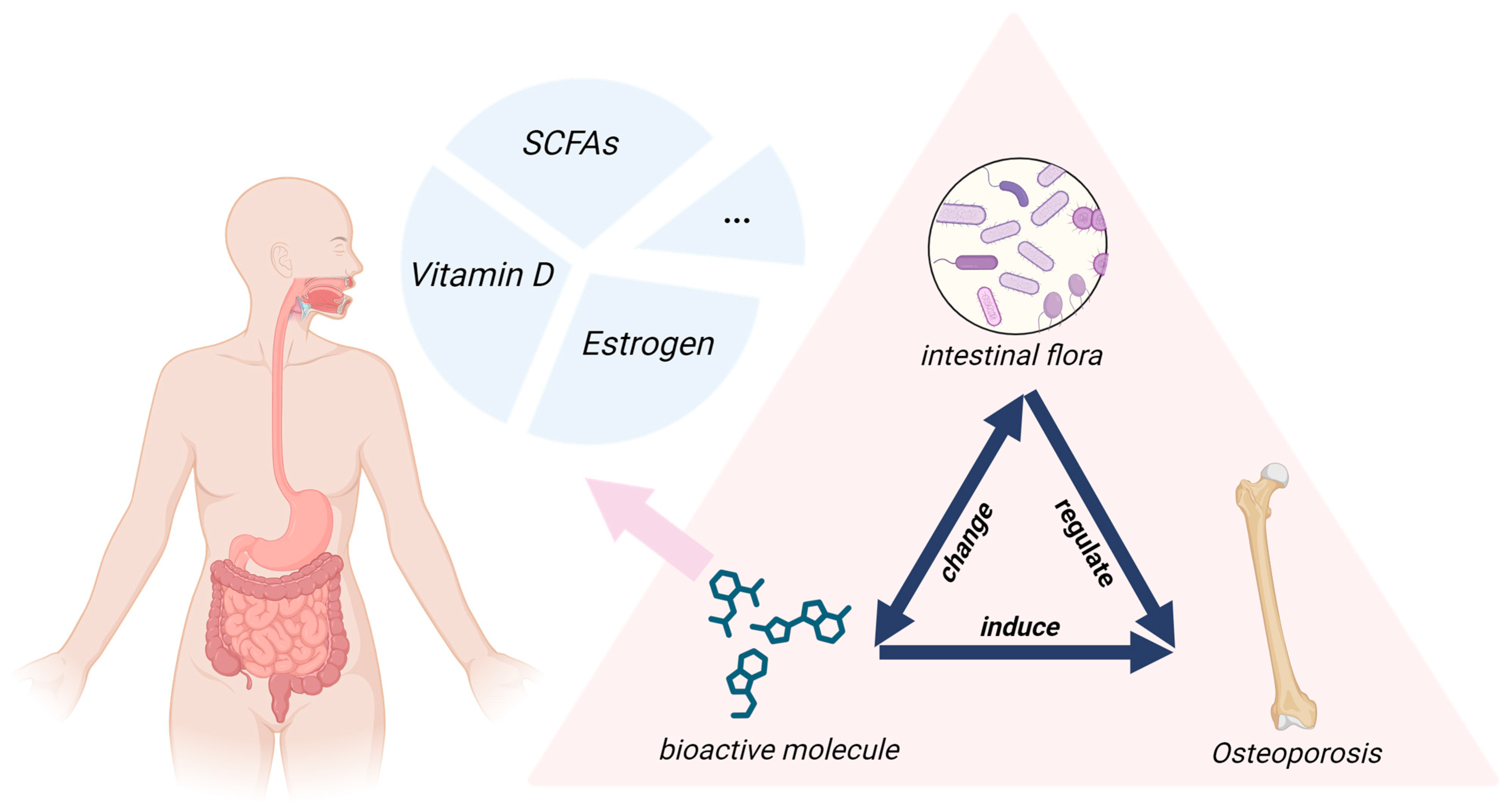
The Mediating Role of Bioactive Molecules in Gut Microbiota–Bone Metabolism Crosstalk
Abstract
1. Introduction
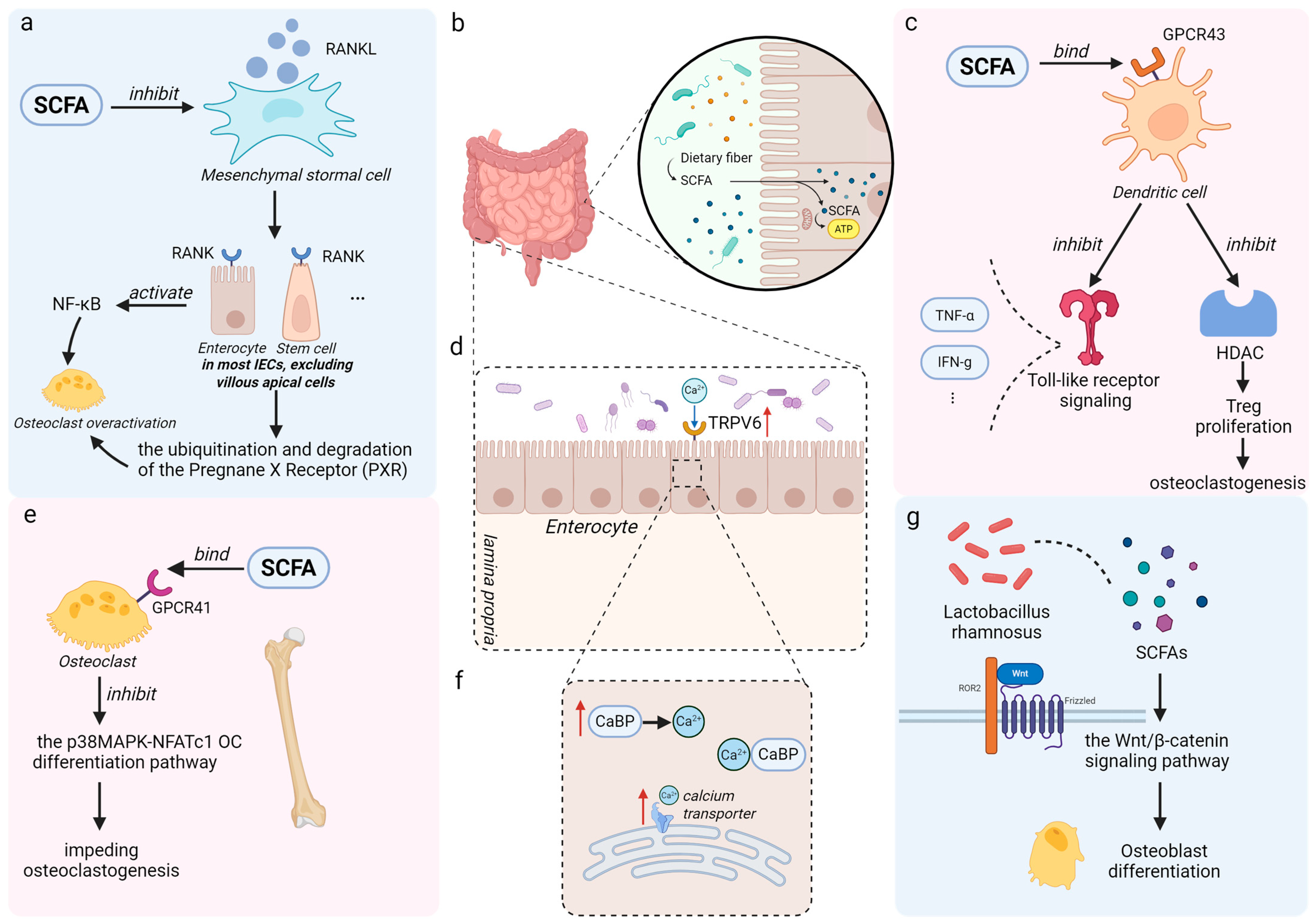
2. Short-Chain Fatty Acids (SCFAs)
2.1. Endocrine Signaling Pathways Mediating SCFAs-Induced Osteoporosis Alleviation
| SCFA Type | Representative Producing Microbes | Proposed Bone-Protective Mechanisms |
|---|---|---|
| Acetate | Akkermansia muciniphila Bacteroides spp. Bifidobacterium spp. | 1. Maintains systemic metabolic homeostasis [47]. 2. Lowers intestinal pH, enhancing the solubility and absorption of minerals (e.g., calcium) [48]. 3. Activates GPR43 to indirectly inhibit bone resorption [49]. |
| Propionate | Bacteroides spp. Prevotella spp. Veillonella spp. | 1. Suppresses NF-κB signaling by activating GPCRs and inhibiting HDAC, reducing osteoclast differentiation [50]. 2. Promotes Treg expansion and inhibits pro-osteoclastogenic cytokines (e.g., TNF-α, IL-1β) [51]. |
| Butyrate | Faecalibacterium prausnitzii Eubacterium rectale Roseburia spp. | 1. Inhibits HDAC, leading to histone hyperacetylation-mediated suppression of osteoclastogenic genes (e.g., NFATc1) [52]. 2. Promotes osteoblast differentiation and mineralization through Wnt/β-catenin pathway activation [45]. 3. Maintains intestinal barrier integrity, thus reducing systemic inflammation from bacterial toxins [25]. |
2.2. Factors Associated with Inflammation Affecting the Modulation of SCFAs in Osteoporosis
2.2.1. Probiotics, Prebiotics, and SCFAs Production
2.2.2. Dietary Intervention and Environmental Factors
2.3. Potential Approaches to Relieve Osteoporosis via SCFAs
| Category | Treatment Method | Microbiota Targeted | SCFAs Produced | Mechanism |
|---|---|---|---|---|
| Biomaterials/Engineered Nanoparticles | Spirulina platensis (SP) | Turicibacter Firmicutes Bacteroidetes | Propionate Butyrate | Reduces oxidative stress, enhances Wnt signaling, and suppresses osteoclast formation, significantly improving bone mineral density (BMD) [86]. |
| Sheep bone protein Hydrolysate | Thick-walled Bacteria Proteobacteria Verrucomicrobia | Propionate Butyrate |
| |
| Β-TCP/P (3 HB) bracket | — | 3-Hydroxybutyric acid | Converts 3-hydroxybutyrate to 3-hydroxybutyric acid, supporting tissue health and reducing osteoporosis [87]. | |
| Colon-targeted engineered postbiotics nanoparticles | Shigella dysenteriae Alistipes | Butyrate |
| |
| Prebiotics | Inulin | Allobaculum Bifidobacterium | Acetate Propionate Butyrate |
|
| Cistanche deserticola Polysaccharide (CDPS) | Butyrate-producing bacteria (e.g., Lachnospiraceae NK4A136 group) | Butyrate | Suppresses overactivation of the SRC/EGFR/PI3K/AKT signaling axis [89]. | |
| Lycium barbarum polysaccharide (LBP) | Sclerobacillus Lactobacillus Turicibacter Clostridium_sensu_stricto_1 Faecalibacterium Adlercreutzia | Acetate Propionate Butyrate | Upregulates alkaline phosphatase (ALP) * biosynthesis and enzymatic activity, promoting osteoblast differentiation and maturation [90]. | |
| Fructooligosaccharide (FOS) | Bifidobacterium | Butyrate | Enhances peak bone mass (PBM) and prevents estrogen deficiency-induced bone loss by selectively stimulating new bone formation [68]. | |
| Diet | Green tea | Akkermansia | Butyrate | Significantly modulates gut microbiota, enhances intestinal antioxidant capacity, and regulates bone metabolism [91,92]. |
| Calcium-fortified diets | Acinetobacter Propionibacterium | Acetate, Propionate | Increases luminal soluble/available calcium and stimulates expression of calcium absorption-related genes, ultimately improving bone mineral density (BMD), bone mineral content (BMC), and femoral mechanical strength [64]. | |
| Mediterranean diet | Bacteroidetes Thick-walled Bacteria | Propionate Butyrate | Modulates specific gut microbiota associated with osteoclast suppression and promotes SCFA production [93]. | |
| Vegetarian Diet | Bacteroides Prevotella | Acetate Propionate Butyrate | Reduces bone metabolic disorders, provided adequate intake of calcium, vitamin D, and protein is maintained [9]. |
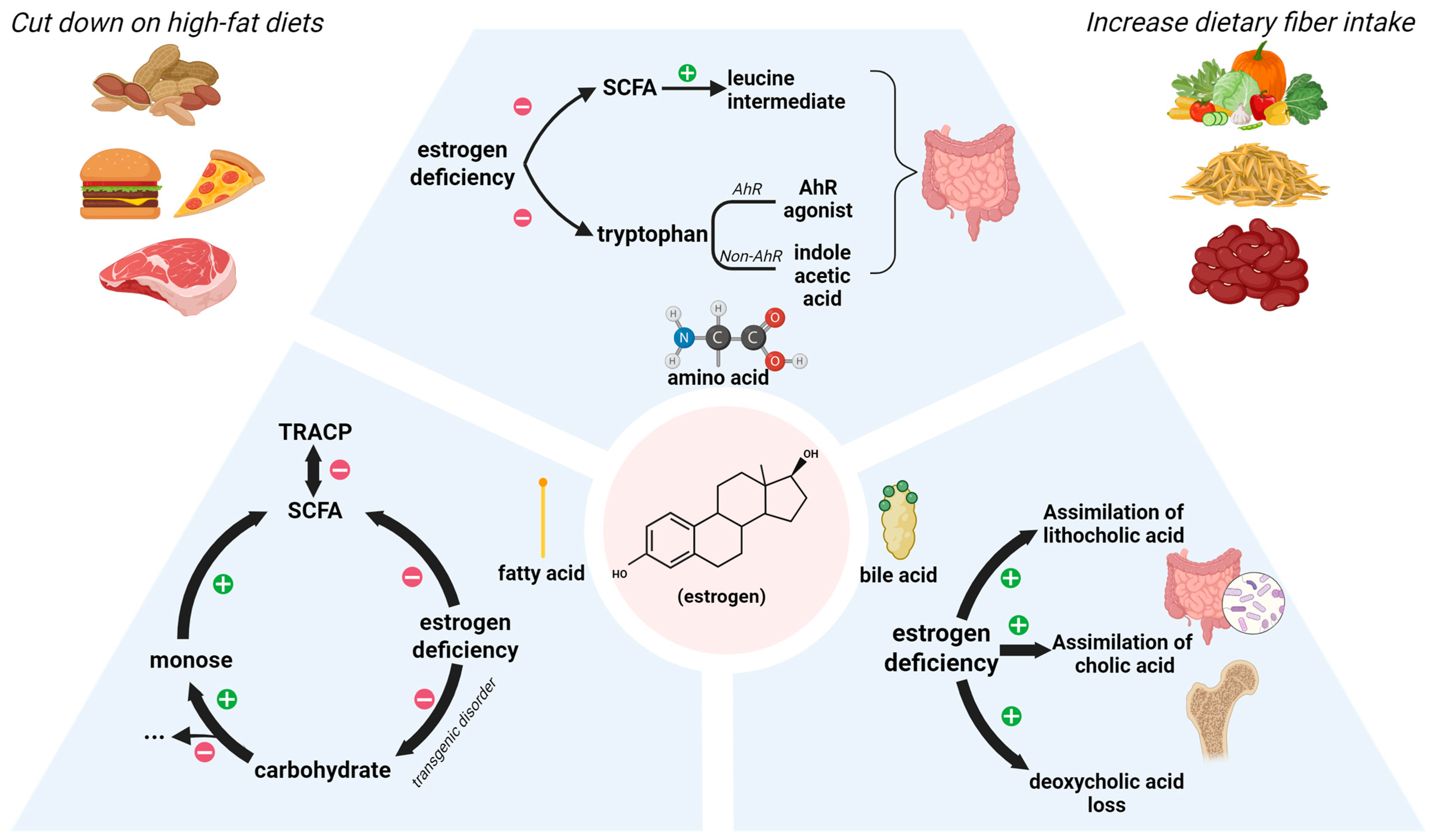
3. Estrogen
3.1. The Role of Estrogen in Intestinal Metabolism
3.1.1. Lipid Metabolism
3.1.2. Amino Acid Metabolism
3.1.3. Bile Acid Metabolism
3.1.4. Practical Therapeutic Implications and Clinical Relevance
3.2. Research Progress on Estrogen–Gut Microbiota Interactions and Associated Limitations
3.3. ERα/β and Metabolite Receptor and Gut Microbiota Dysbiosis-Induced Osteoporosis
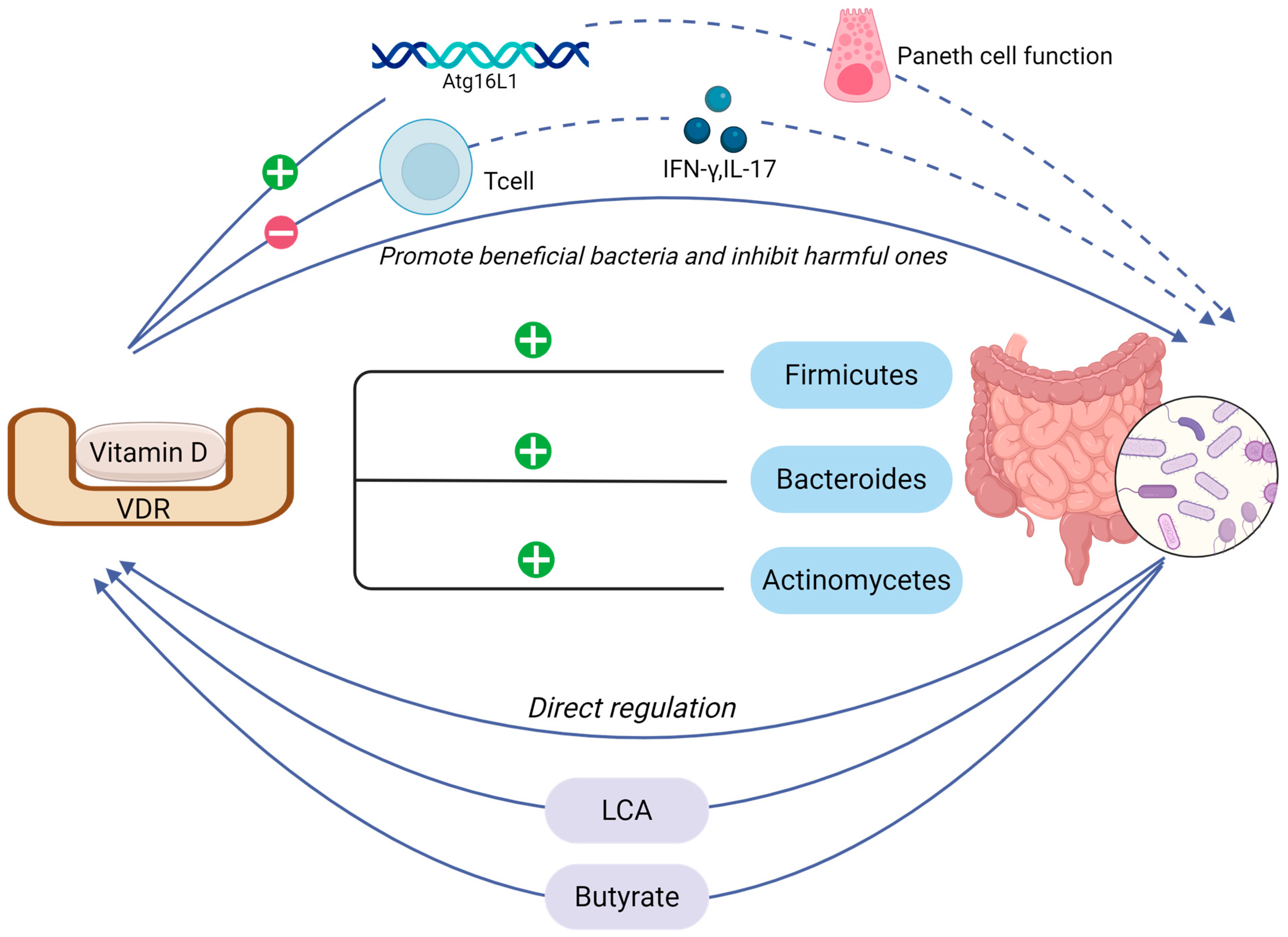
4. Vitamin D
4.1. The Symphonic Interplay Between Vitamin D Signaling and Intestinal Mechanical Barrier Homeostasis
4.2. Interaction Between Vitamin D and Intestinal Microbiota
4.3. Vitamin D-Driven Recombination of Intestinal Flora for Osteoporosis Treatment
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCFAs | short-chain fatty acids |
| BMD | bone mineral density |
| OVX | ovariectomized |
| ORX | orchiectomized |
| OP | osteoporosis |
| HC | healthy controls |
| PA | propionic acid |
| MS | multiple sclerosis |
| RANKL | Receptor Activator of Nuclear Factor Kappa-B Ligand |
| PXR | Pregnane X Receptor |
| GPCRs | G protein-coupled receptors |
| FFAR2/GPR43 | free fatty acid receptor 2 |
| LPS | lipopolysaccharide |
| HIO | high-fat diet-induced obese |
| NO | non-obese |
| PTH | parathyroid hormone |
| IGF-1 | insulin-like growth factor-1 |
| GF | germ-free |
| CaBP | calbindin-D9k |
| TRPV6 | transient receptor potential vanilloid 6 |
| TBP | Tuna Bone Powder |
| GNPs | Gold Nanoparticles |
| scFOS | short-chain fructooligosaccharides |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| FOS | fructooligosaccharide |
| GOS | galactooligosaccharides |
| HFD | high-fat diet |
| DIO | disuse-induced osteoporotic |
| EDCs | endocrine-disrupting chemicals |
| ZEA | zearalenone |
| ER | estrogen receptor |
| HPG | hypothalamic–pituitary–gonadal |
| HRT | hormone replacement therapy |
| PMO | postmenopausal osteoporosis |
| IL-10 | interleukin-10 |
| LCA | lithocholic acid |
| VDR | vitamin D receptor |
| UV | ultraviolet |
| FMT | fecal microbiota transplantation |
References
- Lin, L.; Guo, Z.; He, E.; Long, X.; Wang, D.; Zhang, Y.; Guo, W.; Wei, Q.; He, W.; Wu, W.; et al. SIRT2 regulates extracellular vesicle-mediated liver-bone communication. Nat. Metab. 2023, 5, 821–841. [Google Scholar] [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Vázquez-Lorente, H.; García-Gavilán, J.F.; Shyam, S.; Konieczna, J.; Martínez, J.A.; Martín-Sánchez, V.; Fitó, M.; Ruiz-Canela, M.; Paz-Graniel, I.; Curto, A.; et al. Mediterranean Diet, Physical Activity, and Bone Health in Older Adults: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2025, 8, e253710. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, C.; Deng, Z.; Xiang, T.; Tan, J.; Xu, J.; Sun, D.; Luo, F. Alterations of gut virome with close interaction in the progression of estrogen deficiency-induced osteoporosis. Gut Microbes 2024, 16, 2437250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xie, J.; Wang, Z.; Zhong, Y.; Liu, L.; Liu, J.; Zhang, W.; Pi, Y.; Tang, F.; Liu, Z.; et al. Androgen deficiency-induced loss of Lactobacillus salivarius extracellular vesicles is associated with the pathogenesis of osteoporosis. Microbiol. Res. 2025, 293, 128047. [Google Scholar] [CrossRef]
- Han, Y.X.; Mo, Y.Y.; Wu, H.X.; Iqbal, J.; Cai, J.M.; Li, L.; Bu, Y.H.; Xiao, F.; Jiang, H.L.; Wen, Y.; et al. Safety and efficacy of sequential treatments for postmenopausal osteoporosis: A network meta-analysis of randomised controlled trials. eClinicalMedicine 2024, 68, 102425. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, C.; Kieser, S.; Çolakoğlu, M.; Hadadi, N.; Brun, J.; Rigo, D.; Suárez-Zamorano, N.; Spiljar, M.; Fabbiano, S.; Busse, B.; et al. Warmth Prevents Bone Loss Through the Gut Microbiota. Cell Metab. 2020, 32, 575–590.e577. [Google Scholar] [CrossRef]
- Xu, Q.; Li, D.; Chen, J.; Yang, J.; Yan, J.; Xia, Y.; Zhang, F.; Wang, X.; Cao, H. Crosstalk between the gut microbiota and postmenopausal osteoporosis: Mechanisms and applications. Int. Immunopharmacol. 2022, 110, 108998. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Song, P.R.; Wang, S.C.; Liu, H.; Shi, Z.M.; Su, J.C. Diets intervene osteoporosis via gut-bone axis. Gut Microbes 2024, 16, 2295432. [Google Scholar] [CrossRef]
- Lawenius, L.; Cowardin, C.; Grahnemo, L.; Scheffler, J.M.; Horkeby, K.; Engdahl, C.; Wu, J.; Vandenput, L.; Koskela, A.; Tuukkanen, J.; et al. Transplantation of gut microbiota from old mice into young healthy mice reduces lean mass but not bone mass. Gut Microbes 2023, 15, 2236755. [Google Scholar] [CrossRef]
- Grahnemo, L.; Kambur, O.; Lahti, L.; Jousilahti, P.; Niiranen, T.; Knight, R.; Salomaa, V.; Havulinna, A.S.; Ohlsson, C. Associations between gut microbiota and incident fractures in the FINRISK cohort. NPJ Biofilms Microbiomes 2024, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Sun, R.; Yang, W.; Gu, T.; Ying, X.; Ye, L.; Zheng, Y.; Fan, S.; Zeng, X.; Yao, S. Exercise ameliorates osteopenia in mice via intestinal microbial-mediated bile acid metabolism pathway. Theranostics 2025, 15, 1741–1759. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, W.; Liu, Z.; Huang, D.; Kang, H.; Wang, J.; Jiang, G.; Gao, A. Gut microbiota dysbiosis involved in decabromodiphenyl ether-induced bone homeostasis disorder through inflammaging. Environ. Pollut. 2025, 368, 125710. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; McCauley, K.E.; Lynch, S.V.; Nayak, R.R.; King, N.J.; Patel, S.; Kim, T.Y.; Condra, K.; Fadrosh, D.; Nguyen, D.; et al. Alteration in the gut microbiome is associated with changes in bone metabolism after laparoscopic sleeve gastrectomy. J. Bone Miner. Res. 2024, 39, 95–105. [Google Scholar] [CrossRef]
- Yang, Y.; Hong, Q.; Zhang, X.; Liu, Z. Rheumatoid arthritis and the intestinal microbiome: Probiotics as a potential therapy. Front. Immunol. 2024, 15, 1331486. [Google Scholar] [CrossRef]
- Lawenius, L.; Colldén, H.; Horkeby, K.; Wu, J.; Grahnemo, L.; Vandenput, L.; Ohlsson, C.; Sjögren, K. A probiotic mix partially protects against castration-induced bone loss in male mice. J. Endocrinol. 2022, 254, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xiao, H.M.; Liu, H.M.; Lv, W.Q.; Greenbaum, J.; Gong, R.; Zhang, Q.; Chen, Y.C.; Peng, C.; Xu, X.J.; et al. Gut microbiota impacts bone via Bacteroides vulgatus-valeric acid-related pathways. Nat. Commun. 2023, 14, 6853. [Google Scholar] [CrossRef]
- Wang, D.; Cai, J.; Pei, Q.; Yan, Z.; Zhu, F.; Zhao, Z.; Liu, R.; Guo, X.; Sun, T.; Liu, J.; et al. Gut microbial alterations in arginine metabolism determine bone mechanical adaptation. Cell Metab. 2024, 36, 1252–1268.e1258. [Google Scholar] [CrossRef]
- Huang, S.; Chen, J.; Cui, Z.; Ma, K.; Wu, D.; Luo, J.; Li, F.; Xiong, W.; Rao, S.; Xiang, Q.; et al. Lachnospiraceae-derived butyrate mediates protection of high fermentable fiber against placental inflammation in gestational diabetes mellitus. Sci. Adv. 2023, 9, eadi7337. [Google Scholar] [CrossRef]
- Akinsuyi, O.S.; Roesch, L.F.W. Meta-Analysis Reveals Compositional and Functional Microbial Changes Associated with Osteoporosis. Microbiol. Spectr. 2023, 11, e0032223. [Google Scholar] [CrossRef]
- Duscha, A.; Hegelmaier, T.; Dürholz, K.; Desel, C.; Gold, R.; Zaiss, M.M.; Haghikia, A. Propionic acid beneficially modifies osteoporosis biomarkers in patients with multiple sclerosis. Ther. Adv. Neurol. Disord. 2022, 15, 17562864221103935. [Google Scholar] [CrossRef]
- Takahashi, D.; Hoshina, N.; Kabumoto, Y.; Maeda, Y.; Suzuki, A.; Tanabe, H.; Isobe, J.; Yamada, T.; Muroi, K.; Yanagisawa, Y.; et al. Microbiota-derived butyrate limits the autoimmune response by promoting the differentiation of follicular regulatory T cells. eBioMedicine 2020, 58, 102913. [Google Scholar] [CrossRef]
- Tyagi, A.M.; Yu, M.; Darby, T.M.; Vaccaro, C.; Li, J.Y.; Owens, J.A.; Hsu, E.; Adams, J.; Weitzmann, M.N.; Jones, R.M.; et al. The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression. Immunity 2018, 49, 1116–1131.e1117. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Fan, P.X.; Li, L.S.; Qiao, S.Y.; Zhang, G.L.; Li, D.F. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J. Anim. Sci. 2012, 90 (Suppl. 4), 266–268. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Jia, L.; Mo, L.; Yuan, S.; Zheng, X.; He, J.; Chen, V.; Guo, Q.; Zheng, L.; Yuan, Q.; et al. Berberine Ameliorates Periodontal Bone Loss by Regulating Gut Microbiota. J. Dent. Res. 2019, 98, 107–116. [Google Scholar] [CrossRef]
- Yang, K.L.; Mullins, B.J.; Lejeune, A.; Ivanova, E.; Shin, J.; Bajwa, S.; Possemato, R.; Cadwell, K.; Scher, J.U.; Koralov, S.B. Mitigation of Osteoclast-Mediated Arthritic Bone Remodeling By Short Chain Fatty Acids. Arthritis Rheumatol. 2024, 76, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef]
- Kerezoudi, E.N.; Mitsou, E.K.; Gioti, K.; Terzi, E.; Avgousti, I.; Panagiotou, A.; Koutrotsios, G.; Zervakis, G.I.; Mountzouris, K.C.; Tenta, R.; et al. Fermentation of Pleurotus ostreatus and Ganoderma lucidum mushrooms and their extracts by the gut microbiota of healthy and osteopenic women: Potential prebiotic effect and impact of mushroom fermentation products on human osteoblasts. Food Funct. 2021, 12, 1529–1546. [Google Scholar] [CrossRef]
- Peng, R.; Song, C.; Gou, S.; Liu, H.; Kang, H.; Dong, Y.; Xu, Y.; Hu, P.; Cai, K.; Feng, Q.; et al. Gut Clostridium sporogenes-derived indole propionic acid suppresses osteoclast formation by activating pregnane X receptor. Pharmacol. Res. 2024, 202, 107121. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, L.; Wu, X.; Hu, S.; Zhang, S.; Ning, M.; Yu, J.; Chen, M. Decreased TMIGD1 aggravates colitis and intestinal barrier dysfunction via the BANF1-NF-κB pathway in Crohn’s disease. BMC Med. 2023, 21, 287. [Google Scholar] [CrossRef]
- Onji, M.; Sigl, V.; Lendl, T.; Novatchkova, M.; Ullate-Agote, A.; Andersson-Rolf, A.; Kozieradzki, I.; Koglgruber, R.; Pai, T.P.; Lichtscheidl, D.; et al. RANK drives structured intestinal epithelial expansion during pregnancy. Nature 2025, 637, 156–166. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Nakajima, A.; Nakatani, A.; Hasegawa, S.; Irie, J.; Ozawa, K.; Tsujimoto, G.; Suganami, T.; Itoh, H.; Kimura, I. The short chain fatty acid receptor GPR43 regulates inflammatory signals in adipose tissue M2-type macrophages. PLoS ONE 2017, 12, e0179696. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, X.; Kwan, T.K.; Loh, Y.W.; Singer, J.; Liu, Y.; Ma, J.; Tan, J.; Macia, L.; Mackay, C.R.; et al. Dietary Fiber Protects against Diabetic Nephropathy through Short-Chain Fatty Acid-Mediated Activation of G Protein-Coupled Receptors GPR43 and GPR109A. J. Am. Soc. Nephrol. 2020, 31, 1267–1281. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Sheng, Q.; Bai, Y.; Li, L.; Ning, X.; Liu, Y.; Song, C.; Wang, T.; Dong, X.; Luo, Y.; et al. Obesity, but not high-fat diet, is associated with bone loss that is reversed via CD4(+)CD25(+)Foxp3(+) Tregs-mediated gut microbiome of non-obese mice. NPJ Sci. Food 2023, 7, 14. [Google Scholar] [CrossRef]
- Li, J.Y.; Yu, M.; Pal, S.; Tyagi, A.M.; Dar, H.; Adams, J.; Weitzmann, M.N.; Jones, R.M.; Pacifici, R. Parathyroid hormone-dependent bone formation requires butyrate production by intestinal microbiota. J. Clin. Investig. 2020, 130, 1767–1781. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yan, Q.; Ma, Y.; Li, P.; Yang, Y.; Wang, Y.; Li, W.; Wan, X.; Li, Y.; Zhu, R.; et al. Modified Zuo Gui Wan Ameliorates Ovariectomy-Induced Osteoporosis in Rats by Regulating the SCFA-GPR41-p38MAPK Signaling Pathway. Drug Des. Devel Ther. 2024, 18, 6359–6377. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Kespohl, M.; Vachharajani, N.; Luu, M.; Harb, H.; Pautz, S.; Wolff, S.; Sillner, N.; Walker, A.; Schmitt-Kopplin, P.; Boettger, T.; et al. The Microbial Metabolite Butyrate Induces Expression of Th1-Associated Factors in CD4(+) T Cells. Front. Immunol. 2017, 8, 1036. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Lee, A.R.; Kim, H.; Jhun, J.; Lee, S.Y.; Choi, J.W.; Jeong, Y.; Park, M.S.; Ji, G.E.; Cho, M.L.; et al. Faecalibacterium prausnitzii alleviates inflammatory arthritis and regulates IL-17 production, short chain fatty acids, and the intestinal microbial flora in experimental mouse model for rheumatoid arthritis. Arthritis Res. Ther. 2023, 25, 130. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, J.; Wang, H.; Chen, C.; Han, M.; Gao, L.; Tang, C.; Sun, P.; Zhao, X.; Guo, F.; et al. Trojan Horse Nanocapsule Enabled In Situ Modulation of the Phenotypic Conversion of Th17 Cells to Treg Cells for the Treatment of Multiple Sclerosis in Mice. Adv. Mater. 2023, 35, e2210262. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W.; et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Wang, L.; Chen, Y.; Han, X.; Sun, L.; Chen, H.; Chen, Q. Effect of Bifidobacterium on osteoclasts: TNF-α/NF-κB inflammatory signal pathway-mediated mechanism. Front. Endocrinol. 2023, 14, 1109296. [Google Scholar] [CrossRef]
- Docampo, M.D.; da Silva, M.B.; Lazrak, A.; Nichols, K.B.; Lieberman, S.R.; Slingerland, A.E.; Armijo, G.K.; Shono, Y.; Nguyen, C.; Monette, S.; et al. Alloreactive T cells deficient of the short-chain fatty acid receptor GPR109A induce less graft-versus-host disease. Blood 2022, 139, 2392–2405. [Google Scholar] [CrossRef]
- Trinidad, T.P.; Wolever, T.M.; Thompson, L.U. Effect of acetate and propionate on calcium absorption from the rectum and distal colon of humans. Am. J. Clin. Nutr. 1996, 63, 574–578. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Yang, M.; Wang, Y.; Yang, C.; Shafiq, M.; Wang, G.; Li, L. Sodium propionate protect the blood-milk barrier integrity, relieve lipopolysaccharide-induced inflammatory injury and cells apoptosis. Life Sci. 2021, 270, 119138. [Google Scholar] [CrossRef]
- Bai, Y.; Li, Y.; Marion, T.; Tong, Y.; Zaiss, M.M.; Tang, Z.; Zhang, Q.; Liu, Y.; Luo, Y. Resistant starch intake alleviates collagen-induced arthritis in mice by modulating gut microbiota and promoting concomitant propionate production. J. Autoimmun. 2021, 116, 102564. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, J.; Liu, H.; Wu, Q.; Chen, J.; Chen, G.Q. The mechanism of anti-osteoporosis effects of 3-hydroxybutyrate and derivatives under simulated microgravity. Biomaterials 2014, 35, 8273–8283. [Google Scholar] [CrossRef]
- Li, J.Y.; Adams, J.; Calvi, L.M.; Lane, T.F.; DiPaolo, R.; Weitzmann, M.N.; Pacifici, R. PTH expands short-term murine hemopoietic stem cells through T cells. Blood 2012, 120, 4352–4362. [Google Scholar] [CrossRef][Green Version]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef]
- Akiba, Y.; Inoue, T.; Kaji, I.; Higashiyama, M.; Narimatsu, K.; Iwamoto, K.; Watanabe, M.; Guth, P.H.; Engel, E.; Kuwahara, A.; et al. Short-chain fatty acid sensing in rat duodenum. J. Physiol. 2015, 593, 585–599. [Google Scholar] [CrossRef]
- Tatsuoka, M.; Osaki, Y.; Ohsaka, F.; Tsuruta, T.; Kadota, Y.; Tochio, T.; Hino, S.; Morita, T.; Sonoyama, K. Consumption of indigestible saccharides and administration of Bifidobacterium pseudolongum reduce mucosal serotonin in murine colonic mucosa. Br. J. Nutr. 2022, 127, 513–525. [Google Scholar] [CrossRef]
- Li, K.; Wei, W.; Xu, C.; Lian, X.; Bao, J.; Yang, S.; Wang, S.; Zhang, X.; Zheng, X.; Wang, Y.; et al. Prebiotic inulin alleviates anxiety and depression-like behavior in alcohol withdrawal mice by modulating the gut microbiota and 5-HT metabolism. Phytomedicine 2024, 135, 156181. [Google Scholar] [CrossRef] [PubMed]
- Sugisawa, E.; Takayama, Y.; Takemura, N.; Kondo, T.; Hatakeyama, S.; Kumagai, Y.; Sunagawa, M.; Tominaga, M.; Maruyama, K. RNA Sensing by Gut Piezo1 Is Essential for Systemic Serotonin Synthesis. Cell 2020, 182, 609–624.e21. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Sun, X.; Hao, S.; Li, X.; Qian, M.; Dou, L.; Zhang, M.; Hou, P.; Su, L.; Zhao, L.; et al. Effect of sheep bone protein hydrolysate on promoting calcium absorption and enhancing bone quality in low-calcium diet fed rats. Food Chem. 2024, 446, 138763. [Google Scholar] [CrossRef]
- Whisner, C.M.; Castillo, L.F. Prebiotics, Bone and Mineral Metabolism. Calcif. Tissue Int. 2018, 102, 443–479. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhou, Z. Effect of Xylo-Oligosaccharides Supplementation by Drinking Water on the Bone Properties and Related Calcium Transporters in Growing Mice. Nutrients 2020, 12, 3542. [Google Scholar] [CrossRef]
- Zárate, G.; Morata De Ambrosini, V.; Perez Chaia, A.; González, S. Some factors affecting the adherence of probiotic Propionibacterium acidipropionici CRL 1198 to intestinal epithelial cells. Can. J. Microbiol. 2002, 48, 449–457. [Google Scholar] [CrossRef]
- Bandali, E.; Wang, Y.; Lan, Y.; Rogers, M.A.; Shapses, S.A. The influence of dietary fat and intestinal pH on calcium bioaccessibility: An in vitro study. Food Funct. 2018, 9, 1809–1815. [Google Scholar] [CrossRef]
- He, W.; Xie, Z.; Thøgersen, R.; Rasmussen, M.K.; Zachariassen, L.F.; Jørgensen, N.R.; Nørgaard, J.V.; Andersen, H.J.; Nielsen, D.S.; Hansen, A.K.; et al. Effects of Calcium Source, Inulin, and Lactose on Gut-Bone Associations in an Ovarierectomized Rat Model. Mol. Nutr. Food Res. 2022, 66, e2100883. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, M.; Tomassi, D.; Rizzoli, R.; Tenon, M.; Berton, T.; Harney, S.; Fança-Berthon, P. Effect of a Hop Extract Standardized in 8-Prenylnaringenin on Bone Health and Gut Microbiome in Postmenopausal Women with Osteopenia: A One-Year Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2023, 15, 2688. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, M.; Lu, C.; Han, J.; Tang, S.; Zhou, J.; Li, Y.; Ming, T.; Wang, Z.J.; Su, X. Tuna Bone Powder Alleviates Glucocorticoid-Induced Osteoporosis via Coregulation of the NF-κB and Wnt/β-Catenin Signaling Pathways and Modulation of Gut Microbiota Composition and Metabolism. Mol. Nutr. Food Res. 2020, 64, e1900861. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Yang, C.; Xiang, T.; Dou, C.; Sun, D.; Dai, Q.; Ling, Z.; Xu, J.; Luo, F.; Chen, Y. Gold nanoparticles exhibit anti-osteoarthritic effects via modulating interaction of the “microbiota-gut-joint” axis. J. Nanobiotechnol. 2024, 22, 157. [Google Scholar] [CrossRef]
- Porwal, K.; Pal, S.; Kulkarni, C.; Singh, P.; Sharma, S.; Singh, P.; Prajapati, G.; Gayen, J.R.; Ampapathi, R.S.; Mullick, A.; et al. A prebiotic, short-chain fructo-oligosaccharides promotes peak bone mass and maintains bone mass in ovariectomized rats by an osteogenic mechanism. Biomed. Pharmacother. 2020, 129, 110448. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Bäumler, A.J. Colonocyte metabolism shapes the gut microbiota. Science 2018, 362, eaat9076. [Google Scholar] [CrossRef]
- Nan, X.; Zhao, W.; Liu, W.H.; Li, Y.; Li, N.; Hong, Y.; Cui, J.; Shang, X.; Feng, H.; Hung, W.L.; et al. Bifidobacterium animalis subsp. lactis BL-99 ameliorates colitis-related lung injury in mice by modulating short-chain fatty acid production and inflammatory monocytes/macrophages. Food Funct. 2023, 14, 1099–1112. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, E.J.; Lee, J.C.; Kim, W.K.; Kim, H.S. Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: Involvement of NF-kappaB and ERK signaling pathways. Int. Immunopharmacol. 2007, 7, 70–77. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, X.; Tian, X.; Zhao, M.; Mu, Y.; Yi, H.; Zhang, Z.; Zhang, L. Bifidobacterium improves oestrogen-deficiency-induced osteoporosis in mice by modulating intestinal immunity. Food Funct. 2024, 15, 1840–1851. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, Y.; Xu, Y.; Diao, J.; Zheng, S.; Yuan, C. Gut Microbial Metabolite Butyrate Regulates Treg/Th17 Cell Balance to Alleviate Diabetic Periodontitis. J. Clin. Periodontol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, M.; Wang, Y.; Gong, S.; Yao, W.; Li, W.; Gao, H.; Wei, M. Puerarin improves the bone micro-environment to inhibit OVX-induced osteoporosis via modulating SCFAs released by the gut microbiota and repairing intestinal mucosal integrity. Biomed. Pharmacother. 2020, 132, 110923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, T.; Meng, Y.; Hu, M.; Shu, L.; Jiang, H.; Gao, R.; Ma, J.; Wang, C.; Zhou, X. FOS/GOS attenuates high-fat diet induced bone loss via reversing microbiota dysbiosis, high intestinal permeability and systemic inflammation in mice. Metabolism 2021, 119, 154767. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, C.; Huang, Y.; Yang, J.; Zhou, F.; Zeng, J.; Lin, Y. Jiangu granule ameliorated OVX rats bone loss by modulating gut microbiota-SCFAs-Treg/Th17 axis. Biomed. Pharmacother. 2022, 150, 112975. [Google Scholar] [CrossRef]
- Moreno-Torres, M.; Guzmán, C.; Petrov, P.D.; Jover, R. Valproate and Short-Chain Fatty Acids Activate Transcription of the Human Vitamin D Receptor Gene through a Proximal GC-Rich DNA Region Containing Two Putative Sp1 Binding Sites. Nutrients 2022, 14, 2673. [Google Scholar] [CrossRef]
- Jayasinghe, T.; Jenkins, J.; Medara, N.; Choowong, P.; Dharmarathne, G.; Kong, F.; Cho, H.; Kim, S.H.; Zhang, Y.; Franco-Duarte, R.; et al. Dietary Fibre Modulates Body Composition, Blood Glucose, Inflammation, Microbiome, and Metabolome in a Murine Model of Periodontitis. Nutrients 2025, 17, 1146. [Google Scholar] [CrossRef]
- Wilde, S.; Johnson, A.F.; LaRock, C.N. Playing With Fire: Proinflammatory Virulence Mechanisms of Group A Streptococcus. Front. Cell Infect. Microbiol. 2021, 11, 704099. [Google Scholar] [CrossRef]
- Tran, H.Q.; Bretin, A.; Adeshirlarijaney, A.; Yeoh, B.S.; Vijay-Kumar, M.; Zou, J.; Denning, T.L.; Chassaing, B.; Gewirtz, A.T. “Western Diet”-Induced Adipose Inflammation Requires a Complex Gut Microbiota. Cell Mol. Gastroenterol. Hepatol. 2020, 9, 313–333. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Ngo, V.L.; Wang, Y.; Wang, Y.; Gewirtz, A.T. Maternal fiber deprivation alters microbiota in offspring, resulting in low-grade inflammation and predisposition to obesity. Cell Host Microbe 2023, 31, 45–57.e47. [Google Scholar] [CrossRef]
- Zuo, H.; Zheng, T.; Wu, K.; Yang, T.; Wang, L.; Nima, Q.; Bai, H.; Dong, K.; Fan, Z.; Huang, S.; et al. High-altitude exposure decreases bone mineral density and its relationship with gut microbiota: Results from the China multi-ethnic cohort (CMEC) study. Environ. Res. 2022, 215, 114206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, L.; Zhu, R.; Zhang, S.; Liu, S.; Wang, Y.; Wu, Y.; Xing, S.; Liao, X.; Mi, J. Porcine gut microbiota in mediating host metabolic adaptation to cold stress. NPJ Biofilms Microbiomes 2022, 8, 18. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, Z.; Kwok, L.Y.; Zhao, Z.; Wang, K.; Li, Y.; Sun, Z.; Zhao, J.; Zhang, H. Bifidobacterium lactis Probio-M8 improves bone metabolism in patients with postmenopausal osteoporosis, possibly by modulating the gut microbiota. Eur. J. Nutr. 2023, 62, 965–976. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Bai, N.; Sun, Y.; Li, K.; Ruan, H.; Yan, B.; Hu, J.; Zhang, N.; Zhang, H.; et al. Spirulina platensis components mitigate bone density loss induced by simulated microgravity: A mechanistic insight. Food Chem. 2025, 463, 141361. [Google Scholar] [CrossRef]
- Skibiński, S.; Czechowska, J.P.; Cichoń, E.; Seta, M.; Gondek, A.; Cudnoch-Jędrzejewska, A.; Ślósarczyk, A.; Guzik, M.; Zima, A. Study on βTCP/P(3HB) Scaffolds-Physicochemical Properties and Biological Performance in Low Oxygen Concentration. Int. J. Mol. Sci. 2022, 23, 11587. [Google Scholar] [CrossRef]
- Yu, T.; Bai, R.; Wang, Z.; Qin, Y.; Wang, J.; Wei, Y.; Zhao, R.; Nie, G.; Han, B. Colon-targeted engineered postbiotics nanoparticles alleviate osteoporosis through the gut-bone axis. Nat. Commun. 2024, 15, 10893. [Google Scholar] [CrossRef]
- Qiao, M.; Xue, T.; Zhu, Y.; Yang, J.; Hu, J. Polysaccharides from Cistanche deserticola mitigate inflammatory bowel disease via modulating intestinal microbiota and SRC/EGFR/PI3K/AKT signaling pathways. Int. J. Biol. Macromol. 2025, 308, 142452. [Google Scholar] [CrossRef]
- Li, Z.X.; Zhuo, J.L.; Yang, N.; Gao, M.B.; Qu, Z.H.; Han, T. Effect of Lycium barbarum polysaccharide on osteoblast proliferation and differentiation in postmenopausal osteoporosis. Int. J. Biol. Macromol. 2024, 271, 132415. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (‒)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2022, 24, 340. [Google Scholar] [CrossRef]
- Seethaler, B.; Nguyen, N.K.; Basrai, M.; Kiechle, M.; Walter, J.; Delzenne, N.M.; Bischoff, S.C. Short-chain fatty acids are key mediators of the favorable effects of the Mediterranean diet on intestinal barrier integrity: Data from the randomized controlled LIBRE trial. Am. J. Clin. Nutr. 2022, 116, 928–942. [Google Scholar] [CrossRef]
- Jia, X.; Yang, R.; Li, J.; Zhao, L.; Zhou, X.; Xu, X. Gut-Bone Axis: A Non-Negligible Contributor to Periodontitis. Front. Cell Infect. Microbiol. 2021, 11, 752708. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chang, T.; Yuan, Q.; Wei, W.; Wang, P.; Song, X.; Yuan, H. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front. Immunol. 2022, 13, 930244. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, J.; Liu, L.; Wang, C.; Xie, Y.; Yu, X.; Tian, L. Grape seed extract prevents oestrogen deficiency-induced bone loss by modulating the gut microbiota and metabolites. Microb. Biotechnol. 2024, 17, e14485. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Kikut, J.; Maciejewska, D.; Kulpa, D.; Celewicz, Z.; Ziętek, M. The Associations of SCFA with Anthropometric Parameters and Carbohydrate Metabolism in Pregnant Women. Int. J. Mol. Sci. 2020, 21, 9212. [Google Scholar] [CrossRef]
- Yu, Z.; Huang, J.; Zhou, Z. Icariin protects against cage layer osteoporosis by intervening in steroid biosynthesis and glycerophospholipid metabolism. Anim. Dis. 2021, 1, 1. [Google Scholar] [CrossRef]
- Clapp Organski, A.; Reddivari, A.; Reddivari, L.; Brubaker, D.K.; Fuller, K.N.Z.; Thyfault, J.P.; Cross, T.L. Oral Contraceptives Induce Time- and Intestinal Segment-Dependent Shifts in the Gut Microbiota. Nutrients 2025, 17, 2591. [Google Scholar] [CrossRef]
- Wang, S.; Wang, S.; Wang, X.; Xu, Y.; Zhang, X.; Han, Y.; Yan, H.; Liu, L.; Wang, L.; Ye, H.; et al. Effects of Icariin on Modulating Gut Microbiota and Regulating Metabolite Alterations to Prevent Bone Loss in Ovariectomized Rat Model. Front. Endocrinol. 2022, 13, 874849. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Z.; Lei, H.; Zhang, C.; Wu, M.; Huang, S.; Li, X.; Xie, D.; Liu, M.; Zhang, L.; et al. Microbial Tryptophan Metabolites Ameliorate Ovariectomy-Induced Bone Loss by Repairing Intestinal AhR-Mediated Gut-Bone Signaling Pathway. Adv. Sci. 2024, 11, e2404545. [Google Scholar] [CrossRef]
- Li, M.; Han, X.; Sun, L.; Liu, X.; Zhang, W.; Hao, J. Indole-3-acetic acid alleviates DSS-induced colitis by promoting the production of R-equol from Bifidobacterium pseudolongum. Gut Microbes 2024, 16, 2329147. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Song, Y.W.; Zhang, L.; Zheng, F.J.; Wang, X.M.; Zhuang, X.H.; Wu, F.; Liu, J. Association between bile acid metabolism and bone mineral density in postmenopausal women. Clinics 2020, 75, e1486. [Google Scholar] [CrossRef]
- Qiao, X.; Zhang, K.; Li, X.; Lv, Z.; Wei, W.; Zhou, R.; Yan, L.; Pan, Y.; Yang, S.; Sun, X.; et al. Gut microbiota and fecal metabolic signatures in rat models of disuse-induced osteoporosis. Front. Cell Infect. Microbiol. 2022, 12, 1018897. [Google Scholar] [CrossRef]
- Schoeler, M.; Ellero-Simatos, S.; Birkner, T.; Mayneris-Perxachs, J.; Olsson, L.; Brolin, H.; Loeber, U.; Kraft, J.D.; Polizzi, A.; Martí-Navas, M.; et al. The interplay between dietary fatty acids and gut microbiota influences host metabolism and hepatic steatosis. Nat. Commun. 2023, 14, 5329. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Xuanqi, Z.; Zhu, J.; Yuan, W.; Jia, J.; Zhang, C.; Sun, T.; Leng, H.; Jiang, C.; Xu, Y.; et al. Estrogen deficiency induces bone loss through the gut microbiota. Pharmacol. Res. 2023, 196, 106930. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Lin, B.; Xiao, W.; Wang, F.; Xu, P.; Wang, N. Specnuezhenide Alleviates Senile Osteoporosis by Activating TGR5/FXR Signaling in Bone Marrow Mesenchymal Stem Cells and RANKL-Induced Osteoclasts. Drug Des. Devel Ther. 2025, 19, 1595–1608. [Google Scholar] [CrossRef]
- Hathaway-Schrader, J.D.; Steinkamp, H.M.; Chavez, M.B.; Poulides, N.A.; Kirkpatrick, J.E.; Chew, M.E.; Huang, E.; Alekseyenko, A.V.; Aguirre, J.I.; Novince, C.M. Antibiotic Perturbation of Gut Microbiota Dysregulates Osteoimmune Cross Talk in Postpubertal Skeletal Development. Am. J. Pathol. 2019, 189, 370–390. [Google Scholar] [CrossRef]
- Guan, Z.; Jia, J.; Zhang, C.; Sun, T.; Zhang, W.; Yuan, W.; Leng, H.; Song, C. Gut microbiome dysbiosis alleviates the progression of osteoarthritis in mice. Clin. Sci. 2020, 134, 3159–3174. [Google Scholar] [CrossRef] [PubMed]
- Medeot, A.C.; Boaglio, A.C.; Salas, G.; Maidagan, P.M.; Miszczuk, G.S.; Barosso, I.R.; Sánchez Pozzi, E.J.; Crocenzi, F.A.; Roma, M.G. Tauroursodeoxycholate prevents estradiol 17β-d-glucuronide-induced cholestasis and endocytosis of canalicular transporters by switching off pro-cholestatic signaling pathways. Life Sci. 2024, 352, 122839. [Google Scholar] [CrossRef]
- Perino, A.; Schoonjans, K. Metabolic Messengers: Bile acids. Nat. Metab. 2022, 4, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Fan, Y.; Xu, L.; Yu, Z.; Wang, S.; Xu, H.; Zhang, J.; Zhang, L.; Liu, W.; Wu, L.; et al. Microbiome and tryptophan metabolomics analysis in adolescent depression: Roles of the gut microbiota in the regulation of tryptophan-derived neurotransmitters and behaviors in human and mice. Microbiome 2023, 11, 145. [Google Scholar] [CrossRef]
- Van de Wiele, T.; Vanhaecke, L.; Boeckaert, C.; Peru, K.; Headley, J.; Verstraete, W.; Siciliano, S. Human colon microbiota transform polycyclic aromatic hydrocarbons to estrogenic metabolites. Environ. Health Perspect. 2005, 113, 6–10. [Google Scholar] [CrossRef]
- Zhao, L.; Fang, J.; Tang, S.; Deng, F.; Liu, X.; Shen, Y.; Liu, Y.; Kong, F.; Du, Y.; Cui, L.; et al. PM2.5 and Serum Metabolome and Insulin Resistance, Potential Mediation by the Gut Microbiome: A Population-Based Panel Study of Older Adults in China. Environ. Health Perspect. 2022, 130, 27007. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Pu, Z.; Chi, X.; Yang, J. Multi-omics data reveals the disturbance of glycerophospholipid metabolism and linoleic acid metabolism caused by disordered gut microbiota in PM2.5 gastrointestinal exposed rats. Ecotoxicol. Environ. Saf. 2023, 262, 115182. [Google Scholar] [CrossRef]
- Han, X.; Huangfu, B.; Xu, T.; Huang, K.; He, X. Zearalenone exacerbates lipid metabolism disorders by promoting liver lipid droplet formation and disrupting gut microbiota. Ecotoxicol. Environ. Saf. 2025, 289, 117664. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Chang, J.; Yang, G.; Deng, T.; Liu, P.; Wang, J.; Xu, C. Prophylactic supplementation with selenium nanoparticles protects against foodborne toxin zearalenone-induced intestinal barrier dysfunction. Ecotoxicol. Environ. Saf. 2024, 284, 116914. [Google Scholar] [CrossRef] [PubMed]
- Nosal, B.M.; Thornton, S.N.; Darooghegi Mofrad, M.; Sakaki, J.R.; Mahoney, K.J.; Macdonald, Z.; Daddi, L.; Tran, T.D.B.; Weinstock, G.; Zhou, Y.; et al. Blackcurrants shape gut microbiota profile and reduce risk of postmenopausal osteoporosis via the gut-bone axis: Evidence from a pilot randomized controlled trial. J. Nutr. Biochem. 2024, 133, 109701. [Google Scholar] [CrossRef] [PubMed]
- Damani, J.J.; De Souza, M.J.; VanEvery, H.L.; Strock, N.C.A.; Rogers, C.J. The Role of Prunes in Modulating Inflammatory Pathways to Improve Bone Health in Postmenopausal Women. Adv. Nutr. 2022, 13, 1476–1492. [Google Scholar] [CrossRef]
- Cross, T.L.; Simpson, A.M.R.; Lin, C.Y.; Hottmann, N.M.; Bhatt, A.P.; Pellock, S.J.; Nelson, E.R.; Loman, B.R.; Wallig, M.A.; Vivas, E.I.; et al. Gut microbiome responds to alteration in female sex hormone status and exacerbates metabolic dysfunction. Gut Microbes 2024, 16, 2295429. [Google Scholar] [CrossRef]
- Gao, J.; Mao, K.; Wang, X.; Mi, S.; Fu, M.; Li, X.; Xiao, J.; Simal-Gandara, J.; Sang, Y. Tibet Kefir Milk Regulated Metabolic Changes Induced by High-Fat Diet via Amino Acids, Bile Acids, and Equol Metabolism in Human-Microbiota-Associated Rats. J. Agric. Food Chem. 2021, 69, 6720–6732. [Google Scholar] [CrossRef]
- Singh, V.; Park, Y.J.; Lee, G.; Unno, T.; Shin, J.H. Dietary regulations for microbiota dysbiosis among post-menopausal women with type 2 diabetes. Crit. Rev. Food Sci. Nutr. 2023, 63, 9961–9976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Q.; Zhang, X.; Du, S.; Zhang, Y.; Wang, X.; Liu, Y.; Fang, B.; Chen, J.; Liu, R.; et al. Effects of synbiotics surpass probiotics alone in improving type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2025, 44, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.A.; Voigt, R.M.; Cantu-Jungles, T.M.; Hamaker, B.; Engen, P.A.; Shaikh, M.; Raeisi, S.; Green, S.J.; Naqib, A.; Forsyth, C.B.; et al. An open label, non-randomized study assessing a prebiotic fiber intervention in a small cohort of Parkinson’s disease participants. Nat. Commun. 2023, 14, 926. [Google Scholar] [CrossRef] [PubMed]
- Organski, A.C.; Rajwa, B.; Reddivari, A.; Jorgensen, J.S.; Cross, T.L. Gut microbiome-driven regulation of sex hormone homeostasis: A potential neuroendocrine connection. Gut Microbes 2025, 17, 2476562. [Google Scholar] [CrossRef]
- Guo, M.; Liu, H.; Yu, Y.; Zhu, X.; Xie, H.; Wei, C.; Mei, C.; Shi, Y.; Zhou, N.; Qin, K.; et al. Lactobacillus rhamnosus GG ameliorates osteoporosis in ovariectomized rats by regulating the Th17/Treg balance and gut microbiota structure. Gut Microbes 2023, 15, 2190304. [Google Scholar] [CrossRef]
- Yan, L.; Wang, X.; Yu, T.; Qi, Z.; Li, H.; Nan, H.; Wang, K.; Luo, D.; Hua, F.; Wang, W. Characteristics of the gut microbiota and serum metabolites in postmenopausal women with reduced bone mineral density. Front. Cell Infect. Microbiol. 2024, 14, 1367325. [Google Scholar] [CrossRef]
- Song, C.H.; Kim, N.; Sohn, S.H.; Lee, S.M.; Nam, R.H.; Na, H.Y.; Lee, D.H.; Surh, Y.J. Effects of 17β-Estradiol on Colonic Permeability and Inflammation in an Azoxymethane/Dextran Sulfate Sodium-Induced Colitis Mouse Model. Gut Liver 2018, 12, 682–693. [Google Scholar] [CrossRef]
- Lee, J.; Peesh, P.; Quaicoe, V.; Tan, C.; Banerjee, A.; Mooz, P.; Ganesh, B.P.; Petrosino, J.; Bryan, R.M., Jr.; McCullough, L.D.; et al. Estradiol mediates colonic epithelial protection in aged mice after stroke and is associated with shifts in the gut microbiome. Gut Microbes 2023, 15, 2271629. [Google Scholar] [CrossRef]
- Jia, L.; Tu, Y.; Jia, X.; Du, Q.; Zheng, X.; Yuan, Q.; Zheng, L.; Zhou, X.; Xu, X. Probiotics ameliorate alveolar bone loss by regulating gut microbiota. Cell Prolif. 2021, 54, e13075. [Google Scholar] [CrossRef]
- Tu, M.Y.; Han, K.Y.; Chang, G.R.; Lai, G.D.; Chang, K.Y.; Chen, C.F.; Lai, J.C.; Lai, C.Y.; Chen, H.L.; Chen, C.M. Kefir Peptides Prevent Estrogen Deficiency-Induced Bone Loss and Modulate the Structure of the Gut Microbiota in Ovariectomized Mice. Nutrients 2020, 12, 3432. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef]
- Aguilera, M.; Gálvez-Ontiveros, Y.; Rivas, A. Endobolome, a New Concept for Determining the Influence of Microbiota Disrupting Chemicals (MDC) in Relation to Specific Endocrine Pathogenesis. Front. Microbiol. 2020, 11, 578007. [Google Scholar] [CrossRef]
- Li, D.; Meng, K.; Liu, G.; Wen, Z.; Han, Y.; Liu, W.; Xu, X.; Song, L.; Cai, H.; Yang, P. Lactiplantibacillus plantarum FRT4 protects against fatty liver hemorrhage syndrome: Regulating gut microbiota and FoxO/TLR-4/NF-κB signaling pathway in laying hens. Microbiome 2025, 13, 88. [Google Scholar] [CrossRef]
- Wen, J.; Feng, Y.; Xue, L.; Yuan, S.; Chen, Q.; Luo, A.; Wang, S.; Zhang, J. High-fat diet-induced L-saccharopine accumulation inhibits estradiol synthesis and damages oocyte quality by disturbing mitochondrial homeostasis. Gut Microbes 2024, 16, 2412381. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Wu, Z.; Zhao, Y.; Cao, M.; Shi, B.; Chen, B.; Chen, N.; Guo, H.; Li, N.; et al. Gut microbiota signatures and fecal metabolites in postmenopausal women with osteoporosis. Gut Pathog. 2023, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, D.; Kubota, R.; Maeno, T.; Abdelhakim, M.; Hitosugi, N. Association between gut microbiota, bone metabolism, and fracture risk in postmenopausal Japanese women. Osteoporos. Int. 2021, 32, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Lin, J.; Qi, Q.; Usyk, M.; Isasi, C.R.; Mossavar-Rahmani, Y.; Derby, C.A.; Santoro, N.; Perreira, K.M.; Daviglus, M.L.; et al. Menopause Is Associated with an Altered Gut Microbiome and Estrobolome, with Implications for Adverse Cardiometabolic Risk in the Hispanic Community Health Study/Study of Latinos. mSystems 2022, 7, e0027322. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Liu, Q.; Jiang, Z.; Zhou, H.; Wang, Q. Global burden of low bone mineral density related fractures in pre- and post-menopausal women from 1990 to 2021, with projections to 2050. Osteoporos. Int. 2025, 36, 1593–1605. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Arnoriaga-Rodríguez, M.; Luque-Córdoba, D.; Priego-Capote, F.; Pérez-Brocal, V.; Moya, A.; Burokas, A.; Maldonado, R.; Fernández-Real, J.M. Gut microbiota steroid sexual dimorphism and its impact on gonadal steroids: Influences of obesity and menopausal status. Microbiome 2020, 8, 136. [Google Scholar] [CrossRef]
- Zhu, J.; Liao, M.; Yao, Z.; Liang, W.; Li, Q.; Liu, J.; Yang, H.; Ji, Y.; Wei, W.; Tan, A.; et al. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome 2018, 6, 136. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Q.; Zhang, W.; Kang, M.; Ma, J.; Zhao, L. Gut microbial beta-glucuronidase: A vital regulator in female estrogen metabolism. Gut Microbes 2023, 15, 2236749. [Google Scholar] [CrossRef]
- Kim, J.; Munster, P.N. Estrogens and breast cancer. Ann. Oncol. 2025, 36, 134–148. [Google Scholar] [CrossRef]
- Teede, H.J. Hormone replacement therapy and the prevention of cardiovascular disease. Hum. Reprod. Update 2002, 8, 201–215. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Lv, J.; Zeng, Y.; Li, Q.; Wang, L.; Zhang, Y.; Gao, W.; Wang, J. Gut Microbiome Signature Are Correlated With Bone Mineral Density Alterations in the Chinese Elders. Front. Cell Infect. Microbiol. 2022, 12, 827575. [Google Scholar] [CrossRef]
- Pérez-Prieto, I.; Vargas, E.; Salas-Espejo, E.; Lüll, K.; Canha-Gouveia, A.; Pérez, L.A.; Fontes, J.; Salumets, A.; Andreson, R.; Aasmets, O.; et al. Gut microbiome in endometriosis: A cohort study on 1000 individuals. BMC Med. 2024, 22, 294. [Google Scholar] [CrossRef]
- Ausenda, F.; Barbera, E.; Cotti, E.; Romeo, E.; Natto, Z.S.; Valente, N.A. Clinical, microbiological and immunological short, medium and long-term effects of different strains of probiotics as an adjunct to non-surgical periodontal therapy in patients with periodontitis. Systematic review with meta-analysis. Jpn. Dent. Sci. Rev. 2023, 59, 62–103. [Google Scholar] [CrossRef]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.; Trujillo, S.; Ghoneim, S.; Thomas, C.; Fass, R. Effect of Hormonal Replacement Therapy on Gastroesophageal Reflux Disease and its Complications in Postmenopausal Women. Clin. Gastroenterol. Hepatol. 2023, 21, 549–551.e543. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R. The history and basic science development of soy isoflavones. Menopause 2017, 24, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhang, J.; Hong, G.; Chen, Z.; Deng, W.; He, W.; Chen, M.H. Icariin promotes osteogenic differentiation of rat bone marrow stromal cells by activating the ERα-Wnt/β-catenin signaling pathway. Biomed. Pharmacother. 2016, 84, 931–939. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, Y.; Yao, Y.; Li, J.; Wang, W.; Wu, X. Equol Induces Mitochondria-Dependent Apoptosis in Human Gastric Cancer Cells via the Sustained Activation of ERK1/2 Pathway. Mol. Cells 2016, 39, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Alfa, H.H.; Arroo, R.R. Over 3 decades of research on dietary flavonoid antioxidants and cancer prevention: What have we achieved? Phytochem. Rev. 2019, 18, 989–1004. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, T.; Li, X.; Kong, A.; Xiao, R.; Xie, R.; Gao, J.; Wang, Z.; Cai, Y.; Zou, J.; et al. Estrogen receptor β deficiency impairs gut microbiota: A possible mechanism of IBD-induced anxiety-like behavior. Microbiome 2022, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Shogry, F.F.; Hayes, K.N.; Kim, S.; Burden, A.M.; Tadrous, M.; Aggarwal, S.; Cadarette, S.M. The rise and fall of raloxifene use for osteoporosis, 1999–2022. Osteoporos. Int. 2025, 36, 1277–1286. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Wang, F.; Li, W.; Wu, X.; Zhao, C.; Zhao, J.; Wei, H.; Wu, Z.; Qian, M.; et al. Dual Targeting of Bile Acid Receptor-1 (TGR5) and Farnesoid X Receptor (FXR) Prevents Estrogen-Dependent Bone Loss in Mice. J. Bone Miner. Res. 2019, 34, 765–776. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Feng, X.; Lin, Q.; Deng, J.; Yuan, Y.; Li, M.; Zhai, B.; Chen, J. Folic acid supplementation prevents high body fat-induced bone loss through TGR5 signaling pathways. Food Funct. 2024, 15, 4193–4206. [Google Scholar] [CrossRef]
- De Martinis, M.; Ginaldi, L.; Sirufo, M.M.; Bassino, E.M.; De Pietro, F.; Pioggia, G.; Gangemi, S. IL-33/Vitamin D Crosstalk in Psoriasis-Associated Osteoporosis. Front. Immunol. 2020, 11, 604055. [Google Scholar] [CrossRef]
- Li, Q.; Chan, H.; Liu, W.X.; Liu, C.A.; Zhou, Y.; Huang, D.; Wang, X.; Li, X.; Xie, C.; Liu, W.Y.; et al. Carnobacterium maltaromaticum boosts intestinal vitamin D production to suppress colorectal cancer in female mice. Cancer Cell 2023, 41, 1450–1465.e1458. [Google Scholar] [CrossRef]
- Lungaro, L.; Manza, F.; Costanzini, A.; Barbalinardo, M.; Gentili, D.; Caputo, F.; Guarino, M.; Zoli, G.; Volta, U.; De Giorgio, R.; et al. Osteoporosis and Celiac Disease: Updates and Hidden Pitfalls. Nutrients 2023, 15, 1089. [Google Scholar] [CrossRef]
- Wang, H.; Gong, W.; Gao, J.; Cheng, W.; Hu, Y.; Hu, C. Effects of vitamin D deficiency on chronic alcoholic liver injury. Free Radic. Biol. Med. 2024, 224, 220–231. [Google Scholar] [CrossRef]
- Gubatan, J.; Moss, A.C. Vitamin D in inflammatory bowel disease: More than just a supplement. Curr. Opin. Gastroenterol. 2018, 34, 217–225. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Y.; Sun, J. Breast and gut microbiome in health and cancer. Genes. Dis. 2021, 8, 581–589. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.; Claud, E.C.; Sun, J. Lack of Vitamin D Receptor Leads to Hyperfunction of Claudin-2 in Intestinal Inflammatory Responses. Inflamm. Bowel Dis. 2019, 25, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Pagnini, C.; Picchianti-Diamanti, A.; Bruzzese, V.; Lorenzetti, R.; Luchetti, M.M.; Martin, L.S.M.; Pica, R.; Scolieri, P.; Scribano, M.L.; Zampaletta, C.; et al. Vitamin D Signaling in Gastro-Rheumatology: From Immuno-Modulation to Potential Clinical Applications. Int. J. Mol. Sci. 2021, 22, 2456. [Google Scholar] [CrossRef]
- Otero, R.; Ishizawa, M.; Numoto, N.; Ikura, T.; Ito, N.; Tokiwa, H.; Mouriño, A.; Makishima, M.; Yamada, S. 25 S-Adamantyl-23-yne-26,27-dinor-1α,25-dihydroxyvitamin D(3): Synthesis, Tissue Selective Biological Activities, and X-ray Crystal Structural Analysis of Its Vitamin D Receptor Complex. J. Med. Chem. 2018, 61, 6658–6673. [Google Scholar] [CrossRef]
- Wang, B.; Qian, J.Y.; Tang, T.T.; Lin, L.L.; Yu, N.; Guo, H.L.; Ni, W.J.; Lv, L.L.; Wen, Y.; Li, Z.L.; et al. VDR/Atg3 Axis Regulates Slit Diaphragm to Tight Junction Transition via p62-Mediated Autophagy Pathway in Diabetic Nephropathy. Diabetes 2021, 70, 2639–2651. [Google Scholar] [CrossRef] [PubMed]
- Cruchet Muñoz, S.; Verbeke Palma, S.; Lera Marqués, L.; Espinosa Pizarro, M.N.; Malig Mechasqui, J.; Sorensen, K. Effects of Bifidobacterium longum 35624 in Children and Adolescents with Irritable Bowel Syndrome. Nutrients 2024, 16, 1967. [Google Scholar] [CrossRef]
- Forsgård, R.A.; Rode, J.; Lobenius-Palmér, K.; Kamm, A.; Patil, S.; Tacken, M.G.J.; Lentjes, M.A.H.; Axelsson, J.; Grompone, G.; Montgomery, S.; et al. Limosilactobacillus reuteri DSM 17938 supplementation and SARS-CoV-2 specific antibody response in healthy adults: A randomized, triple-blinded, placebo-controlled trial. Gut Microbes 2023, 15, 2229938. [Google Scholar] [CrossRef]
- Lin, H.R.; Xu, F.; Chen, D.; Xie, K.; Yang, Y.; Hu, W.; Li, B.Y.; Jiang, Z.; Liang, Y.; Tang, X.Y.; et al. The gut microbiota-bile acid axis mediates the beneficial associations between plasma vitamin D and metabolic syndrome in Chinese adults: A prospective study. Clin. Nutr. 2023, 42, 887–898. [Google Scholar] [CrossRef]
- Nehring, J.A.; Zierold, C.; DeLuca, H.F. Lithocholic acid can carry out in vivo functions of vitamin D. Proc. Natl. Acad. Sci. USA 2007, 104, 10006–10009. [Google Scholar] [CrossRef]
- Morato-Martínez, M.; López-Plaza, B.; Santurino, C.; Palma-Milla, S.; Gómez-Candela, C. A Dairy Product to Reconstitute Enriched with Bioactive Nutrients Stops Bone Loss in High-Risk Menopausal Women without Pharmacological Treatment. Nutrients 2020, 12, 2203. [Google Scholar] [CrossRef] [PubMed]
- Boughanem, H.; Ruiz-Limón, P.; Pilo, J.; Lisbona-Montañez, J.M.; Tinahones, F.J.; Moreno Indias, I.; Macías-González, M. Linking serum vitamin D levels with gut microbiota after 1-year lifestyle intervention with Mediterranean diet in patients with obesity and metabolic syndrome: A nested cross-sectional and prospective study. Gut Microbes 2023, 15, 2249150. [Google Scholar] [CrossRef] [PubMed]
- Giampazolias, E.; Pereira da Costa, M.; Lam, K.C.; Lim, K.H.J.; Cardoso, A.; Piot, C.; Chakravarty, P.; Blasche, S.; Patel, S.; Biram, A.; et al. Vitamin D regulates microbiome-dependent cancer immunity. Science 2024, 384, 428–437. [Google Scholar] [CrossRef]
- Bellerba, F.; Muzio, V.; Gnagnarella, P.; Facciotti, F.; Chiocca, S.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Serrano, D.; Raimondi, S.; et al. The Association between Vitamin D and Gut Microbiota: A Systematic Review of Human Studies. Nutrients 2021, 13, 3378. [Google Scholar] [CrossRef]
- Tangestani, H.; Boroujeni, H.K.; Djafarian, K.; Emamat, H.; Shab-Bidar, S. Vitamin D and The Gut Microbiota: A Narrative Literature Review. Clin. Nutr. Res. 2021, 10, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Hridayanka, K.S.N.; Duttaroy, A.K. Bioactives and their roles in bone metabolism of osteoarthritis: Evidence and mechanisms on gut-bone axis. Front. Immunol. 2023, 14, 1323233. [Google Scholar] [CrossRef]
- Wang, J.; Mei, L.; Hao, Y.; Xu, Y.; Yang, Q.; Dai, Z.; Yang, Y.; Wu, Z.; Ji, Y. Contemporary Perspectives on the Role of Vitamin D in Enhancing Gut Health and Its Implications for Preventing and Managing Intestinal Diseases. Nutrients 2024, 16, 2352. [Google Scholar] [CrossRef]
- Bashir, M.; Prietl, B.; Tauschmann, M.; Mautner, S.I.; Kump, P.K.; Treiber, G.; Wurm, P.; Gorkiewicz, G.; Högenauer, C.; Pieber, T.R. Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur. J. Nutr. 2016, 55, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, M.; Hope, B.; Krause, L.; Morrison, M.; Protani, M.M.; Zakrzewski, M.; Neale, R.E. Vitamin D and the gut microbiome: A systematic review of in vivo studies. Eur. J. Nutr. 2019, 58, 2895–2910. [Google Scholar] [CrossRef]
- Vijayendra Chary, A.; Hemalatha, R.; Seshacharyulu, M.; Vasudeva Murali, M.; Jayaprakash, D.; Dinesh Kumar, B. Vitamin D deficiency in pregnant women impairs regulatory T cell function. J. Steroid Biochem. Mol. Biol. 2015, 147, 48–55. [Google Scholar] [CrossRef]
- Bastyte, D.; Tamasauskiene, L.; Stakaitiene, I.; Briede, K.; Ugenskiene, R.; Valiukeviciene, S.; Gradauskiene, B. Relation of T Cell Profile with Vitamin D Receptor and Vitamin D-Binding Protein Gene Polymorphisms in Atopy. Int. J. Mol. Sci. 2024, 25, 9021. [Google Scholar] [CrossRef]
- Martens, P.J.; Centelles-Lodeiro, J.; Ellis, D.; Cook, D.P.; Sassi, G.; Verlinden, L.; Verstuyf, A.; Raes, J.; Mathieu, C.; Gysemans, C. High Serum Vitamin D Concentrations, Induced via Diet, Trigger Immune and Intestinal Microbiota Alterations Leading to Type 1 Diabetes Protection in NOD Mice. Front. Immunol. 2022, 13, 902678. [Google Scholar] [CrossRef] [PubMed]
- Bora, S.A.; Kennett, M.J.; Smith, P.B.; Patterson, A.D.; Cantorna, M.T. Regulation of vitamin D metabolism following disruption of the microbiota using broad spectrum antibiotics. J. Nutr. Biochem. 2018, 56, 65–73. [Google Scholar] [CrossRef]
- Wu, S.; Yoon, S.; Zhang, Y.G.; Lu, R.; Xia, Y.; Wan, J.; Petrof, E.O.; Claud, E.C.; Chen, D.; Sun, J. Vitamin D receptor pathway is required for probiotic protection in colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G341–G349. [Google Scholar] [CrossRef]
- Wei, M.; Huang, F.; Zhao, L.; Zhang, Y.; Yang, W.; Wang, S.; Li, M.; Han, X.; Ge, K.; Qu, C.; et al. A dysregulated bile acid-gut microbiota axis contributes to obesity susceptibility. eBioMedicine 2020, 55, 102766. [Google Scholar] [CrossRef]
- Xie, X.; Huang, R.; Zhang, W.; Zhang, R. Cofactor-dependence alteration of 7β-hydroxysteroid dehydrogenase: Enhancing one-pot synthesis efficiency of chenodeoxycholic acid to ursodeoxycholic acid through cofactor self-recycling. Int. J. Biol. Macromol. 2024, 280, 136328. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.; Alegre, M.L. Tiny heroes: Microbiota-modified bile acid metabolites protect against drug-induced gut damage. Am. J. Transplant. 2024, 24, 1105–1107. [Google Scholar] [CrossRef]
- Ishizawa, M.; Akagi, D.; Makishima, M. Lithocholic Acid Is a Vitamin D Receptor Ligand That Acts Preferentially in the Ileum. Int. J. Mol. Sci. 2018, 19, 1975. [Google Scholar] [CrossRef]
- Fang, Y.; Qin, M.; Zheng, Q.; Wang, K.; Han, X.; Yang, Q.; Sang, X.; Cao, G. Role of Bile Acid Receptors in the Development and Function of Diabetic Nephropathy. Kidney Int. Rep. 2024, 9, 3116–3133. [Google Scholar] [CrossRef]
- Li, N.; Ma, P.; Li, Y.; Shang, X.; Nan, X.; Shi, L.; Han, X.; Liu, J.; Hong, Y.; Li, Q.; et al. Gut microbiota-derived 12-ketolithocholic acid suppresses the IL-17A secretion from colonic group 3 innate lymphoid cells to prevent the acute exacerbation of ulcerative colitis. Gut Microbes 2023, 15, 2290315. [Google Scholar] [CrossRef]
- Dou, J.Y.; Cui, Z.Y.; Xuan, M.Y.; Gao, C.; Li, Z.X.; Lian, L.H.; Cui, H.Z.; Nan, J.X.; Wu, Y.L. Diallyl disulfide, the bioactive component of Allium species, ameliorates pulmonary fibrosis by mediating the crosstalk of farnesoid X receptor and yes-associated protein 1 signaling pathway. Phytother. Res. 2024, 38, 4009–4021. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Dabbas, B.; Laperriere, D.; Bitton, A.J.; Soualhine, H.; Tavera-Mendoza, L.E.; Dionne, S.; Servant, M.J.; Bitton, A.; Seidman, E.G.; et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J. Biol. Chem. 2010, 285, 2227–2231. [Google Scholar] [CrossRef]
- Thomas, R.L.; Jiang, L.; Adams, J.S.; Xu, Z.Z.; Shen, J.; Janssen, S.; Ackermann, G.; Vanderschueren, D.; Pauwels, S.; Knight, R.; et al. Vitamin D metabolites and the gut microbiome in older men. Nat. Commun. 2020, 11, 5997. [Google Scholar] [CrossRef] [PubMed]
- Kanhere, M.; Chassaing, B.; Gewirtz, A.T.; Tangpricha, V. Role of vitamin D on gut microbiota in cystic fibrosis. J. Steroid Biochem. Mol. Biol. 2018, 175, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, K.; Xiong, Q.; Zhang, J.; Cai, B.; Huang, Z.; Yang, B.; Wei, B.; Chen, J.; Niu, Q. Gut microbiota in pre-clinical rheumatoid arthritis: From pathogenesis to preventing progression. J. Autoimmun. 2023, 141, 103001. [Google Scholar] [CrossRef]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef]
- Maeda, Y.; Rachez, C.; Hawel, L., 3rd; Byus, C.V.; Freedman, L.P.; Sladek, F.M. Polyamines modulate the interaction between nuclear receptors and vitamin D receptor-interacting protein 205. Mol. Endocrinol. 2002, 16, 1502–1510. [Google Scholar] [CrossRef]
- Pham, H.; Waterhouse, M.; Rahman, S.; Baxter, C.; Duarte Romero, B.; McLeod, D.S.A.; Ebeling, P.R.; English, D.R.; Hartel, G.; O’Connell, R.L.; et al. The effect of vitamin D supplementation on the gut microbiome in older Australians-Results from analyses of the D-Health Trial. Gut Microbes 2023, 15, 2221429. [Google Scholar] [CrossRef] [PubMed]
- Midttun, M.; Overgaard, K.; Zerahn, B.; Pedersen, M.; Rashid, A.; Østergren, P.B.; Paulin, T.K.; Pødenphanth, T.W.; Karlsson, L.K.; Rosendahl, E.; et al. Beneficial effects of exercise, testosterone, vitamin D, calcium and protein in older men-A randomized clinical trial. J. Cachexia Sarcopenia Muscle 2024, 15, 1451–1462. [Google Scholar] [CrossRef]
- Arnqvist, H.J.; Leanderson, P.; Spångeus, A. Vitamin D status in longstanding type 1 diabetes and controls. Association with upper extremity impairments. Ups. J. Med. Sci. 2023, 128, e9888. [Google Scholar] [CrossRef]
- Bosman, E.S.; Albert, A.Y.; Lui, H.; Dutz, J.P.; Vallance, B.A. Skin Exposure to Narrow Band Ultraviolet (UVB) Light Modulates the Human Intestinal Microbiome. Front. Microbiol. 2019, 10, 2410. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, J.; Dong, C.; Fu, Y.; Liu, H. Gut microbiome-mediated changes in bone metabolism upon infrared light exposure in rats. J. Photochem. Photobiol. B 2021, 217, 112156. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Carlberg, C.; Raczyk, M.; Zawrotna, N. Vitamin D: A master example of nutrigenomics. Redox Biol. 2023, 62, 102695. [Google Scholar] [CrossRef]
- Vitkova, M.; Diouf, I.; Malpas, C.; Horakova, D.; Kubala Havrdova, E.; Patti, F.; Ozakbas, S.; Izquierdo, G.; Eichau, S.; Shaygannejad, V.; et al. Association of Latitude and Exposure to Ultraviolet B Radiation With Severity of Multiple Sclerosis: An International Registry Study. Neurology 2022, 98, e2401–e2412. [Google Scholar] [CrossRef]
- Gomes, S.; Teixeira-Guedes, C.; Silva, E.; Baltazar, F.; Preto, A. Colon microbiota modulation by dairy-derived diet: New strategy for prevention and treatment of colorectal cancer. Food Funct. 2022, 13, 9183–9194. [Google Scholar] [CrossRef] [PubMed]
- Rakab, M.S.; Rateb, R.M.; Maamoun, A.; Radwan, N.; Shubietah, A.; Manasrah, A.; Rajab, I.; Scichilone, G.; Tussing-Humphreys, L.; Mahmoud, A.M. Impact of Probiotic/Synbiotic Supplementation on Post-Bariatric Surgery Anthropometric and Cardiometabolic Outcomes: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2025, 17, 2193. [Google Scholar] [CrossRef]
- Cheng, J.; Zhai, J.; Zhong, W.; Zhao, J.; Zhou, L.; Wang, B. Lactobacillus rhamnosus GG Promotes Intestinal Vitamin D Absorption by Upregulating Vitamin D Transporters in Senile Osteoporosis. Calcif. Tissue Int. 2022, 111, 162–170. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, T.; Shen, H.; Jiang, Y.; Yang, Q.; Su, S.; Wu, L.; Fan, X.; Gao, M.; Wu, Y.; et al. Mixed probiotics modulated gut microbiota to improve spermatogenesis in bisphenol A-exposed male mice. Ecotoxicol. Environ. Saf. 2024, 270, 115922. [Google Scholar] [CrossRef]
- Ghaly, S.; Kaakoush, N.O.; Lloyd, F.; McGonigle, T.; Mok, D.; Baird, A.; Klopcic, B.; Gordon, L.; Gorman, S.; Forest, C.; et al. High Dose Vitamin D supplementation alters faecal microbiome and predisposes mice to more severe colitis. Sci. Rep. 2018, 8, 11511. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Z.; Gao, X.; Bao, Y.; Hong, Y.; He, X.; Zhu, W.; Li, Y.; Huang, W.; Zheng, N.; et al. Gut microbiota remodeling improves natural aging-related disorders through Akkermansia muciniphila and its derived acetic acid. Pharmacol. Res. 2023, 189, 106687. [Google Scholar] [CrossRef]
- Yang, A.; Lv, Q.; Han, Z.; Dai, S.; Li, Y.; Hao, M.; Yu, R.; Zhu, J.; Yang, C.; Shi, Z.; et al. The Effects of Vitamin D on Muscle Strength Are Influenced by Testosterone Levels. J. Cachexia Sarcopenia Muscle 2025, 16, e13733. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Gong, S.; Yao, W.; Gao, H.; Liu, M.; Wei, M. Puerarin improves OVX-induced osteoporosis by regulating phospholipid metabolism and biosynthesis of unsaturated fatty acids based on serum metabolomics. Phytomedicine 2022, 102, 154198. [Google Scholar] [CrossRef]
- Teede, H.J. The menopause and HRT. Hormone replacement therapy, cardiovascular and cerebrovascular disease. Best. Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 73–90. [Google Scholar] [CrossRef]
- Wu, K.C.; Cao, S.; Weaver, C.M.; King, N.J.; Patel, S.; Kingman, H.; Sellmeyer, D.E.; McCauley, K.; Li, D.; Lynch, S.V.; et al. Prebiotic to Improve Calcium Absorption in Postmenopausal Women After Gastric Bypass: A Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2022, 107, 1053–1064. [Google Scholar] [CrossRef]
- Collins, F.L.; Irwin, R.; Bierhalter, H.; Schepper, J.; Britton, R.A.; Parameswaran, N.; McCabe, L.R. Lactobacillus reuteri 6475 Increases Bone Density in Intact Females Only under an Inflammatory Setting. PLoS ONE 2016, 11, e0153180. [Google Scholar] [CrossRef]
- Bunyavanich, S. Food allergy: Could the gut microbiota hold the key? Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 201–202. [Google Scholar] [CrossRef]
- Resciniti, S.M.; Biesiekierski, J.R.; Ghasem-Zadeh, A.; Moschonis, G. The Effectiveness of a Lactobacilli-Based Probiotic Food Supplement on Bone Mineral Density and Bone Metabolism in Australian Early Postmenopausal Women: Protocol for a Double-Blind Randomized Placebo-Controlled Trial. Nutrients 2024, 16, 1150. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, H.; Chen, Y.; Tang, Y.; Tang, Y.; Sarmento, B.; He, C.; Cai, Z.; Cui, W. Self-Replenishable Metabolically Augmented Synbiotic Microspheres Remodel Gut-Bone Homeostasis. Adv. Mater. 2025, 37, e2500746. [Google Scholar] [CrossRef]
- Li, Z.; Deng, X.; Qiu, C.; Wang, Z.; Tang, F.; Pi, Y.; He, Z.; Zheng, C.; Luo, J. Orally administered degradable nanoarmor-assisted probiotics for remodeling the gut microenvironment and treating osteoporosis. J. Nanobiotechnol. 2025, 23, 660. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, Z.; Zhang, X.; Ren, H.; Ji, H.; Yang, F.; Fu, Z.; Kong, X.; Cheng, X.; Li, J.; et al. Metagenomic analysis revealing links between age, gut microbiota and bone loss in Chinese adults. NPJ Metab. Health Dis. 2025, 3, 18. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Wang, L. The Mediating Role of Bioactive Molecules in Gut Microbiota–Bone Metabolism Crosstalk. Nutrients 2025, 17, 3421. https://doi.org/10.3390/nu17213421
Liang X, Wang L. The Mediating Role of Bioactive Molecules in Gut Microbiota–Bone Metabolism Crosstalk. Nutrients. 2025; 17(21):3421. https://doi.org/10.3390/nu17213421
Chicago/Turabian StyleLiang, Xinping, and Luoyang Wang. 2025. "The Mediating Role of Bioactive Molecules in Gut Microbiota–Bone Metabolism Crosstalk" Nutrients 17, no. 21: 3421. https://doi.org/10.3390/nu17213421
APA StyleLiang, X., & Wang, L. (2025). The Mediating Role of Bioactive Molecules in Gut Microbiota–Bone Metabolism Crosstalk. Nutrients, 17(21), 3421. https://doi.org/10.3390/nu17213421





