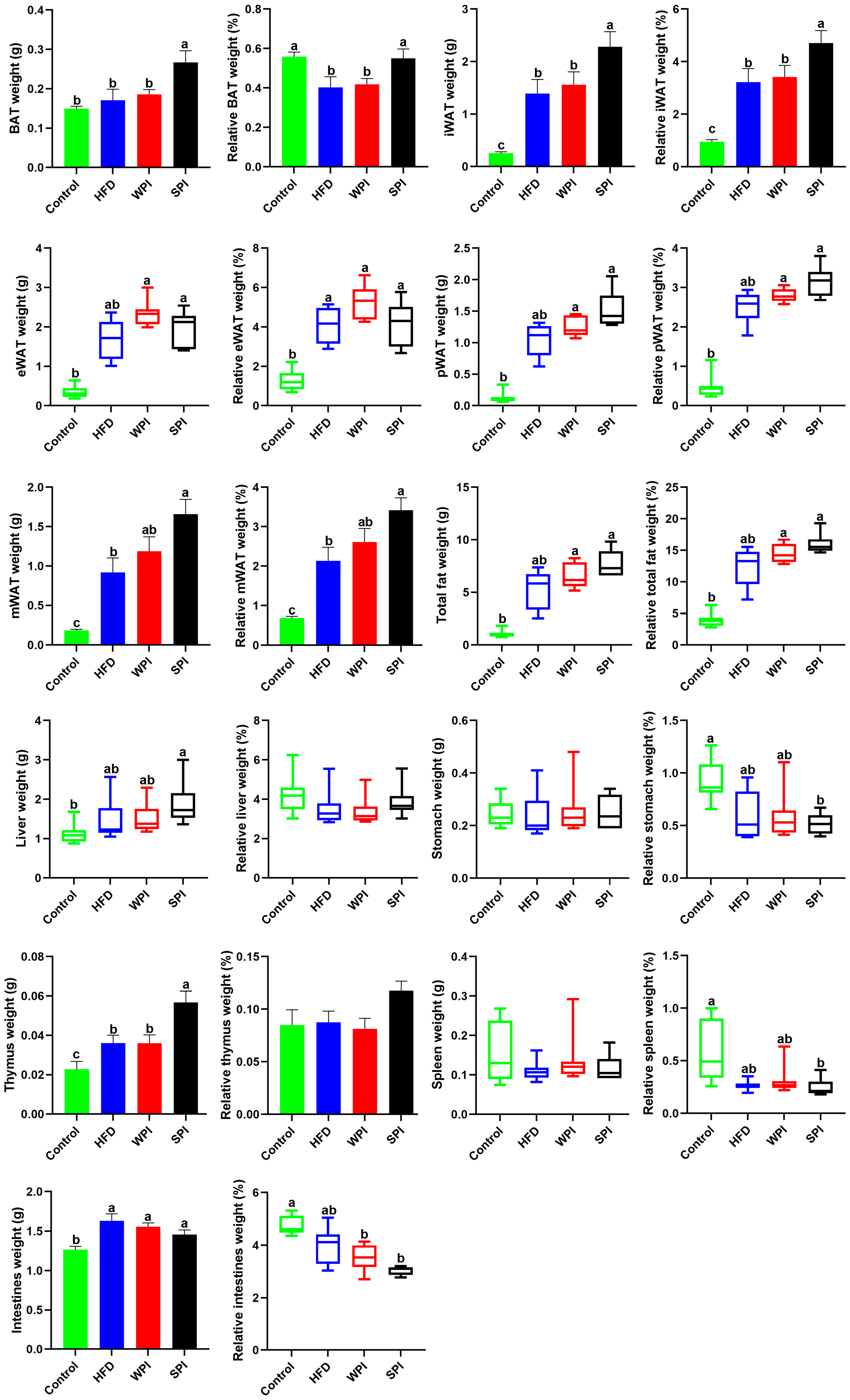
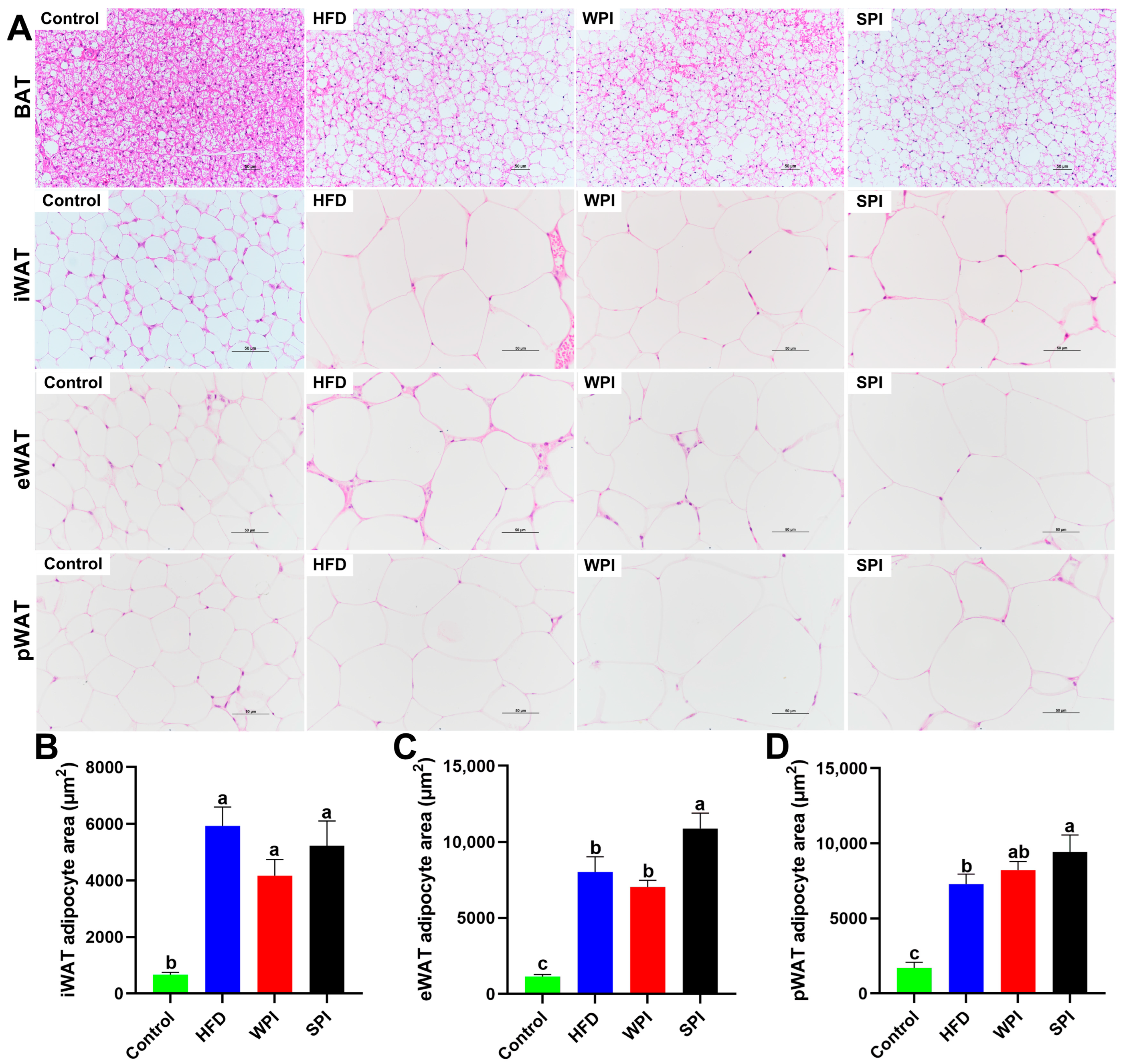
3.3. Effect of WPI and SPI on Adipose Tissue and Organ Mass
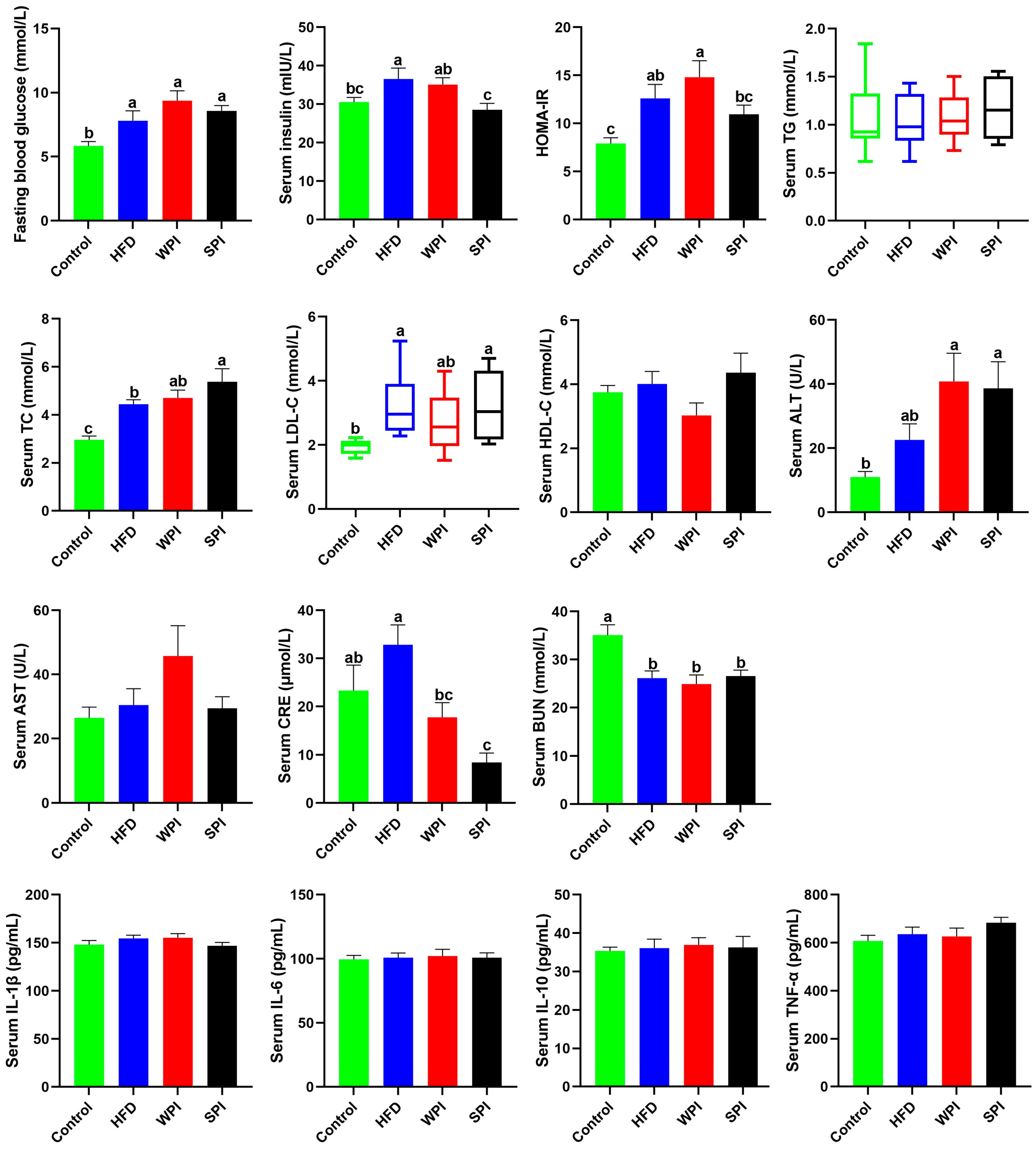
3.4. Effect of WPI and SPI on Serum Parameters
3.5. Effect of WPI and SPI on Serum Appetite-Related Hormone Levels
3.6. Effects of WPI and SPI on Fecal TG and TC Levels
3.7. Effects of WPI and SPI on Adipose Tissue Morphology
3.8. Effects of WPI and SPI on Liver Morphology
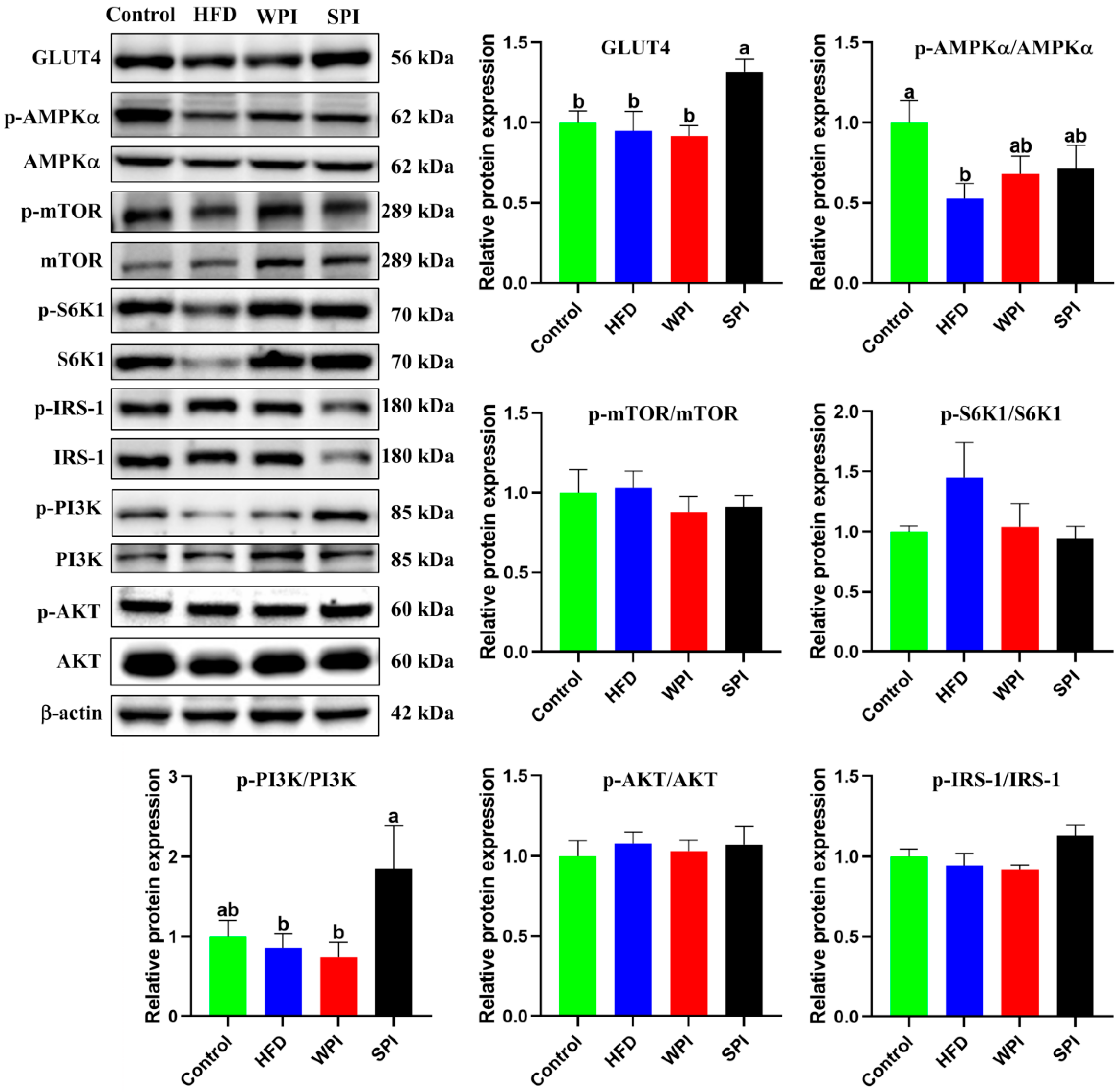
3.9. Effects of WPI and SPI on the Expression of Hepatic Insulin Signaling-Related Proteins
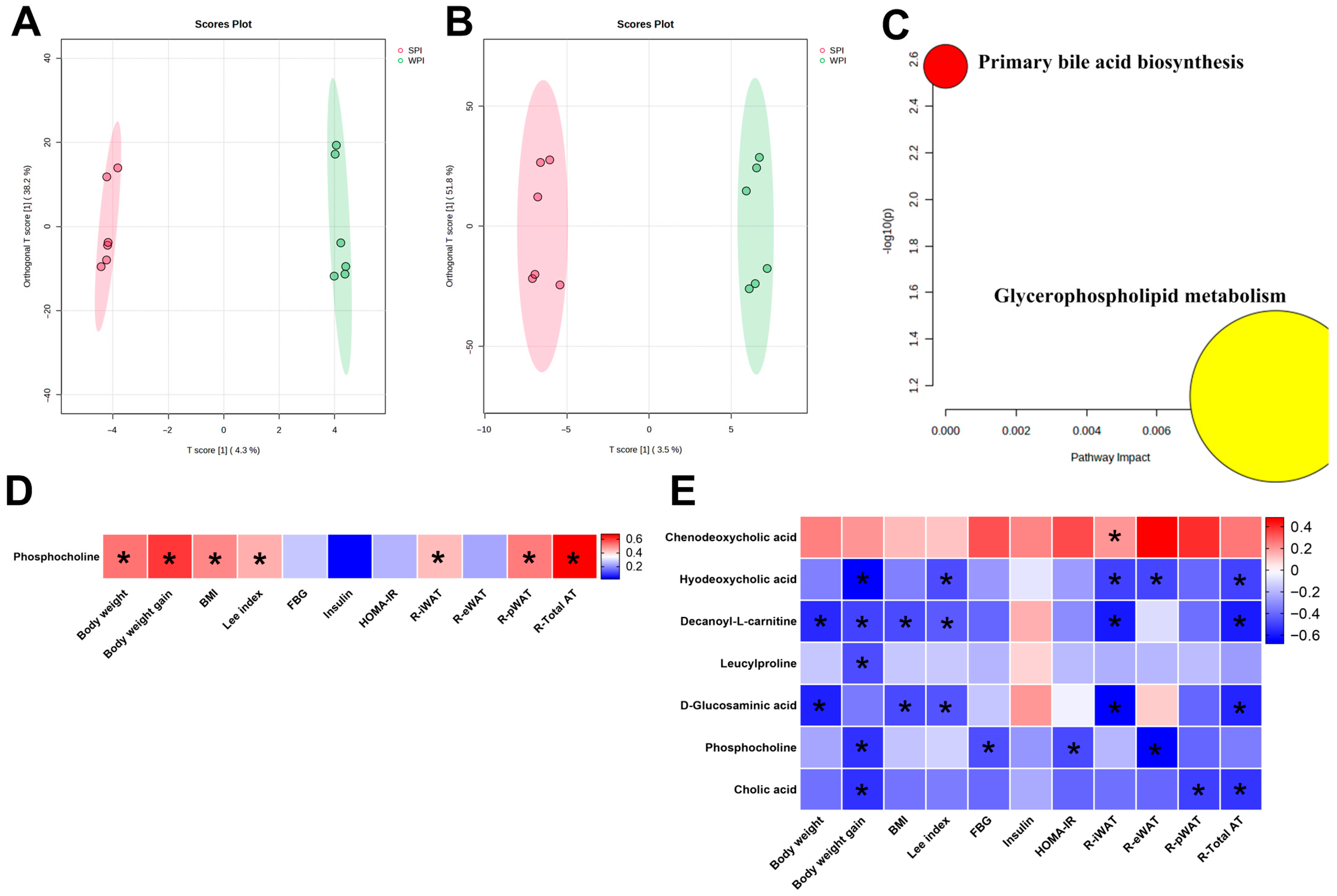
3.10. Effects of WPI and SPI on Serum and Liver Metabolic Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Obesity Federation. World Obesity Atlas 2025; World Obesity Federation: London, UK, 2025; Available online: https://data.worldobesity.org/publications/?cat=23 (accessed on 15 October 2025).
- International Diabetes Federation. IDF Diabetes Atlas, 11th ed.; International Diabetes Federation: Brussels, Belgium, 2025; Available online: https://diabetesatlas.org (accessed on 15 October 2025).
- Liisberg, U.; Myrmel, L.S.; Fjære, E.; Rønnevik, A.K.; Bjelland, S.; Fauske, K.R.; Holm, J.B.; Basse, A.L.; Hansen, J.B.; Liaset, B.; et al. The protein source determines the potential of high protein diets to attenuate obesity development in C57BL/6J mice. Adipocyte 2016, 5, 196–211. [Google Scholar] [CrossRef] [PubMed]
- McAllan, L.; Skuse, P.; Cotter, P.D.; O’Connor, P.; Cryan, J.F.; Ross, R.P.; Fitzgerald, G.; Roche, H.M.; Nilaweera, K.N. Protein quality and the protein to carbohydrate ratio within a high fat diet influences energy balance and the gut microbiota in C57BL/6J mice. PLoS ONE 2014, 9, e88904. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Clifton, P.; Gannon, M.C.; Krauss, R.M.; Nuttall, F.Q. Protein in optimal health: Heart disease and type 2 diabetes. Am. J. Clin. Nutr. 2008, 87, 1571s–1575s. [Google Scholar] [CrossRef]
- Chalvon-Demersay, T.; Azzout-Marniche, D.; Arfsten, J.; Egli, L.; Gaudichon, C.; Karagounis, L.G.; Tomé, D. A Systematic Review of the Effects of Plant Compared with Animal Protein Sources on Features of Metabolic Syndrome. J. Nutr. 2017, 147, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Yang, Y.; Yang, Z.; Feng, X.; Wang, X.; Zhang, Z.; Hu, R. Insulin resistance in Chinese patients with type 2 diabetes is associated with C-reactive protein independent of abdominal obesity. Cardiovasc. Diabetol. 2010, 9, 92. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Bickel, P.E.; Scherer, P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug Discov. 2016, 15, 639–660. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Pal, S.; Radavelli-Bagatini, S. The effects of whey protein on cardiometabolic risk factors. Obes. Rev. 2013, 14, 324–343. [Google Scholar] [CrossRef]
- Badely, M.; Sepandi, M.; Samadi, M.; Parastouei, K.; Taghdir, M. The effect of whey protein on the components of metabolic syndrome in overweight and obese individuals; a systematic review and meta-analysis. Diabetes Metab. Syndr. 2019, 13, 3121–3131. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Bhathena, S.J. Role of dietary soy protein in obesity. Int. J. Med. Sci. 2007, 4, 72–82. [Google Scholar] [CrossRef]
- Zhou, S.; Cheng, F.; He, J.; Xu, T.; Zhang, X.; Wan, S.; Qi, J.; He, J.; Chen, F.; Luo, J.; et al. Effects of high-quality protein supplementation on cardiovascular risk factors in individuals with metabolic diseases: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2024, 43, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Tahavorgar, A.; Vafa, M.; Shidfar, F.; Gohari, M.; Heydari, I. Whey protein preloads are more beneficial than soy protein preloads in regulating appetite, calorie intake, anthropometry, and body composition of overweight and obese men. Nutr. Res. 2014, 34, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Baer, D.J.; Stote, K.S.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Clevidence, B.A. Whey protein but not soy protein supplementation alters body weight and composition in free-living overweight and obese adults. J. Nutr. 2011, 141, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Silva Ton, W.T.; das Graças de Almeida, C.; de Morais Cardoso, L.; Marvila Girondoli, Y.; Feliciano Pereira, P.; Viana Gomes Schitini, J.K.; Galvão Cândido, F.; Marques Arbex, P.; de Cássia Gonçalves Alfenas, R. Effect of different protein types on second meal postprandial glycaemia in normal weight and normoglycemic subjects. Nutr. Hosp. 2014, 29, 553–558. [Google Scholar]
- Wróblewska, B.; Juśkiewicz, J. The effects of whey and soy proteins on growth performance, gastrointestinal digestion, and selected physiological responses in rats. Food Funct. 2018, 9, 1500–1509. [Google Scholar] [CrossRef]
- Jia, Y.; Leng, Y.; Cruz, A.L.P.; Bao, C.L.; Bao, B.; Wu, W.; Wang, P.; Ma, M. The Effect of Oral Nutritional Formula With Three Different Proteins on Type 2 Diabetes Mellitus in vivo. Front. Nutr. 2021, 8, 680700. [Google Scholar] [CrossRef]
- Wei, T.; Jia, Y.; Xue, W.; Ma, M.; Wu, W. Nutritional Effects of the Enteral Nutritional Formula on Regulation of Gut Microbiota and Metabolic Level in Type 2 Diabetes Mellitus Mice. Diabetes Metab. Syndr. Obes. 2021, 14, 1855–1869. [Google Scholar] [CrossRef]
- Aoyama, T.; Fukui, K.; Nakamori, T.; Hashimoto, Y.; Yamamoto, T.; Takamatsu, K.; Sugano, M. Effect of soy and milk whey protein isolates and their hydrolysates on weight reduction in genetically obese mice. Biosci. Biotechnol. Biochem. 2000, 64, 2594–2600. [Google Scholar] [CrossRef]
- Cain, J.; Banz, W.J.; Butteiger, D.; Davis, J.E. Soy protein isolate modified metabolic phenotype and hepatic Wnt signaling in obese Zucker rats. Horm. Metab. Res. 2011, 43, 774–781. [Google Scholar] [CrossRef]
- Ji, A.; Chen, W.; Zhang, T. Whey protein and soy protein prevent obesity by upregulating uncoupling protein 1 to activate brown adipose tissue and promote white adipose tissue browning in high-fat diet-fed mice. Food Funct. 2022, 13, 12836–12851. [Google Scholar] [CrossRef]
- Ji, A.; Chen, W.; Liu, C.; Zhang, T. Soy protein compared with whey protein ameliorates insulin resistance by regulating lipid metabolism, AMPK/mTOR pathway and gut microbiota in high-fat diet-fed mice. Food Funct. 2023, 14, 5752–5767. [Google Scholar] [CrossRef]
- Tong, X. Effect of Whey Protein on Combating Obesity and Potential Mechanisms. Ph.D. Thesis, Soochow University, Suzhou, China, 2018. [Google Scholar]
- McAllan, L.; Keane, D.; Schellekens, H.; Roche, H.M.; Korpela, R.; Cryan, J.F.; Nilaweera, K.N. Whey protein isolate counteracts the effects of a high-fat diet on energy intake and hypothalamic and adipose tissue expression of energy balance-related genes. Br. J. Nutr. 2013, 110, 2114–2126. [Google Scholar] [CrossRef]
- Zhang, X.; Hartmann, P. How to calculate sample size in animal and human studies. Front. Med. 2023, 10, 1215927. [Google Scholar] [CrossRef]
- Lin, X.; Yang, Y.; Qu, J.; Wang, X. Aerobic exercise decreases chemerin/CMKLR1 in the serum and peripheral metabolic organs of obesity and diabetes rats by increasing PPARγ. Nutr. Metab. 2019, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, X. Effects of cyclophosphamide on immune system and gut microbiota in mice. Microbiol. Res. 2015, 171, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ni, X.; Song, X.; Wen, B.; Zhou, Y.; Zou, F.; Yang, M.; Peng, Z.; Zhu, H.; Zeng, Y.; et al. Fermented Yupingfeng polysaccharides enhance immunity by improving the foregut microflora and intestinal barrier in weaning rex rabbits. Appl. Microbiol. Biotechnol. 2016, 100, 8105–8120. [Google Scholar] [CrossRef]
- Xiao, P.; Huang, H.; Li, X.; Chen, J.; Duan, J.A. Characterization, evaluation of nutritional parameters of Radix isatidis protein and its antioxidant activity in D-galactose induced ageing mice. BMC Complement. Altern. Med. 2019, 19, 297. [Google Scholar] [CrossRef]
- Alexander, M.; Cho, E. Pathology of Diabetes-Induced Immune Dysfunction. Int. J. Mol. Sci. 2024, 25, 7105. [Google Scholar] [CrossRef]
- Qin, W.; Sun, L.; Dong, M.; An, G.; Zhang, K.; Zhang, C.; Meng, X. Regulatory T Cells and Diabetes Mellitus. Hum. Gene Ther. 2021, 32, 875–881. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J. Effects of exercise intervention on serum-related factors and immunity in a rat model of insulin resistance. Chin. J. Tissue Eng. Res. 2016, 20, 4075–4082. (In Chinese) [Google Scholar]
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005, 111, 1448–1454. [Google Scholar] [CrossRef]
- Segal, K.R.; Landt, M.; Klein, S. Relationship between insulin sensitivity and plasma leptin concentration in lean and obese men. Diabetes 1996, 45, 988–991. [Google Scholar] [CrossRef]
- Yadav, A.; Jyoti, P.; Jain, S.K.; Bhattacharjee, J. Correlation of adiponectin and leptin with insulin resistance: A pilot study in healthy north Indian population. Indian J. Clin. Biochem. 2011, 26, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Kanemaru, Y.; Harada, N.; Shimazu-Kuwahara, S.; Yamane, S.; Ikeguchi, E.; Murata, Y.; Kiyobayashi, S.; Hatoko, T.; Inagaki, N. Absence of GIP secretion alleviates age-related obesity and insulin resistance. J. Endocrinol. 2020, 245, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Nasteska, D.; Harada, N.; Suzuki, K.; Yamane, S.; Hamasaki, A.; Joo, E.; Iwasaki, K.; Shibue, K.; Harada, T.; Inagaki, N. Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high-fat diet conditions. Diabetes 2014, 63, 2332–2343. [Google Scholar] [CrossRef] [PubMed]
- Shimazu-Kuwahara, S.; Harada, N.; Yamane, S.; Joo, E.; Sankoda, A.; Kieffer, T.J.; Inagaki, N. Attenuated secretion of glucose-dependent insulinotropic polypeptide (GIP) does not alleviate hyperphagic obesity and insulin resistance in ob/ob mice. Mol. Metab. 2017, 6, 288–294. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]
- James, D.E.; Stöckli, J. The aetiology and molecular landscape of insulin resistance. Nat. Rev. Mol. Cell Biol. 2021, 22, 751–771. [Google Scholar] [CrossRef]
- Yan, J.; Wang, C.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, Z.; Liu, K.; Sun, H. Catalpol ameliorates hepatic insulin resistance in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway. Pharmacol. Res. 2018, 130, 466–480. [Google Scholar] [CrossRef]
- Tremblay, F.; Marette, A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J. Biol. Chem. 2001, 276, 38052–38060. [Google Scholar] [CrossRef]
- Das, D.; Afzal, N.U.; Wann, S.B.; Kalita, J.; Manna, P. A ~24 kDa protein isolated from protein isolates of Hawaijar, popular fermented soy food of North-East India exhibited promising antidiabetic potential via stimulating PI3K/AKT/GLUT4 signaling pathway of muscle glucose metabolism. Int. J. Biol. Macromol. 2023, 224, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, Z.; Liu, M.; Ren, Y.; Ruan, Y.; Li, D. Relationship between the n-3 index, serum metabolites and breast cancer risk. Food Funct. 2021, 12, 7741–7748. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Wang, J.; He, X.; Huang, M.; Yang, X.; He, L.; Qiu, Y.; Lou, Y. Plasma metabolites, especially lipid metabolites, are altered in pregnant women with gestational diabetes mellitus. Clin. Chim. Acta 2021, 517, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Hatch, G.M.; Wang, Y.; Yu, F.; Wang, M. The relationship between phospholipids and insulin resistance: From clinical to experimental studies. J. Cell. Mol. Med. 2019, 23, 702–710. [Google Scholar] [CrossRef]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef]
- Fukase, N.; Igarashi, M.; Takahashi, H.; Manaka, H.; Yamatani, K.; Daimon, M.; Tominaga, M.; Sasaki, H. Hypersecretion of truncated glucagon-like peptide-1 and gastric inhibitory polypeptide in obese patients. Diabet. Med. 1993, 10, 44–49. [Google Scholar] [CrossRef]
- Eckel, R.H.; Fujimoto, W.Y.; Brunzell, J.D. Gastric inhibitory polypeptide enhanced lipoprotein lipase activity in cultured preadipocytes. Diabetes 1979, 28, 1141–1142. [Google Scholar] [CrossRef]
- Getty-Kaushik, L.; Song, D.H.; Boylan, M.O.; Corkey, B.E.; Wolfe, M.M. Glucose-dependent insulinotropic polypeptide modulates adipocyte lipolysis and reesterification. Obesity 2006, 14, 1124–1131. [Google Scholar] [CrossRef]
- Song, D.H.; Wolfe, M.M. Glucose-dependent insulinotropic polypeptide and its role in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 46–51. [Google Scholar] [CrossRef]
- Blanco Mejia, S.; Messina, M.; Li, S.S.; Viguiliouk, E.; Chiavaroli, L.; Khan, T.A.; Srichaikul, K.; Mirrahimi, A.; Sievenpiper, J.L.; Kris-Etherton, P.; et al. A Meta-Analysis of 46 Studies Identified by the FDA Demonstrates that Soy Protein Decreases Circulating LDL and Total Cholesterol Concentrations in Adults. J. Nutr. 2019, 149, 968–981. [Google Scholar] [CrossRef]
- Maki, K.C.; Butteiger, D.N.; Rains, T.M.; Lawless, A.; Reeves, M.S.; Schasteen, C.; Krul, E.S. Effects of soy protein on lipoprotein lipids and fecal bile acid excretion in men and women with moderate hypercholesterolemia. J. Clin. Lipidol. 2010, 4, 531–542. [Google Scholar] [CrossRef]
- Wilson, T.A.; DeSimone, A.P.; Romano, C.A.; Nicolosi, R.J. Corn fiber oil lowers plasma cholesterol levels and increases cholesterol excretion greater than corn oil and similar to diets containing soy sterols and soy stanols in hamsters. J. Nutr. Biochem. 2000, 11, 443–449. [Google Scholar] [CrossRef]
- Lin, Y.; Meijer, G.W.; Vermeer, M.A.; Trautwein, E.A. Soy protein enhances the cholesterol-lowering effect of plant sterol esters in cholesterol-fed hamsters. J. Nutr. 2004, 134, 143–148. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, A.; Qi, Y.; Zhao, K.; Niu, J.; Shi, R.; Qi, Z.; Zhou, L.; Zhao, C.; Li, D. Soy Protein Outperforms Whey Protein in Ameliorating Insulin Resistance but Not Obesity in High-Fat Diet-Induced Obese Mice. Nutrients 2025, 17, 3427. https://doi.org/10.3390/nu17213427
Ji A, Qi Y, Zhao K, Niu J, Shi R, Qi Z, Zhou L, Zhao C, Li D. Soy Protein Outperforms Whey Protein in Ameliorating Insulin Resistance but Not Obesity in High-Fat Diet-Induced Obese Mice. Nutrients. 2025; 17(21):3427. https://doi.org/10.3390/nu17213427
Chicago/Turabian StyleJi, Andong, Yuxia Qi, Kuan Zhao, Juanjuan Niu, Runjia Shi, Zhongshi Qi, Liying Zhou, Chunhui Zhao, and Duo Li. 2025. "Soy Protein Outperforms Whey Protein in Ameliorating Insulin Resistance but Not Obesity in High-Fat Diet-Induced Obese Mice" Nutrients 17, no. 21: 3427. https://doi.org/10.3390/nu17213427
APA StyleJi, A., Qi, Y., Zhao, K., Niu, J., Shi, R., Qi, Z., Zhou, L., Zhao, C., & Li, D. (2025). Soy Protein Outperforms Whey Protein in Ameliorating Insulin Resistance but Not Obesity in High-Fat Diet-Induced Obese Mice. Nutrients, 17(21), 3427. https://doi.org/10.3390/nu17213427





