The Effect of Leucine-Enriched β-Lactoglobulin Versus an Isonitrogenous Whey Protein Isolate on Skeletal Muscle Protein Anabolism in Young Healthy Males
Abstract
1. Introduction
2. Methodology
2.1. Ethical Approval
2.2. Participant Characteristics and Screening
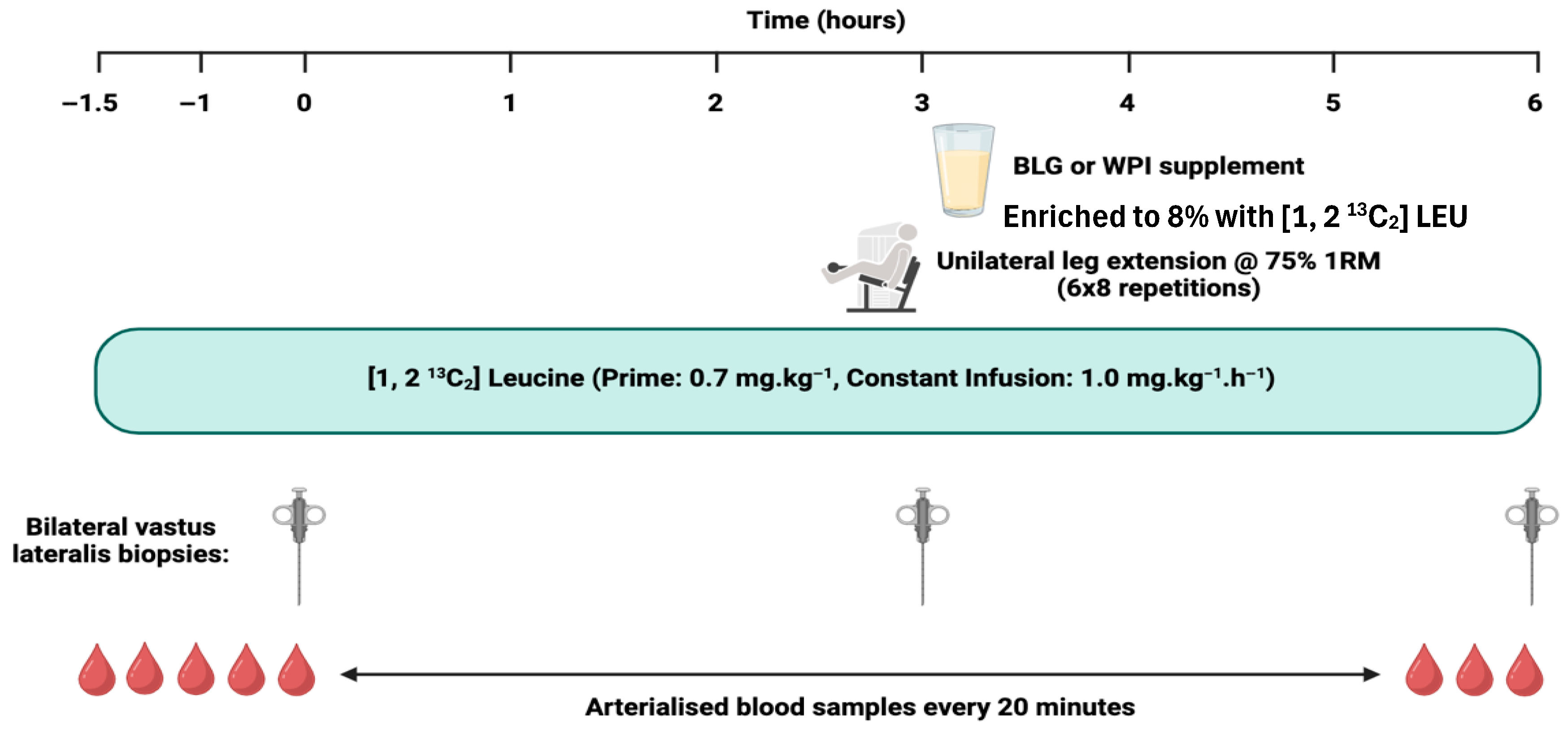
2.3. Experimental Protocol
2.4. Sample Analysis
2.4.1. Plasma Insulin
2.4.2. Plasma EAA Concentrations and α-KIC Enrichment
2.4.3. Myofibrillar Fractional Synthetic Rate
2.5. Statistical Analysis
3. Results
3.1. Plasma Leucine, BCAA, and EAA Concentrations
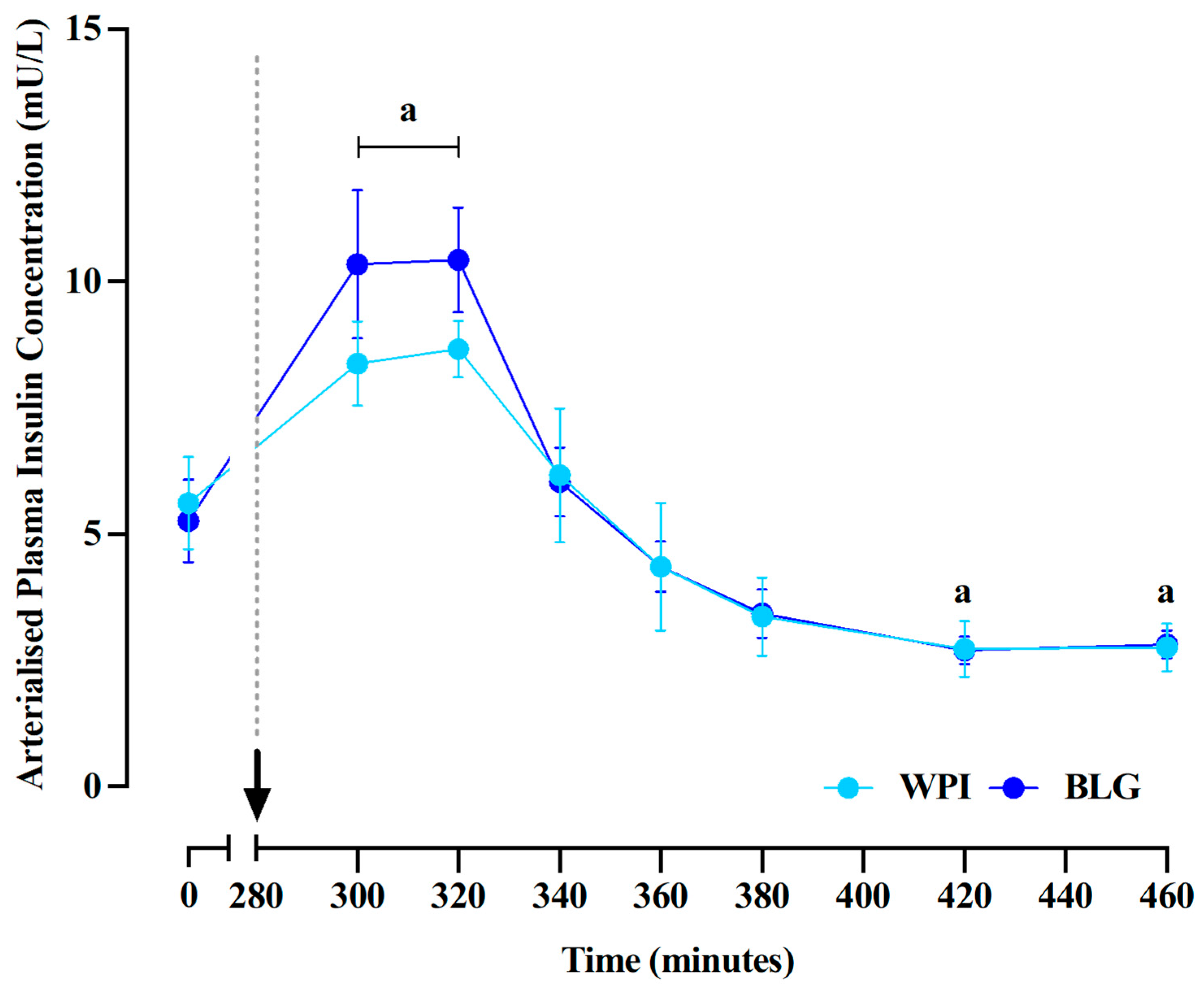
3.2. Plasma Insulin Concentrations
3.3. Muscle Protein Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wolfe, R.R. Update on protein intake: Importance of milk proteins for health status of the elderly. Nutr. Rev. 2015, 73 (Suppl. 1), 41–47. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M. The impact of protein quality on the promotion of resistance exercise-induced changes in muscle mass. Nutr. Metab. 2016, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Deglaire, A.; Bos, C.; Tomé, D.; Moughan, P.J. Ileal digestibility of dietary protein in the growing pig and adult human. Br. J. Nutr. 2009, 102, 1752–1759. [Google Scholar] [CrossRef]
- Mathai, J.K.; Liu, Y.; Stein, H.H. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br. J. Nutr. 2017, 117, 490–499. [Google Scholar] [CrossRef]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.-L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef]
- Mahé, S.; Roos, N.; Benamouzig, R.; Davin, L.; Luengo, C.; Gagnon, L.; Gaussergès, N.; Rautureau, J.; Tomé, D. Gastrojejunal kinetics and the digestion of [15N]beta-lactoglobulin and casein in humans: The influence of the nature and quantity of the protein. Am. J. Clin. Nutr. 1996, 63, 546–552. [Google Scholar] [CrossRef]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Burd, N.A.; Yang, Y.; Moore, D.R.; Tang, J.E.; Tarnopolsky, M.A.; Phillips, S.M. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br. J. Nutr. 2012, 108, 958–962. [Google Scholar] [CrossRef]
- Tipton, K.D.; Elliott, T.A.; Cree, M.G.; Wolf, S.E.; Sanford, A.P.; Wolfe, R.R. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med. Sci. Sports Exerc. 2004, 36, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Reitelseder, S.; Agergaard, J.; Doessing, S.; Helmark, I.C.; Lund, P.; Kristensen, N.B.; Frystyk, J.; Flyvbjerg, A.; Schjerling, P.; van Hall, G.; et al. Whey and casein labeled with L-[1-13C]leucine and muscle protein synthesis: Effect of resistance exercise and protein ingestion. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E231–E242. [Google Scholar] [CrossRef]
- Dideriksen, K.J.; Reitelseder, S.; Petersen, S.G.; Hjort, M.; Helmark, I.C.; Kjaer, M.; Holm, L. Stimulation of muscle protein synthesis by whey and caseinate ingestion after resistance exercise in elderly individuals. Scand. J. Med. Sci. Sports 2011, 21, e372–e383. [Google Scholar] [CrossRef]
- Tulipano, G.; Sibilia, V.; Caroli, A.M.; Cocchi, D. Whey proteins as source of dipeptidyl dipeptidase IV (dipeptidyl peptidase-4) inhibitors. Peptides 2011, 32, 835–838. [Google Scholar] [CrossRef]
- Mose, M.; Møller, N.; Jessen, N.; Mikkelsen, U.R.; Christensen, B.; Rakvaag, E.; Hartmann, B.; Holst, J.J.; Jørgensen, J.O.L.; Rittig, N. β-Lactoglobulin Is Insulinotropic Compared with Casein and Whey Protein Ingestion during Catabolic Conditions in Men in a Double-Blinded Randomized Crossover Trial. J. Nutr. 2021, 151, 1462–1472. [Google Scholar] [CrossRef]
- Farrell, H.M.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the proteins of cows’ milk—sixth revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef]
- Smith, K.; Barua, J.M.; Watt, P.W.; Scrimgeour, C.M.; Rennie, M.J. Flooding with L-[1-13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1-13C]valine. Am. J. Physiol. 1992, 262 Pt 1, E372–E376. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Yoshizawa, F.; Anthony, T.G.; Vary, T.C.; Jefferson, L.S.; Kimball, S.R. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 2000, 130, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Selby, A.; Rankin, D.; Patel, R.; Atherton, P.; Hildebrandt, W.; Williams, J.; Smith, K.; Seynnes, O.; Hiscock, N.; et al. Age-related differences in the dose–response relationship of muscle protein synthesis to resistance exercise in young and old men. J. Physiol. 2009, 587, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Ely, I.A.; Phillips, B.E.; Smith, K.; Wilkinson, D.J.; Piasecki, M.; Breen, L.; Larsen, M.S.; Atherton, P.J. A focus on leucine in the nutritional regulation of human skeletal muscle metabolism in ageing, exercise and unloading states. Clin. Nutr. 2023, 42, 1849–1865. [Google Scholar] [CrossRef]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E381–E387. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Burd, N.A.; Mitchell, C.J.; West, D.W.D.; Philp, A.; Marcotte, G.R.; Baker, S.K.; Baar, K.; Phillips, S.M. Supplementation of a suboptimal protein dose with leucine or essential amino acids: Effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J. Physiol. 2012, 590, 2751–2765. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Breen, L.; Di Donato, D.M.; Hector, A.J.; Mitchell, C.J.; Moore, D.R.; Stellingwerff, T.; Breuille, D.; Offord, E.A.; Baker, S.K.; et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: A double-blind, randomized trial. Am. J. Clin. Nutr. 2014, 99, 276–286. [Google Scholar] [CrossRef]
- Bukhari, S.S.I.; Phillips, B.E.; Wilkinson, D.J.; Limb, M.C.; Rankin, D.; Mitchell, W.K.; Kobayashi, H.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1056–E1065. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Bukhari, S.S.I.; Phillips, B.E.; Limb, M.C.; Cegielski, J.; Brook, M.S.; Rankin, D.; Mitchell, W.K.; Kobayashi, H.; Williams, J.P.; et al. Effects of leucine-enriched essential amino acid and whey protein bolus dosing upon skeletal muscle protein synthesis at rest and after exercise in older women. Clin. Nutr. 2018, 37 Pt A, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Verdijk, L.B.; Beelen, M.; Gorselink, M.; Kruseman, A.N.; Wagenmakers, A.J.M.; Kuipers, H.; van Loon, L.J.C. Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Br. J. Nutr. 2008, 99, 571–580. [Google Scholar] [CrossRef]
- DE Andrade, I.T.; Gualano, B.; Hevia-Larraín, V.; Neves-Junior, J.; Cajueiro, M.; Jardim, F.; Gomes, R.L.; Artioli, G.G.; Phillips, S.M.; Campos-Ferraz, P.; et al. Leucine Supplementation Has No Further Effect on Training-induced Muscle Adaptations. Med. Sci. Sports Exerc. 2020, 52, 1809–1814. [Google Scholar] [CrossRef]
- Verhoeven, S.; Vanschoonbeek, K.; Verdijk, L.B.; Koopman, R.; Wodzig, W.K.W.H.; Dendale, P.; van Loon, L.J.C. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am. J. Clin. Nutr. 2009, 89, 1468–1475. [Google Scholar] [CrossRef]
- Aguiar, A.F.; Grala, A.P.; da Silva, R.A.; Soares-Caldeira, L.F.; Pacagnelli, F.L.; Ribeiro, A.S.; da Silva, D.K.; de Andrade, W.B.; Balvedi, M.C.W. Free leucine supplementation during an 8-week resistance training program does not increase muscle mass and strength in untrained young adult subjects. Amino Acids 2017, 49, 1255–1262. [Google Scholar] [CrossRef]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005, 19, 1–22. [Google Scholar] [CrossRef]
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 2014, 99, 86–95. [Google Scholar] [CrossRef]
- Pennings, B.; Boirie, Y.; Senden, J.M.G.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J.C. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef]
- Xu, Z.; Tan, Z.; Zhang, Q.; Gui, Q.; Yang, Y. The effectiveness of leucine on muscle protein synthesis, lean body mass and leg lean mass accretion in older people: A systematic review and meta-analysis. Br. J. Nutr. 2015, 113, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Abumrad, N.N.; Rabin, D.; Diamond, M.P.; Lacy, W.W. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism 1981, 30, 936–940. [Google Scholar] [CrossRef]
- Atherton, P.J.; Kumar, V.; Selby, A.L.; Rankin, D.; Hildebrandt, W.; Phillips, B.E.; Williams, J.P.; Hiscock, N.; Smith, K. Enriching a protein drink with leucine augments muscle protein synthesis after resistance exercise in young and older men. Clin. Nutr. 2017, 36, 888–895. [Google Scholar] [CrossRef]
- Smedegaard, S.B.; Mose, M.; Hulman, A.; Mikkelsen, U.R.; Møller, N.; Wegener, G.; Jessen, N.; Rittig, N. β-Lactoglobulin Elevates Insulin and Glucagon Concentrations Compared with Whey Protein-A Randomized Double-Blinded Crossover Trial in Patients with Type Two Diabetes Mellitus. Nutrients 2021, 13, 308. [Google Scholar] [CrossRef]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein Ingestion to Stimulate Myofibrillar Protein Synthesis Requires Greater Relative Protein Intakes in Healthy Older Versus Younger Men. J. Gerontol. Ser. A 2015, 70, 57–62. [Google Scholar] [CrossRef]
- Moore, D.R.; Robinson, M.J.; Fry, J.L.; Tang, J.E.; Glover, E.I.; Wilkinson, S.B.; Prior, T.; Tarnopolsky, M.A.; Phillips, S.M. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009, 89, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Reynolds, N.; Downie, S.; Patel, A.; Rennie, M.J. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am. J. Physiol.-Endocrinol. Metab. 1998, 275, E73–E78. [Google Scholar] [CrossRef]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Tarnopolsky, M.A.; Phillips, S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Glynn, E.L.; Fry, C.S.; Drummond, M.J.; Timmerman, K.L.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J. Nutr. 2010, 140, 1970–1976. [Google Scholar] [CrossRef] [PubMed]


| Amino Acid | BLG (g/100 g Protein) | WPI (g/100 g Protein) |
|---|---|---|
| Alanine | 7.0 | 5.0 |
| Arginine | 2.7 | 1.8 |
| Asparagine | 11.9 | 10.7 |
| Cysteine | 3.0 | 2.5 |
| Glutamic Acid | 20.6 | 16.8 |
| Glycine | 1.4 | 1.5 |
| Histidine | 1.6 | 1.6 |
| Isoleucine | 6.4 | 6.4 |
| Leucine | 15.7 (13.6%) | 10.2 (10.4%) |
| Lysine | 12.2 | 9.2 |
| Methionine | 2.8 | 2.1 |
| Phenylalanine | 3.5 | 2.9 |
| Proline | 5.5 | 6.0 |
| Serine | 3.9 | 4.6 |
| Threonine | 5.5 | 7.1 |
| Tryptophan | 2.2 | 1.8 |
| Tyrosine | 3.6 | 2.7 |
| Valine Total EAA (%) | 6.1 115.6 48.4 | 5.6 98.5 47.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ely, I.A.; Paul, M.; Wall, J.J.S.; Cox, J.; Larsen, M.S.; Scaife, P.J.; Lund, J.N.; Breen, L.; Wilkinson, D.J.; Smith, K.; et al. The Effect of Leucine-Enriched β-Lactoglobulin Versus an Isonitrogenous Whey Protein Isolate on Skeletal Muscle Protein Anabolism in Young Healthy Males. Nutrients 2025, 17, 3410. https://doi.org/10.3390/nu17213410
Ely IA, Paul M, Wall JJS, Cox J, Larsen MS, Scaife PJ, Lund JN, Breen L, Wilkinson DJ, Smith K, et al. The Effect of Leucine-Enriched β-Lactoglobulin Versus an Isonitrogenous Whey Protein Isolate on Skeletal Muscle Protein Anabolism in Young Healthy Males. Nutrients. 2025; 17(21):3410. https://doi.org/10.3390/nu17213410
Chicago/Turabian StyleEly, Isabel A., Melanie Paul, Joshua J. S. Wall, Jake Cox, Mads S. Larsen, Paula J. Scaife, Jon N. Lund, Leigh Breen, Daniel J. Wilkinson, Kenneth Smith, and et al. 2025. "The Effect of Leucine-Enriched β-Lactoglobulin Versus an Isonitrogenous Whey Protein Isolate on Skeletal Muscle Protein Anabolism in Young Healthy Males" Nutrients 17, no. 21: 3410. https://doi.org/10.3390/nu17213410
APA StyleEly, I. A., Paul, M., Wall, J. J. S., Cox, J., Larsen, M. S., Scaife, P. J., Lund, J. N., Breen, L., Wilkinson, D. J., Smith, K., Phillips, B. E., & Atherton, P. J. (2025). The Effect of Leucine-Enriched β-Lactoglobulin Versus an Isonitrogenous Whey Protein Isolate on Skeletal Muscle Protein Anabolism in Young Healthy Males. Nutrients, 17(21), 3410. https://doi.org/10.3390/nu17213410








