Dietary Fat Intake and Indices of Blood Profiles in High-Performance Athletes: An Exploratory Study Focusing on Platelet Variables
Abstract
1. Introduction
2. Materials and Methods
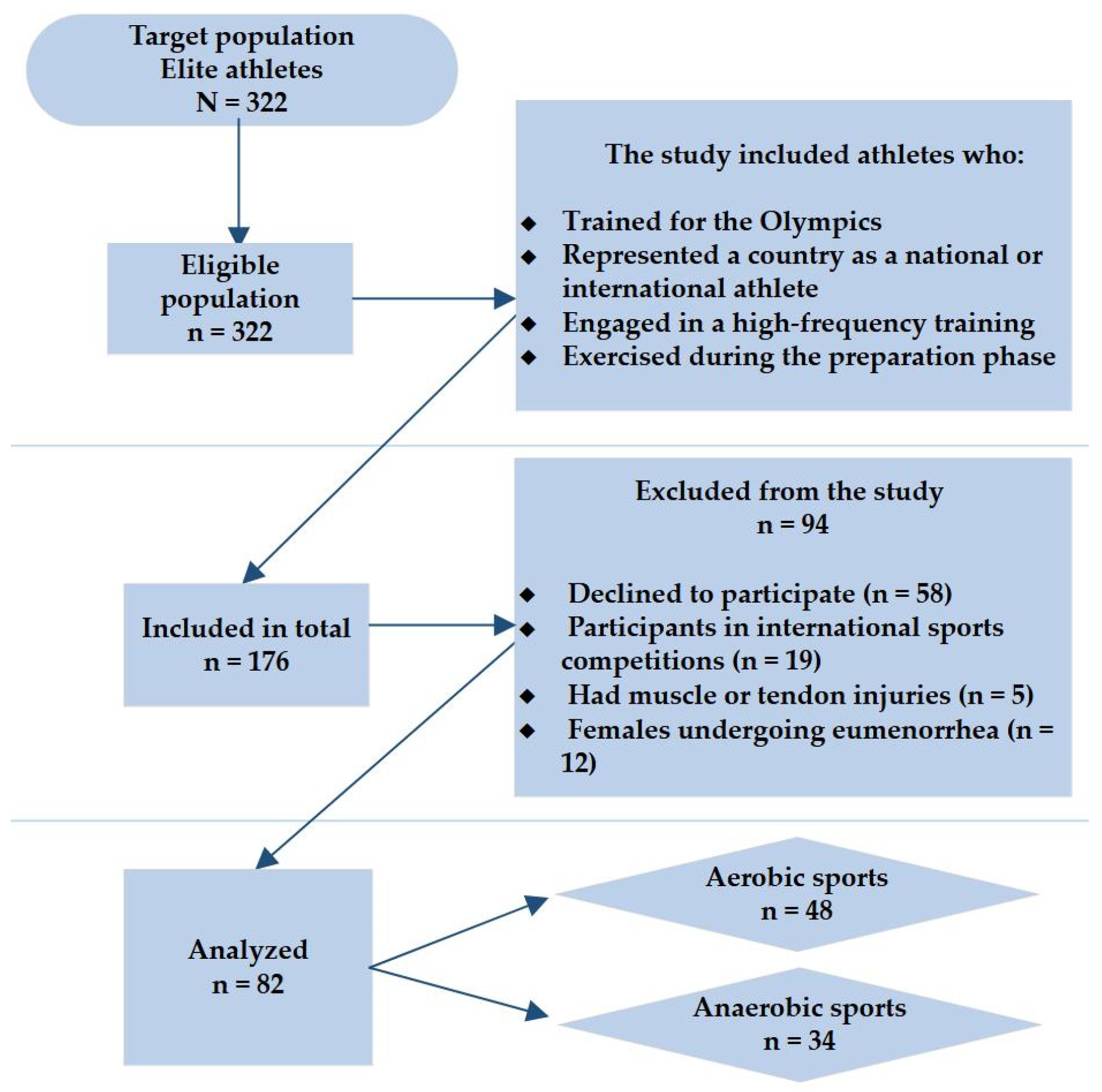
2.1. Study Design, Population, and Data Collection
2.2. Measurements
2.2.1. Dietary and Body Weight Assessment
2.2.2. Blood Collection
2.3. Statistical Data Analysis
3. Results
3.1. Characteristics of Athletes
3.2. Nutritional Status
3.3. Blood Analysis
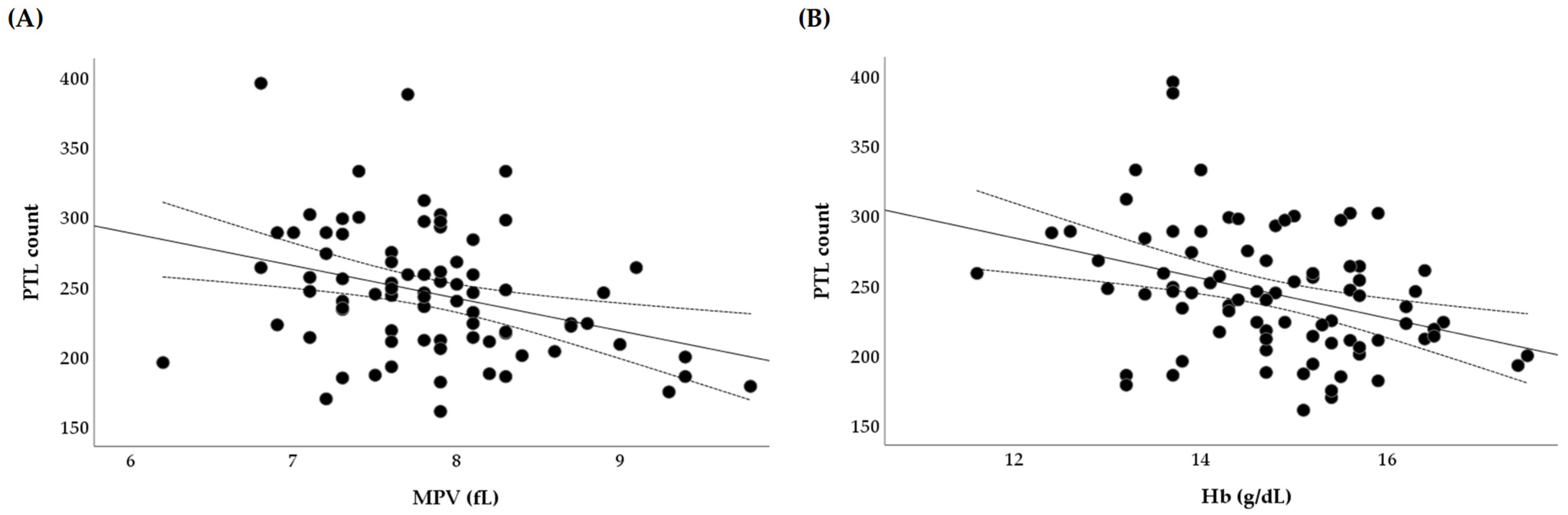
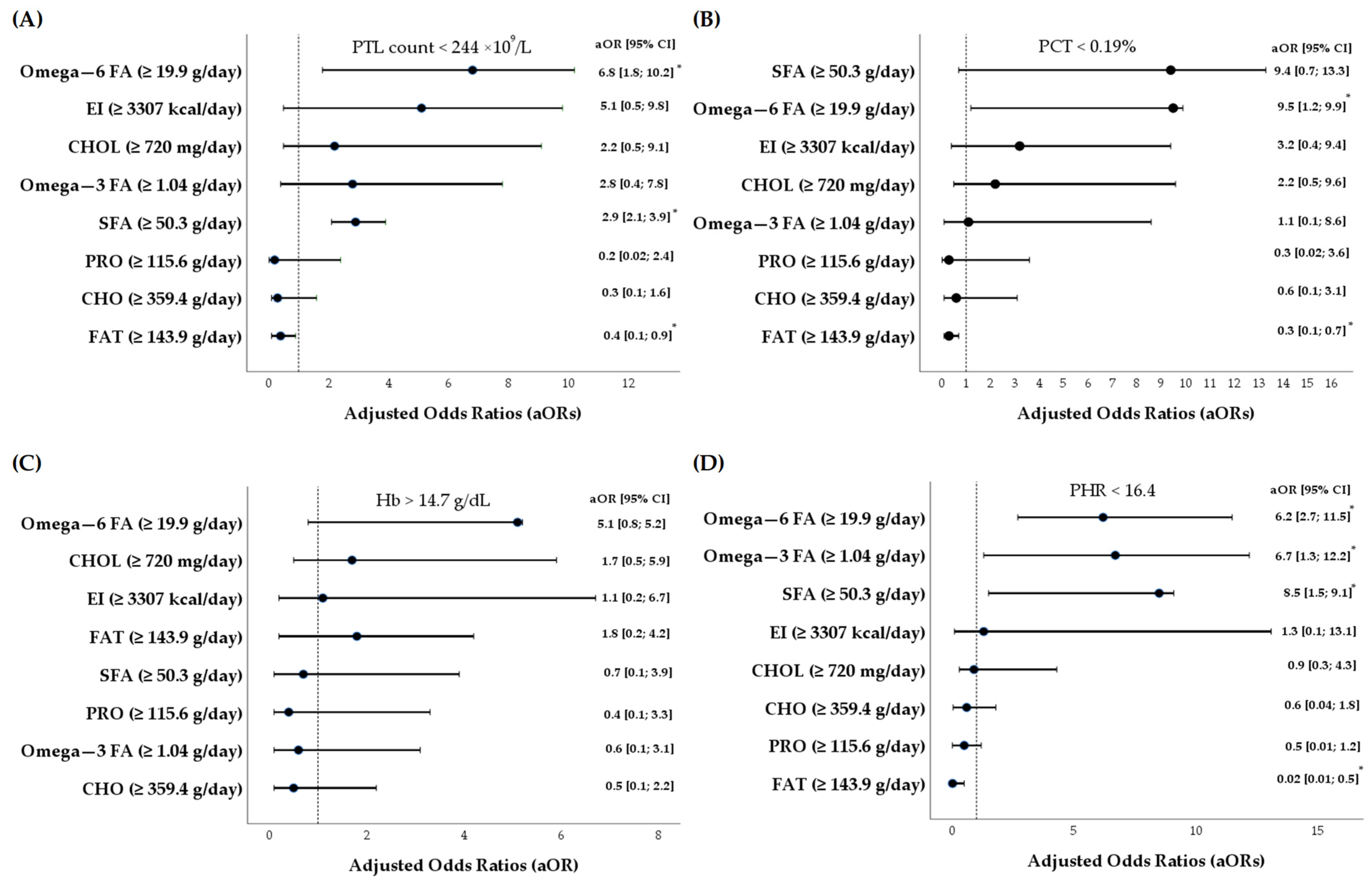
3.4. Associations Between Dietary Intakes and Platelet Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| BIA | Bioelectrical impedance analysis |
| BW | Body weight |
| CI | Confidence interval |
| CVD | Cardiovascular disease |
| DHA | Docosahexaenoic acid |
| EI | Energy intake |
| EN | European Union |
| EPA | Eicosapentaenoic acid |
| FA | Fatty acids |
| Hb | Hemoglobin |
| Ht | Hematocrit |
| ISO | International Organization for Standardization |
| ISSN | International Society of Sports Nutrition |
| LB | Lower bound |
| LDL | Low-density lipoprotein |
| Lym | Lymphocytes |
| LSC | Lithuanian Sports Centre (LSC) |
| LTOK | Lithuanian National Olympic Committee |
| MCH | Mean corpuscular hemoglobin concentration |
| Mo | Monocytes |
| MPV | Mean platelet volume |
| NO | Nitric oxide |
| OR | Odds ratio |
| PCT | Plateletcrit |
| PHR | Platelet-to-hemoglobin ratio |
| PTL | Platelet |
| RBC | Red blood cells |
| RDA | Recommended dietary allowance |
| RDW | Red blood cell distribution width |
| SD | Standard deviation |
| SPSS | Statistical Package for the Social Sciences |
| TxB3 | Thromboxane B3 |
| UB | Upper bound |
| WBC | White blood cells |
References
- Roy, S.; Paul, K. Nourishing the clot: A comprehensive review of dietary habits and their implications for platelet function. Bull. Fac. Phys. Ther. 2024, 29, 7. [Google Scholar] [CrossRef]
- Fabricius, H.-Å.; Starzonek, S.; Lange, T. The role of platelet cell surface P-selectin for the direct platelet-tumor cell contact during metastasis formation in human tumors. Front. Oncol. 2021, 11, 642761. [Google Scholar] [CrossRef]
- Ho-Tin-Noe, B.; Boulaftali, Y.; Camerer, E. Platelets and vascular integrity: How platelets prevent bleeding in inflammation. Blood 2018, 131, 277–288. [Google Scholar] [CrossRef]
- Davi, G.; Patrono, C. Platelet activation and atherothrombosis. N. Engl. J. Med. 2007, 357, 2482–2494. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Datta, T.; Biswas, D.; Duss, R.; O’Kennedy, N.; Duttaroy, A.K. Evaluation of the equivalence of different intakes of Fruitflow in affecting platelet aggregation and thrombin generation capacity in a randomized, double-blinded pilot study in male subjects. BMC Nutr. 2021, 7, 80. [Google Scholar] [CrossRef]
- van der Meijden, P.E.; Heemskerk, J.W. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Qu, M.; Zou, X.; Fang, F.; Wang, S.; Xu, L.; Zeng, Q.; Fan, Z.; Chen, L.; Yue, W.; Xie, X.; et al. Platelet-derived microparticles enhance megakaryocyte differentiation and platelet generation via miR-1915-3p. Nat. Commun. 2020, 11, 4964. [Google Scholar] [CrossRef]
- Menter, D.G.; Kopetz, S.; Hawk, E.; Sood, A.K.; Loree, J.M.; Gresele, P.; Honn, K.V. Platelet “first responders” in wound response, cancer, and metastasis. Cancer Metastasis Rev. 2017, 36, 199–213. [Google Scholar] [CrossRef]
- Martin, J.F.; Kristensen, S.D.; Mathur, A.; Grove, E.L.; Choudry, F.A. The causal role of megakaryocyte–platelet hyperactivity in acute coronary syndromes. Nat. Rev. Cardiol. 2012, 9, 658–670. [Google Scholar] [CrossRef]
- Johansson, P.; Ostrowski, S.; Secher, N. Management of major blood loss: An update. Acta Anaesthesiol. Scand. 2010, 54, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, F.; Massberg, S. Blood coagulation in immunothrombosis-At the frontline of intravascular immunity. Semin. Immunol. 2016, 28, 561–569. [Google Scholar] [CrossRef]
- Nurden, A.T. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 2011, 105, S13–S33. [Google Scholar] [CrossRef]
- Xu, X.R.; Zhang, D.; Oswald, B.E.; Carrim, N.; Wang, X.; Hou, Y.; Zhang, Q.; Lavalle, C.; McKeown, T.; Marshall, A.H.; et al. Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit. Rev. Clin. Lab. Sci. 2016, 53, 409–430. [Google Scholar] [CrossRef]
- Das, D.; Adhikary, S.; Das, R.K.; Banerjee, A.; Radhakrishnan, A.K.; Paul, S.; Pathak, S.; Duttaroy, A.K. Bioactive food components and their inhibitory actions in multiple platelet pathways. J. Food Biochem. 2022, 46, e14476. [Google Scholar] [CrossRef] [PubMed]
- O’Kennedy, N.; Duss, R.; Duttaroy, A.K. Dietary Antiplatelets: A New Perspective on the Health Benefits of the Water-Soluble Tomato Concentrate Fruitflow®. Nutrients 2021, 13, 2184. [Google Scholar] [CrossRef]
- Das, D.; Shruthi, N.R.; Banerjee, A.; Jothimani, G.; Duttaroy, A.K.; Pathak, S. Endothelial dysfunction, platelet hyperactivity, hypertension, and the metabolic syndrome: Molecular insights and combating strategies. Front. Nutr. 2023, 10, 1221438. [Google Scholar] [CrossRef]
- Natarajan, A.; Zaman, A.G.; Marshall, S.M. Platelet hyperactivity in type 2 diabetes: Role of antiplatelet agents. Diab. Vasc. Dis. Res. 2008, 5, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Massberg, S.; Brand, K.; Gruner, S.; Page, S.; Muller, E.; Muller, I.; Bergmeier, W.; Richter, T.; Lorenz, M.; Konrad, I.; et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J. Exp. Med. 2002, 196, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, Z.S.; Jackson, S.P. The role of platelets in atherothrombosis. Hematol. Am. Soc. Hematol. Educ. Program 2011, 2011, 51–61. [Google Scholar] [CrossRef]
- Xu, X.R.; Yousef, G.M.; Ni, H. Cancer and platelet crosstalk: Opportunities and challenges for aspirin and other antiplatelet agents. Blood 2018, 131, 1777–1789. [Google Scholar] [CrossRef]
- Koupenova, M.; Corkrey, H.A.; Vitseva, O.; Manni, G.; Pang, C.J.; Clancy, L.; Yao, C.; Rade, J.; Levy, D.; Wang, J.P.; et al. The role of platelets in mediating a response to human influenza infection. Nat. Commun. 2019, 10, 1780. [Google Scholar] [CrossRef]
- Puurunen, M.K.; Hwang, S.J.; Larson, M.G.; Vasan, R.S.; O’Donnell, C.J.; Tofler, G.; Johnson, A.D. ADP Platelet Hyperreactivity Predicts Cardiovascular Disease in the FHS (Framingham Heart Study). J. Am. Heart Assoc. 2018, 7, e008522. [Google Scholar] [CrossRef]
- Tomczynska, M.; Salata, I.; Bijak, M.; Saluk-Bijak, J. The potential contribution and role of a blood platelets in autoimmune thyroid diseases. J. Cell. Mol. Med. 2018, 22, 6386–6390. [Google Scholar] [CrossRef]
- Garcia-Leon, M.J.; Liboni, C.; Mittelheisser, V.; Bochler, L.; Follain, G.; Mouriaux, C.; Busnelli, I.; Larnicol, A.; Colin, F.; Peralta, M.; et al. Platelets favor the outgrowth of established metastases. Nat. Commun. 2024, 15, 3297. [Google Scholar] [CrossRef]
- El Haouari, M.; Rosado, J.A. Platelet function in hypertension. Blood Cells Mol. Dis. 2009, 42, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Song, Z.; Hu, L.T.; Tong, Y.H.; Hu, J.Y.; Shen, H. Abnormal platelet parameters in inflammatory bowel disease: A systematic review and meta-analysis. BMC Gastroenterol. 2024, 24, 214. [Google Scholar] [CrossRef] [PubMed]
- Harifi, G.; Sibilia, J. Pathogenic role of platelets in rheumatoid arthritis and systemic autoimmune diseases. Perspectives and therapeutic aspects. Saudi Med. J. 2016, 37, 354–360. [Google Scholar] [CrossRef]
- Vannice, G.; Rasmussen, H. Position of the academy of nutrition and dietetics: Dietary fatty acids for healthy adults. J. Acad. Nutr. Diet. 2014, 114, 136–153. [Google Scholar] [CrossRef]
- Podgórska, K.; Derkacz, A.; Szahidewicz-Krupska, E.; Jasiczek, J.; Dobrowolski, P.; Radziwon-Balicka, A.; Skomro, R.; Szuba, A.; Mazur, G.; Doroszko, A. Effect of regular aerobic activity in young healthy athletes on profile of endothelial function and platelet activity. Biomed. Res. Int. 2017, 2017, 8715909. [Google Scholar] [CrossRef] [PubMed]
- Di Francescomarino, S.; Sciartilli, A.; Di Valerio, V.; Di Baldassarre, A.; Gallina, S. The effect of physical exercise on endothelial function. Sports Med. 2009, 39, 797–812. [Google Scholar] [CrossRef]
- Heber, S.; Volf, I. Effects of physical (in)activity on platelet function. Biomed. Res. Int. 2015, 2015, 165078. [Google Scholar] [CrossRef]
- Dutta-Roy, A.K. Dietary components and human platelet activity. Platelets 2002, 13, 67–75. [Google Scholar] [CrossRef] [PubMed]
- O’Kennedy, N.; Raederstorff, D.; Duttaroy, A.K. Fruitflow®: The first European Food Safety Authority-approved natural cardio-protective functional ingredient. Eur. J. Nutr. 2017, 56, 461–482. [Google Scholar]
- Duttaroy, A.K.; Jorgensen, A. Effects of kiwi fruit consumption on platelet aggregation and plasma lipids in healthy human volunteers. Platelets 2004, 15, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Dutta-Roy, A.K.; Gordon, M.J.; Kelly, C.; Hunter, K.; Crosbie, L.; Knight-Carpentar, T.; Williams, B.C. Inhibitory effect of Ginkgo biloba extract on human platelet aggregation. Platelets 1999, 10, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Dutta-Roy, A.K.; Crosbie, L.; Gordon, M.J. Effects of tomato extract on human platelet aggregation in vitro. Platelets 2001, 12, 218–227. [Google Scholar] [CrossRef]
- Larson, M.K.; Shearer, G.C.; Ashmore, J.H.; Anderson-Daniels, J.M.; Graslie, E.L.; Tholen, J.T.; Vogelaar, J.L.; Korth, A.J.; Nareddy, V.; Sprehe, M.; et al. Omega-3 fatty acids modulate collagen signaling in human platelets. Prostaglandins Leukot. Essent. Fat. Acids 2011, 84, 93–98. [Google Scholar]
- De Carvalho, C.C.; Caramujo, M.J. The various roles of fatty acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef]
- Sullivan, K.M. Open Source Statistics for Public Health. Available online: https://www.openepi.com/SampleSize/SSPropor.htm (accessed on 10 February 2022).
- The Lithuanian National Olympic Committee (LTOK). Available online: https://www.ltok.lt/en (accessed on 12 December 2021).
- Skernevičius, J.; Milašius, K.; Raslanas, A.; Dadelienė, R. Sporto treniruotė (Sports training). In Sportininkų Gebėjimai ir jų Ugdymas (Skills and Training of Athletes), 1st ed.; Čepulėnas, A., Saplinskas, J., Paulauskas, R., Eds.; Lithuanian University of Educational Sciences Press: Vilnius, Lithuania, 2011; pp. 165–217. [Google Scholar]
- Deakin, V.; Kerr, D.; Boushey, C. Measuring nutritional status of athletes: Clinical and research perspectives. In Clinical Sports Nutrition, 5th ed.; Burke, L.M., Deakin, V., Eds.; McGraw-Hill: North Ryde, Australia, 2015; pp. 27–53. [Google Scholar]
- Capling, L.; Beck, K.L.; Gifford, J.A.; Slater, G.; Flood, V.M.; O’Connor, H. Validity of dietary assessment in athletes: A systematic review. Nutrients 2017, 9, 1313. [Google Scholar] [CrossRef]
- Barzda, A.; Bartkevičiūtė, R.; Viseckienė, V.; Abaravičius, A.J.; Stukas, R. Maisto Produktų ir Patiekalų Porcijų Nuotraukų Atlasas (Atlas of Foodstuffs and Dishes); Republican Nutrition Center, Vilnius University Faculty of Medicine: Vilnius, Lithuania, 2007; pp. 7–42. Available online: https://www.hi.lt/uploads/Products/product_324/Maisto_produkt_ir_patiekal_porcij_nuotrauk_atlasas_2007_m..pdf (accessed on 28 April 2021).
- Nutrition Surveys and Calculations. Available online: http://www.nutrisurvey.de/ (accessed on 21 January 2021).
- Sučilienė, S.; Abaravičius, A. Maisto Produktų Sudėtis (Food Product Composition); Ministry of Health of the Republic of Lithuania: Vilnius, Lithuania, 2002; pp. 10–315. [Google Scholar]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International Society of Sports Nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14, 33. [Google Scholar] [CrossRef]
- Aragon, A.A.; Schoenfeld, B.; Wildman, R.; Kleiner, S.; Vandusseldorp, T.; Taylor, L.; Earnest, C.P.; Arciero, P.J.; Wilborn, C.; Kalman, D.S.; et al. International Society of Sports Nutrition position stand: Diets and body composition. J. Int. Soc. Sports Nutr. 2017, 14, 16. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.; Meyer, N.; et al. International Olympic Committee (IOC) consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 316–331. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 104–125. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.E.; Smesny, S.; Kim, S.W.; Davey, C.G.; Rice, S.; Sarnyai, Z.; Schlögelhofer, M.; Schäfer, M.R.; Berk, M.; McGorry, P.D.; et al. Omega-6 to omega-3 polyunsaturated fatty acid ratio and subsequent mood disorders in young people with at-risk mental states: A 7-year longitudinal study. Transl. Psychiatry 2017, 7, e1220. [Google Scholar] [CrossRef]
- Harris, J.; Benedict, F. A Biometric Study of Basal Metabolism in Man; Lippincott: Philadelphia, PA, USA, 1919. [Google Scholar]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American college of sports medicine joint position statement. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. Compendium of physical activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- FAO/WHO/UNU. Human Energy Requirements: Principles and Definitions. Report of a Joint FAO/WHO/UNU Expert Consultation; WHO: Geneva, Switzerland, 2001; Available online: https://www.fao.org/4/y5686e/y5686e00.htm (accessed on 19 December 2021).
- EN ISO 13488:2000; Quality Systems—Medical Devices—Particular Requirements for the Application of EN ISO 9002 (Revision of EN 46002:1996) (Identical to ISO 13488:1996). ISO: Geneva, Switzerland, 2000.
- Pratiwi, M.P.; Syakdiyah, N.H.; Sintoro, H.P. Platelet-to-lymphocyte ratio (PLR), platelet-to-hemoglobin ratio (PHR) and systemic immune-inflammation index (SII) as predictive factors of amputation among Diabetic Foot Ulcer (DFU) in Ibnu Sina Gresik Regional Public Hospital, East Java, Indonesia. Indones. J. Biomed. Sci. 2024, 18, 158–161. [Google Scholar] [CrossRef]
- Abdul-Sahib, N.S.; Mahmood, M.M.; Ad’hiah, A.H. The significance of inflammatory markers derived from blood cell counts in predicting rheumatoid arthritis among the elderly. Rev. Colomb. Reumatol. 2024, RCREU-2157, 1–11. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge Academic: New York, NY, USA, 1988. [Google Scholar]
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Van Horn, L.; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Dietary cholesterol and cardiovascular risk: A science advisory from the American Heart Association. Circulation 2020, 141, e39–e53. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.A.S.; Lazarim, F.L.; Brenzikofer, R.; Macedo, D.V. Applicability of the reference interval and reference change value of hematological and biochemical biomarkers to sport science. Int. Perspect. Top. Sports Med. Sports Inj. 2012, 4, 77–98. [Google Scholar]
- Zareifar, S.; Farahmand Far, M.R.; Golfeshan, F.; Cohan, N. Changes in platelet count and mean platelet volume during infectious and inflammatory disease and their correlation with ESR and CRP. J. Clin. Lab. Anal. 2014, 28, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Garai, B.; Chatterjee, S.; Mondal, S.; Mondal, T. Effect of exercise on platelet variables: An overview. Int. J. Phys. Educ. Sports Health 2017, 4, 506–510. [Google Scholar]
- Schumacher, Y.O.; Schmid, A.; Grathwohl, D.; Bültermann, D.I.R.K.; Berg, A. Hematological indices and iron status in athletes of various sports and performances. Med. Sci. Sports Exerc. 2002, 34, 869–875. [Google Scholar] [CrossRef]
- Segal, J.B.; Moliterno, A.R. Platelet counts differ by sex, ethnicity, and age in the United States. Ann. Epidemiol. 2006, 16, 123–130. [Google Scholar] [CrossRef]
- Al-Khelaifi, F.; Diboun, I.; Donati, F.; Botrè, F.; Alsayrafi, M.; Georgakopoulos, C.; Suhre, K.; Yousri, N.A.; Elrayess, M.A. A pilot study comparing the metabolic profiles of elite-level athletes from different sporting disciplines. Sports Med. Open 2018, 4, 2. [Google Scholar] [CrossRef]
- Wang, C.; Harris, W.S.; Chung, M.; Lichtenstein, A.H.; Balk, E.M.; Kupelnick, B.; Jordan, H.S.; Lau, J. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: A systematic review. Am. J. Clin. Nutr. 2006, 84, 5–17. [Google Scholar] [CrossRef]
- Andrioli, G.; Carletto, A.; Guarini, P.; Galvani, S.; Biasi, D.; Bellavite, P.; Corrocher, R. Differential effects of dietary supplementation with fish oil or soy lecithin on human platelet adhesion. Thromb. Haemost. 1999, 82, 1522–1527. [Google Scholar] [CrossRef]
- Fischer, S.; Weber, P.C. Thromboxane A3 (TXA3) is formed in human platelets after dietary eicosapentaenoic acid (C20:5 omega 3). Biochem. Biophys. Res. Commun. 1983, 116, 1091–1099. [Google Scholar] [CrossRef]
- Golanski, J.; Szymanska, P.; Rozalski, M. Effects of omega-3 polyunsaturated fatty acids and their metabolites on haemostasis—Current perspectives in cardiovascular disease. Int. J. Mol. Sci. 2021, 22, 2394. [Google Scholar] [CrossRef] [PubMed]
- Mallick, R.; Basak, S.; Das, R.K.; Banerjee, A.; Paul, S.; Pathak, S.; Duttaroy, A.K. Fatty Acids and their proteins in adipose tissue inflammation. Cell Biochem. Biophys. 2024, 82, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Heileson, J.L.; Abou Sawan, S.; Dickerson, B.L.; Leonard, M.; Kreider, R.B.; Kerksick, C.M.; Cornish, S.M.; Candow, D.G.; Cordingley, D.M.; et al. International society of sports nutrition position stand: Long-chain omega-3 polyunsaturated fatty acids. J. Int. Soc. Sports Nutr. 2025, 22, 2441775. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hossain, S.; Shido, O. Docosahexaenoic acid but not eicosapentaenoic acid withstands dietary cholesterol-induced decreases in platelet membrane fluidity. Mol. Cell. Biochem. 2006, 293, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Svaneborg, N.; Kristensen, S.D.; Hansen, L.M.; Bullow, I.; Husted, S.E.; Schmidt, E.B. The acute and short-time effect of supplementation with the combination of n-3 fatty acids and acetylsalicylic acid on platelet function and plasma lipids. Thromb. Res. 2002, 105, 311–316. [Google Scholar] [CrossRef]
- Eschen, O.; Christensen, J.H.; De Caterina, R.; Schmidt, E.B. Soluble adhesion molecules in healthy subjects: A dose-response study using n-3 fatty acids. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 180–185. [Google Scholar] [CrossRef]
- Vanschoonbeek, K.; Feijge, M.A.; Paquay, M.; Rosing, J.; Saris, W.; Kluft, C.; Giesen, P.L.; de Maat, M.P.; Heemskerk, J.W. Variable hypocoagulant effect of fish oil intake in humans: Modulation of fibrinogen level and thrombin generation. Arter. Thromb. Vasc. Biol. 2004, 24, 1734–1740. [Google Scholar] [CrossRef]
- Din, J.N.; Harding, S.A.; Valerio, C.J.; Sarma, J.; Lyall, K.; Riemersma, R.A.; Newby, D.E.; Flapan, A.D. Dietary intervention with oil rich fish reduces platelet-monocyte aggregation in man. Atherosclerosis 2008, 197, 290–296. [Google Scholar] [CrossRef]
- Vericel, E.; Calzada, C.; Chapuy, P.; Lagarde, M. The influence of low intake of n-3 fatty acids on platelets in elderly people. Atherosclerosis 1999, 147, 187–192. [Google Scholar] [CrossRef]
- Croset, M.; Vericel, E.; Rigaud, M.; Hanss, M.; Courpron, P.; Dechavanne, M.; Lagarde, M. Functions and tocopherol content of blood platelets from elderly people after low intake of purified eicosapentaenoic acid. Thromb. Res. 1990, 57, 1–12. [Google Scholar] [CrossRef]
- Driss, F.; Vericel, E.; Lagarde, M.; Dechavanne, M.; Darcet, P. Inhibition of platelet aggregation and thromboxane synthesis after intake of small amount of icosapentaenoic acid. Thromb. Res. 1984, 36, 389–396. [Google Scholar] [CrossRef]
- Guillot, N.; Caillet, E.; Laville, M.; Calzada, C.; Lagarde, M.; Vericel, E. Increasing intakes of the long-chain omega-3 docosahexaenoic acid: Effects on platelet functions and redox status in healthy men. FASEB J. 2009, 23, 2909–2916. [Google Scholar] [CrossRef]
- Duttaroy, A.K. Functional foods in preventing human blood platelet hyperactivity-mediated diseases—An updated review. Nutrients 2024, 16, 3717. [Google Scholar] [CrossRef]
- Santhakumar, A.B.; Bulmer, A.C.; Singh, I. A review of the mechanisms and effectiveness of dietary polyphenols in reducing oxidative stress and thrombotic risk. J. Hum. Nutr. Diet. 2014, 27, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Saputri, F.C.; Azmi, N.U.; Puteri, M.U.; Damayanti; Novita, V.; Marisi, G.; Oktavira, E.; Sari, A.N.; Ronaningtyas, K.; Herawati, E. High-fat diet enhances platelet activation and is associated with proprotein convertase subtilisin kexin 9: An animal study. Nutrients 2023, 15, 4463. [Google Scholar] [CrossRef]
- Nagy, B., Jr.; Jin, J.; Ashby, B.; Reilly, M.P.; Kunapuli, S.P. Contribution of the P2Y12 receptor-mediated pathway to platelet hyperreactivity in hypercholesterolemia. J. Thromb. Haemost. 2011, 9, 810–819. [Google Scholar] [CrossRef]
- Mariscal-Arcas, M.; Carvajal, C.; Monteagudo, C.; Lahtinen, J.; Fernández de Alba, M.C.; Feriche, B.; Olea-Serrano, F. Nutritional analysis of diet at base camp of a seven thousand-metre mountain in the Himalayas. Rev. Andal. Med. Deporte 2010, 3, 127–132. [Google Scholar]
- Stukas, R.; Pečiukonienė, M.; Kemerytė-Riaubienė, E.; Baškienė, V. Some aspects of fat metabolism in an athlete’s body. Sport Sci. 2009, 2, 44–49. [Google Scholar]
- Fung, T.T.; Rimm, E.B.; Spiegelman, D.; Rifai, N.; Tofler, G.H.; Willett, W.C.; Hu, F.B. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am. J. Clin. Nutr. 2001, 73, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Martinez, K.; Chuang, C.C.; LaPoint, K.; McIntosh, M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: Mechanisms of action and implications. J. Nutr. 2009, 139, 1–4. [Google Scholar] [CrossRef]
| Sports | Skiing and Biathlon | Boxing, Graeco-Roman Wrestling and Judo | Road Cycling | Swimming (50–400 m) and Canoe Paddling (500–2000 m) | Modern Pentathlon | Basketball |
|---|---|---|---|---|---|---|
| Performance investigation by month | August | May–June | April | March–May | June | May |
| Macrocycle phase | Preparation | Preparation | Preparation | Preparation | Preparation | Preparation |
| Stage | Basic training | Special training | Special training | Special training | Special training | Special training |
| Days of exercise per month | 27 | 27 | 26 | 25–27 | 26 | 27 |
| Total physical training (hours per month) | 31–32 | 36–41 | 36 | 40–41 | 40 | 41 |
| Special training is categorized into five zones of intensity depending on ATP production in the muscles | ||||||
| Zone 1: Aerobic strength endurance training, recovery (pulse rate − 130 ± 10 bpm, lactate level up to 2 mmol/L) | 26–36% | 12–18% | 18% | 18–20% | 20% | 18% |
| Zone 2: Aerobic strength training (pulse rate − 150 ± 10 bpm, lactate level is 2–4 mmol/L), muscular power increase at the anaerobic threshold | 37–48% | 26–30% | 50% | 40–50% | 50% | 42% |
| Zone 3: Mixed aerobic and anaerobic glycolytic strength training (pulse rate −170 ± 10 bpm, lactate level is 4–12 mmol/L), increase of VO2max | 15–17% | 40–50% | 32% | 29–35% | 25% | 32% |
| Zone 4: Anaerobic glycolytic strength training (pulse rate ≥181 bpm, lactate level up to 21 mmol/L) | 6–10% | 8–10% | – | 5% | 3% | 5% |
| Zone 5: Anaerobic phosphocreatine strength training (lactate level is 1.5–6 mmol/L) | 2–3% | 2–4% | – | 1–2% | 2% | 3% |
| Energy Intake and Macronutrients | Endurance Athletes (n = 48) | Strength-Power Athletes (n = 34) | RDAs | d |
|---|---|---|---|---|
| Energy intake (kcal/kg/day) | 49 ± 15 | 50 ± 16 | 50 ± 8 [49; 53] | −0.07 |
| Carbohydrates (g/kg/day) | 5.5 ± 1.9 | 5.3 ± 1.9 | 7–10 | −1.63 |
| Carbohydrates (% of EI) | 47.3 ± 7.4 ** | 43.2 ± 6.2 | 45–65 | −1.31 |
| Protein (g/kg/day) | 1.6 ± 0.5 | 1.7 ± 0.5 | 1.2–2.0 | −0.02 |
| Protein (% of EI) | 14.2 ± 3.1 | 14.0 ± 2.6 | 12–20 | −0.82 |
| Lipids (g/kg/day) | 2.0 ± 0.8 | 2.3 ± 0.8 | 1.0–1.5 | 1.06 |
| Lipids (% of EI) | 38.5 ± 6.9 | 42.8 ± 5.7 ** | 25–35 | 1.53 |
| Saturated FA (g/day) | 45.5 ± 19.7 | 60.4 ± 20.5 *** | — | — |
| Saturated FA (% of EI) | 13.4 ± 3.4 | 14.3 ± 2.5 | ≤10 | 1.22 |
| Cholesterol (mg) | 698 ± 391 | 982 ± 478 *** | — | — |
| Monounsaturated FA (g/day) | 63.5 ± 23.6 | 97.2 ± 47.6 *** | — | — |
| Polyunsaturated FA (g/day) | 20.1 ± 10.7 | 27.2 ± 12.0 ** | — | — |
| Polyunsaturated FA (% of EI) | 5.9 ± 2.4 | 6.3 ± 1.7 | 6–10 | −1.86 |
| Omega-6 FA (g/day) | 18.4 ± 9.8 | 25.4 ± 11.5 ** | — | — |
| Omega-6 FA (% of EI) | 5.4 ± 2.2 | 5.9 ± 1.7 | 5–8 | −0.19 |
| Omega-3 FA (g/day) | 1.1 ± 0.8 | 1.3 ± 0.5 | — | — |
| Omega-3 FA (% of EI) | 0.3 ± 0.2 | 0.3 ± 0.1 | ≥2 | −8.50 |
| Omega-6 FA/Omega-3 FA ratio | 18.9 ± 8.1 | 19.4 ± 6.1 | 1:1–4:1 | 2.07 |
| Parameter | Endurance Athletes (n = 48) | Strength-Power Athletes (n = 34) | RIs for Physically Active Individuals 2.5th–97.5th Percentile | d |
|---|---|---|---|---|
| RBC count (×1012/L) | 5.1 ± 0.4 | 5.0 ± 0.5 | 4.4–5.6 | 0.14 |
| Hb (g/dL) | 14.7 ± 1.1 | 14.9 ± 1.2 | 13.0–16.1 | 0.17 |
| MCV (fL) | 86.4 ± 3.4 | 87.2 ± 3.8 | 80.9–94.9 | −0.19 |
| Ht (%) | 44.4 ± 3.7 | 44.0 ± 3.7 | 39.5–48 | 0.13 |
| MCH (pg) | 28.6 ± 1.5 | 29.4 ± 1.6 | 26.1–31.6 | 0.01 |
| RDW (%) | 11.7 ± 0.8 | 11.6 ± 0.7 | 12.1–14.3 | −2.3 |
| WBC count (×109/L) | 6.9 ± 1.6 | 7.2 ± 1.9 | 4.5–10.1 | −0.17 |
| Lym (%) | 34.0 ± 6.3 | 34.5 ± 8.4 | 20–44 | 0.19 |
| Mo (%) | 6.9 ± 1.8 | 6.6 ± 1.6 | 2.0–9.5 | 0.11 |
| PTL count (×109/L) | 247 ± 49 | 241 ± 42 | 140–337 | 0.10 |
| PCT (%) | 0.19 ± 0.03 | 0.20 ± 0.03 | 0.12–0.41 | −0.20 |
| MPV (fL) | 7.8 ± 0.6 | 7.9 ± 0.7 | 5.9–9.9 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranauskas, M.; Kupčiūnaitė, I.; Lieponienė, J.; Stukas, R. Dietary Fat Intake and Indices of Blood Profiles in High-Performance Athletes: An Exploratory Study Focusing on Platelet Variables. Nutrients 2025, 17, 3418. https://doi.org/10.3390/nu17213418
Baranauskas M, Kupčiūnaitė I, Lieponienė J, Stukas R. Dietary Fat Intake and Indices of Blood Profiles in High-Performance Athletes: An Exploratory Study Focusing on Platelet Variables. Nutrients. 2025; 17(21):3418. https://doi.org/10.3390/nu17213418
Chicago/Turabian StyleBaranauskas, Marius, Ingrida Kupčiūnaitė, Jurgita Lieponienė, and Rimantas Stukas. 2025. "Dietary Fat Intake and Indices of Blood Profiles in High-Performance Athletes: An Exploratory Study Focusing on Platelet Variables" Nutrients 17, no. 21: 3418. https://doi.org/10.3390/nu17213418
APA StyleBaranauskas, M., Kupčiūnaitė, I., Lieponienė, J., & Stukas, R. (2025). Dietary Fat Intake and Indices of Blood Profiles in High-Performance Athletes: An Exploratory Study Focusing on Platelet Variables. Nutrients, 17(21), 3418. https://doi.org/10.3390/nu17213418








