Human Milk Fortification and Necrotizing Enterocolitis in Very Low Birthweight Infants: State of Evidence and Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Methods
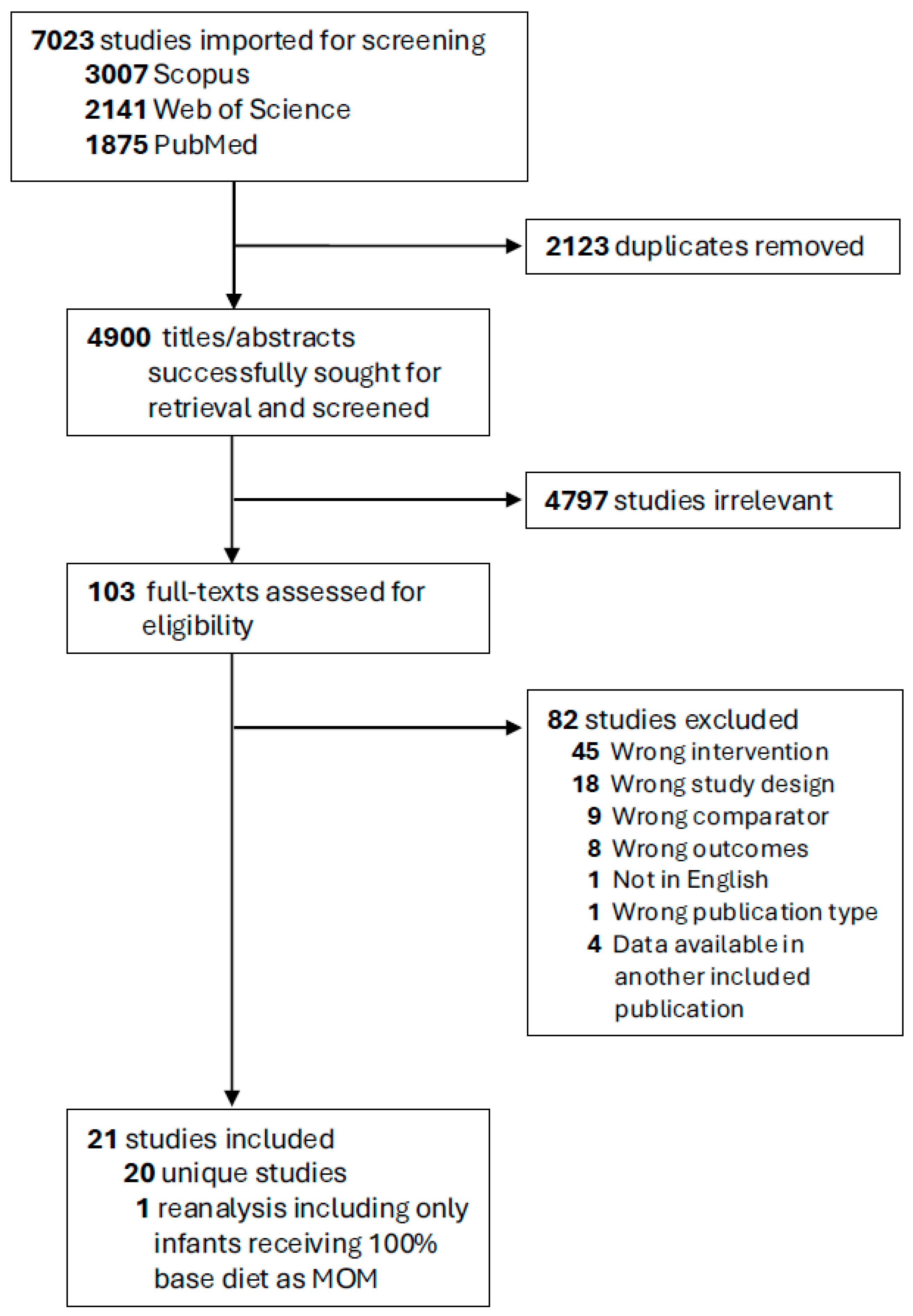
2.1. Search Strategy and Screening
2.2. Study Selection Criteria
2.3. Data Extraction
2.4. Outcome Measures and Data Harmonization
2.5. Risk of Bias Assessment
2.6. Statistical Analyses
3. Results
3.1. Description of Included Studies
| Publication and Location | Study Design | Participant Birth Year | Initial Eligibility Criteria | EHMD Fortification | Control Diet | Sample Size | ||
|---|---|---|---|---|---|---|---|---|
| EHMD | Control | Total | ||||||
| Assad 2016 [16] * US | Single center, retrospective cohort | 2009–2014 | GA ≤ 28 weeks BW ≤ 1500 g | Prolact+ H2MF®: 4 kcal/oz at 120–150 mL/kg/day * | MOM + cow milk-based fortifier @ 4 mL/kg/day at 120–150 mL/kg/day and/or preterm infant formula | 87 | 206 | 293 |
| Bushati 2021 [31] ** US | Single center, retrospective cohort | 2018–2019 | BW ≤ 1000 g | Prolact+ H2MF® @ 6 kcal/oz at 60 mL/kg/day; 8 kcal/oz at 120 mL/kg/day; CR if weight gain <15 g/kg/day on Prolact+8 * | MOM + cow milk-based fortifier @ 2 kcal/oz at 80 mL/kg/day; 4 kcal/oz at 100 mL/kg/day (liquid protein fortifier, microlipids, and Neosure (formula) if inadequate growth) | 15 | 49 | 64 |
| Carome 2021 [32] US | Single center, retrospective cohort | 2012–2017 | ELBW infants born at or transferred to NICU within 24 h of birth | Prolact+ H2MF® @ 4 kcal/oz at 80 mL/kg/day; then 6–8 kcal/oz if growth <15 g/kg/day | Preterm infant formula or MOM + cow milk-based fortifier @ 2 kcal/oz at 80 mL/kg/day; 4 kcal/oz 1 day after initiation; then 6 kcal/oz if growth <15 g/kg/day | 127 | 179 | 306 |
| Colacci 2017 [33] US | Single center, retrospective cohort | 2011–2013 | BW < 1000 g GA < 37 weeks | Not reported | MOM + cow milk-based fortifier and/or preterm formula | 39 | 46 | 85 |
| Cristofalo 2013 [14] US, Austria | Multicenter, RCT | 2007–2008 | BW 500–1250 g whose mothers did not intend to give milk | Prolact+ H2MF® @ 40 mL/kg/day or 100 mL/kg/day | Preterm infant formula | 29 | 24 | 53 |
| Eibensteiner 2019 [34] Austria | Multicenter, retrospective cohort | 2012–2018 | BW < 1000 g and initiation of human milk fortification | Prolact+ H2MF® @ 6 kcal/oz at 100 mL/kg/day or preterm infant formula | MOM + cow milk-based fortifier @ 4.4 kcal/oz at 100 mL/kg/day or preterm infant formula | 96 | 96 | 192 |
| El-Fadeel 2022 [35] US | Single center, retrospective cohort | 2013–2018 | BW < 1250 g who survived until discharge | Prolact+ H2MF® @ 80–120 mL/kg/day | MOM + cow milk-based fortifier @ 80–100 mL/kg/day or preterm infant formula w/fortification at DOL 18 as needed | 56 | 109 | 165 |
| Embleton 2023 [36] UK | Multicenter, RCT | 2017–2019 | GA ≤ 28 6/7 weeks and <72 h age | Prolact+ H2MF® @ 6 kcal at 150 mL/kg/day | MOM + preterm formula @ 150 mL/kg/day | 63 | 63 | 126 |
| Hair 2016 [46] US | Multicenter, retrospective cohort | 2006–2014 | BW < 1250 g | Prolact+ H2MF® @ 60 mL/kg/day or 100–120 mL/kg/day | MOM+ cow milk-based fortifier and/or preterm infant formula | 819 | 768 | 1587 |
| Hanford 2021 [38] US | Single center, retrospective cohort | 2016–2018 | BW < 1100 g GA < 30 weeks | Prolact+ H2MF® @ 4–8 kcal/oz at 80 mL/kg/day depending on growth | MOM + cow milk-based fortifier @ 80 mL/kg/day or preterm infant formula depending on growth | 53 | 36 | 89 |
| Harris 2024 [39] US | Single center, retrospective cohort | Not reported | BW < 1250 g GA < 32 weeks | 4 kcal/oz @ 40 mL/kg/day | Not reported | 105 | 96 | 201 |
| Herrmann 2014 [40] US | Single center, retrospective cohort | 2004–2012 | GA < 33 weeks | Prolact+ H2MF® start/advancement not reported | MOM + cow milk-based fortifier start/advancement not reported | 199 | 443 | 642 |
| Huston 2018 [41] US | Single center, retrospective cohort | 2007–2015 | BW 500–1250 g | Prolact+ H2MF® @ 4 kcal/oz at 40–50 mL/kg/day or 80–100 mL/kg/day; 6 kcal/oz at variable volumes or 130 mL/kg/day, then 8 kcal/oz if fluid restricted to <145 mL/kg/day of enteral feedings or poor growth | MOM+ cow milk-based fortifier @ 4 kcal/oz at 80–100 mL/kg/day; 6 kcal/oz at variable volumes (or 130 mL/kg/day), then 7 kcal/oz Enfacare (formula) if fluid restricted to <145 mL/kg/day of enteral feedings or poor growth | 127 | 252 | 379 |
| Jensen 2024 [19] Sweden | Multicenter, RCT | 2019–2021 | GA 22 + 0–27 + 6 weeks and ability to maintain intervention until PMA 34 weeks | Targeted fortification w/Prolacta Humavant+6 before 100 mL/kg/day (protein goal 4.0–4.5 g/kg/day, then gradually decreased as infant approaches term equivalent) | Targeted fortification w/cow milk-based fortifier before <100 mL/kg/day (protein goal 4.0–4.5 g/kg/day, then gradually decreased as infant approaches term equivalent) | 115 | 113 | 228 |
| O’Connor 2018 [18] Canada | Multicenter, RCT | 2014–2016 | BW < 1250 g and parent consent to donor human milk; enteral feeding started within 14 d of birth | Prolact+ H2MF® 4 kcal/oz at 100 mL/kg/day; 6 kcal at 140 mL/kg/day, then 8 kcal if weight gain <15 g/kg/day after achieving full feeds (160 mL/kg/day) | MOM + cow milk-based fortifier @ 2 kcal/oz at 100 mL/kg/day; 4 kcal/oz at 140 mL/kg/day, then 6 kcal/oz if weight gain <15 g/kg/day after achieving full feeds | 64 | 63 | 127 |
| Sato 2020 [42] ** US | Single center, retrospective cohort | 2012–2018 | BW 1000–1499 g | Prolact+ H2MF® @ 4 kcal/oz at 90 mL/kg/day | MOM+ cow milk-based fortifier @ 4 kcal/oz at 90 mL/kg/day | 265 | 134 | 399 |
| Sullivan 2010 [15] *** US, Austria | Multicenter, RCT | 2007–2008 | BW 500–1250 g | Prolact+ H2MF® @ 40 mL/kg/day or 100 mL/kg/day * | MOM + cow milk-based fortifier @ 100 mL/kg/day or preterm infant formula | 138 | 69 | 207 |
| Swanson 2023 [17] US | Multicenter, retrospective cohort | Not reported | BW < 1000–≤ 1500 g GA < 28–< 32 weeks | Prolact+ H2MF® @ 60 mL/kg/day or 80 mL/kg/day with fortification goal of 26–28 kcal/oz or 26–32 kcal/oz | Cow milk-based fortifier @ 60 mL/kg/day or 80 mL/kg/day with fortification goal of 26–28 kcal/oz or 26–32 kcal/oz | 511 | 526 | 1037 |
| Tetarbe 2024 [43] US | Single center, retrospective cohort | 2015–2016, 2020–2021 | VLBW infants fed MOM or DHM fortified with CMDF or HMDF and ability to maintain intervention until PMA 34 weeks | Prolact+ H2MF® @ 80 mL/kg/day and human milk caloric fortifier (2 kcal/oz) when infants tolerated 120 mL/kg/day of enteral feeds and were off PN | MOM/DHM + cow milk-based fortifier @ 80 mL/kg/day | 57 | 64 | 121 |
| Wickland 2022 [44] US | Single center, retrospective cohort | 2013–2016 | BW ≤ 1250 g GA ≤ 33 weeks | Prolact+ H2MF® @ 4 kcal/oz @ 80 mL/kg/day or Prolact+ H2MF® @ 6 kcal/oz at 80 mL/kg/day fortified to 24 kcal/oz | MOM/DHM + cow milk-based fortifier @ 80 mL/kg/day fortified to 24 kcal/oz | 275 | 218 | 493 |
3.2. Associations of an EHMD with Infant Medical NEC
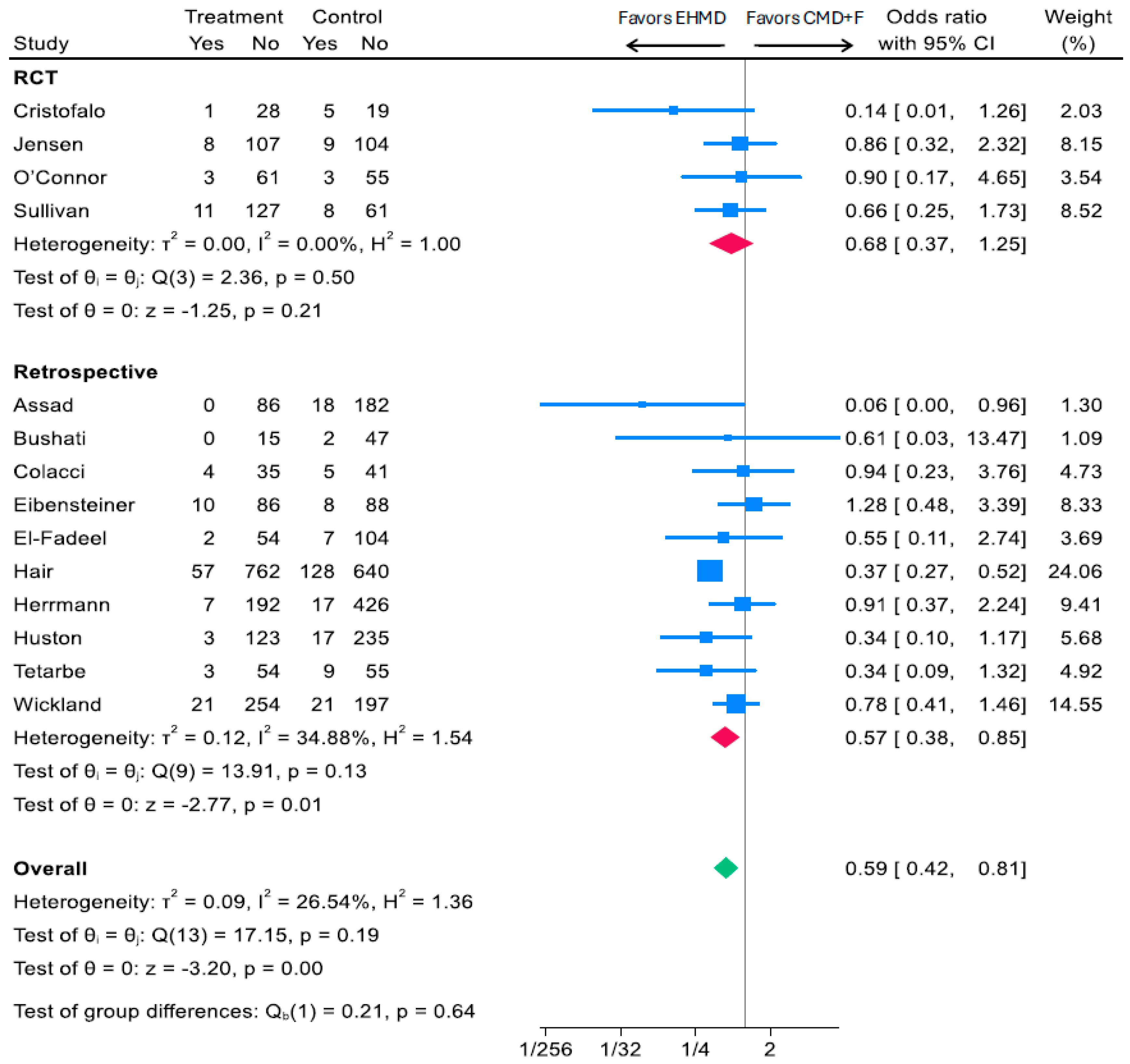
3.2.1. Associations of an EHMD vs. Any Cow Milk-Containing Diet (CMD+F) and Medical NEC
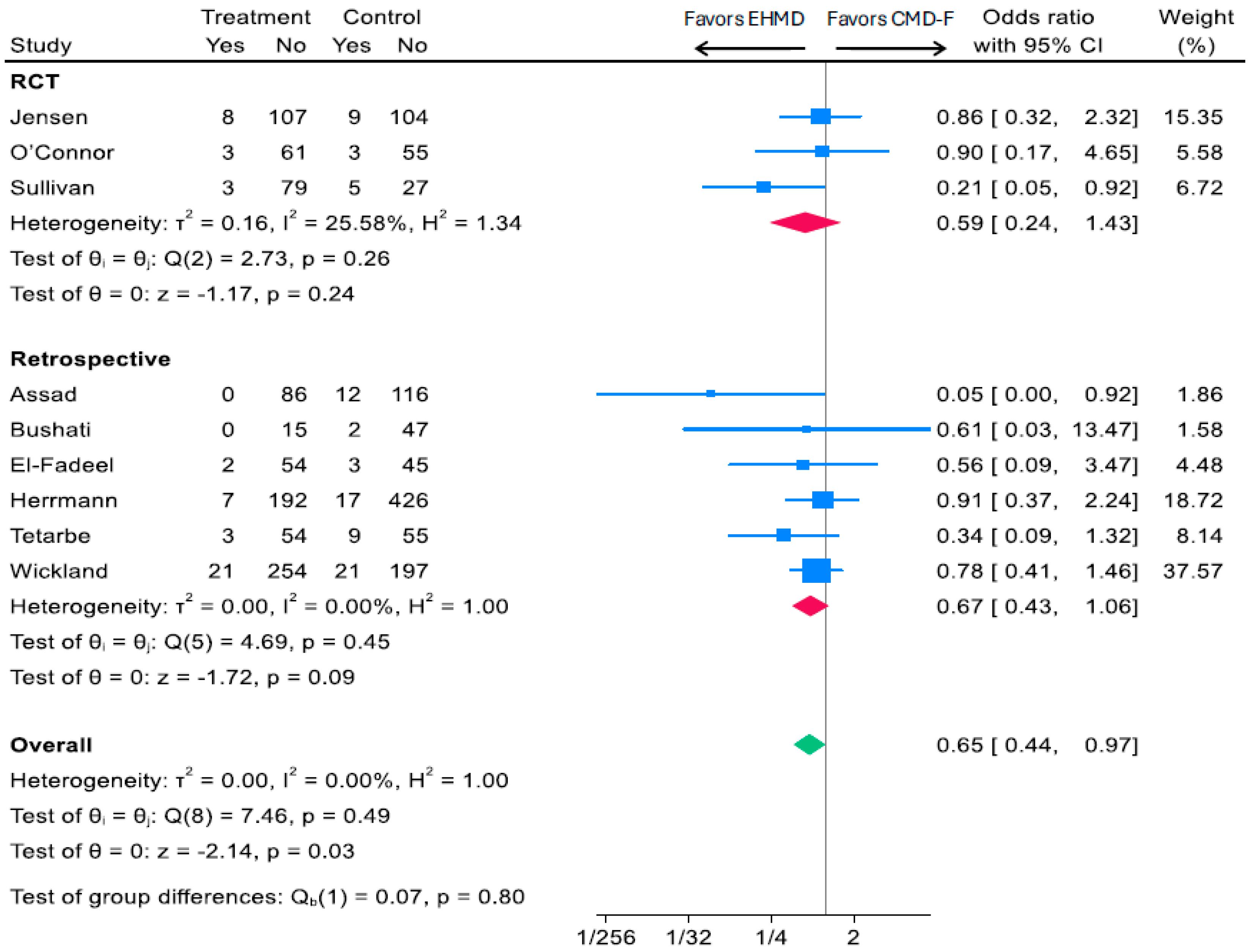
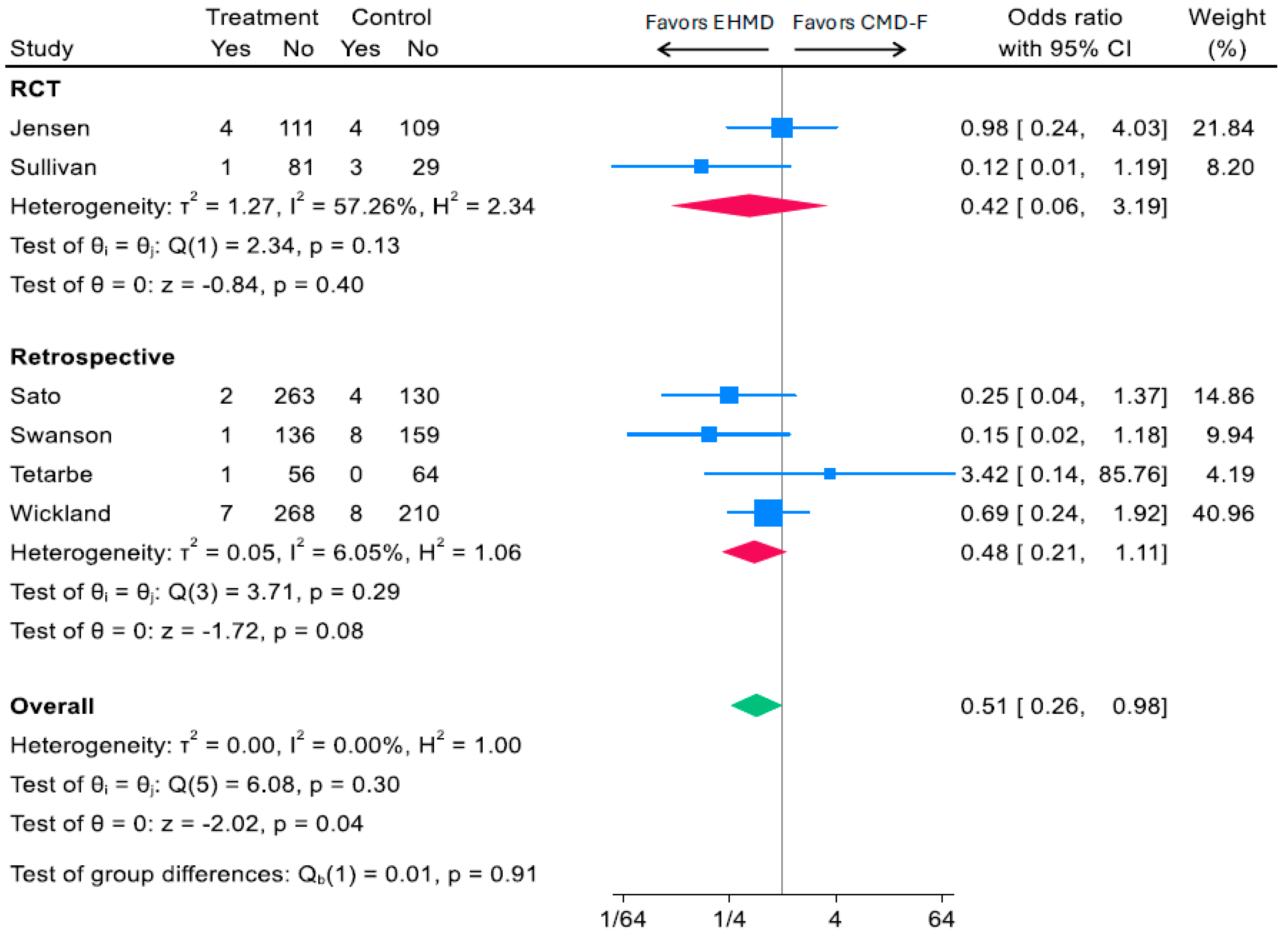
3.2.2. Head-to-Head Comparison of Human vs. Cow Milk-Based Fortifiers and Medical NEC
3.3. Associations of an EHMD with Infant Surgical NEC
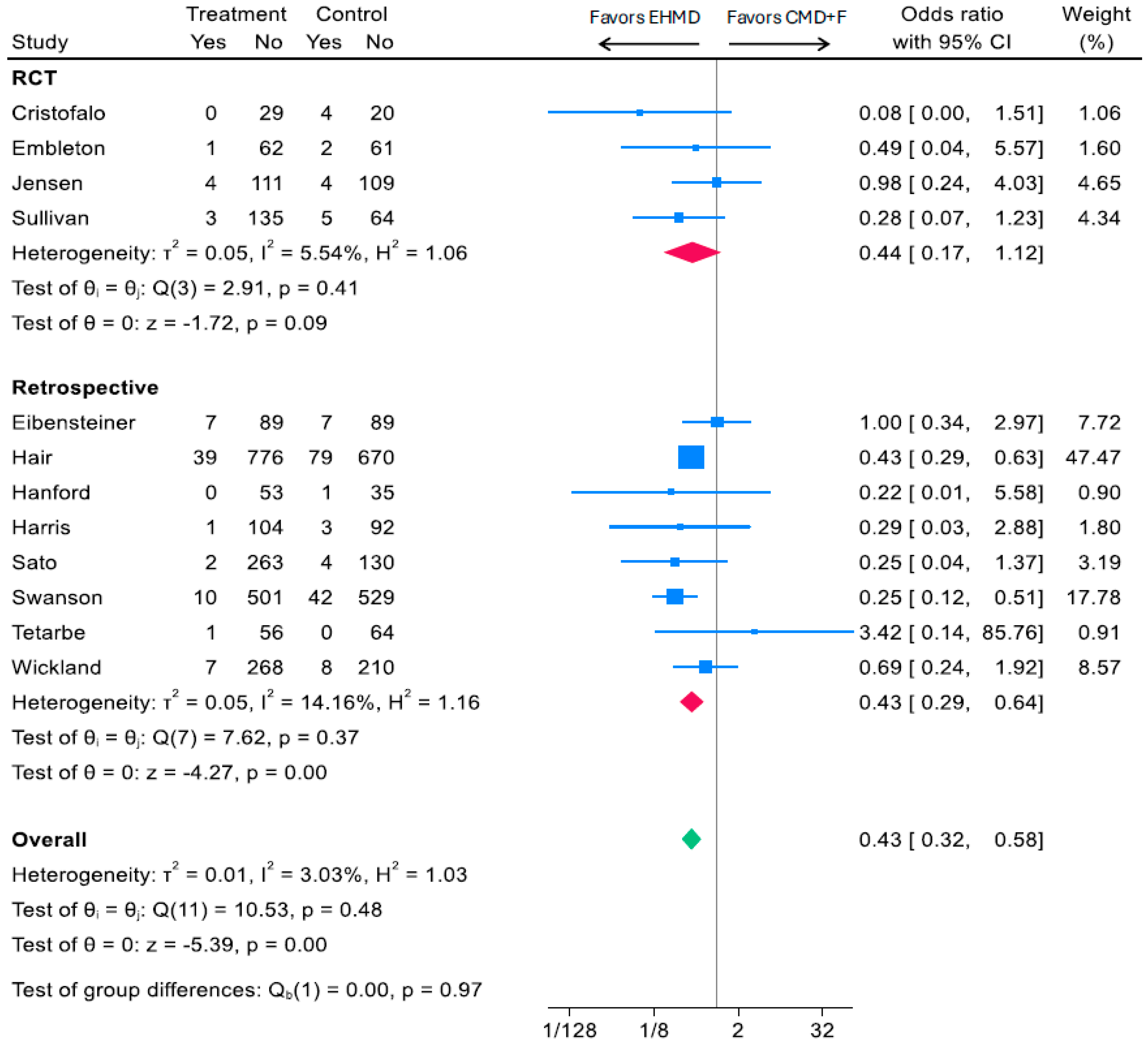
3.3.1. EHMD vs. Any Cow Milk Diet (CMD+F) and Surgical NEC
3.3.2. Head-to-Head Comparison of Human vs. Cow Milk-Based Fortifiers and Surgical NEC
3.4. Quality Assessment and Publication Bias
4. Discussion
4.1. Key Findings
4.2. RCTs, Real-World Data, and Variability in Clinical Practice
4.3. Cost-Effectiveness Considerations
4.4. Ethical Challenges and Evolving Clinical Practice
4.5. Future Directions
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.R.; Rellinger, E.J.; Hatch, L.D.; Weitkamp, J.H.; Speck, K.E.; Danko, M.; Blakely, M.L. Surgical necrotizing enterocolitis. Semin. Perinatol. 2017, 41, 70–79. [Google Scholar] [CrossRef]
- Han, S.M.; Hong, C.R.; Knell, J.; Edwards, E.M.; Morrow, K.A.; Soll, R.F.; Modi, B.P.; Horbar, J.D.; Jaksic, T. Trends in incidence and outcomes of necrotizing enterocolitis over the last 12 years: A multicenter cohort analysis. J. Pediatr. Surg. 2020, 55, 998–1001. [Google Scholar] [CrossRef]
- Fitzgibbons, S.C.; Ching, Y.; Yu, D.; Carpenter, J.; Kenny, M.; Weldon, C.; Lillehei, C.; Valim, C.; Horbar, J.D.; Jaksic, T. Mortality of necrotizing enterocolitis expressed by birth weight categories. J. Pediatr. Surg. 2009, 44, 1072–1075. [Google Scholar] [CrossRef]
- Hull, M.A.; Fisher, J.G.; Gutierrez, I.M.; Jones, B.A.; Kang, K.H.; Kenny, M.; Zurakowski, D.; Modi, B.P.; Horbar, J.D.; Jaksic, T. Mortality and management of surgical necrotizing enterocolitis in very low birth weight neonates: A prospective cohort study. J. Am. Coll. Surg. 2014, 218, 1148–1155. [Google Scholar] [CrossRef]
- Singh, D.K.; Miller, C.M.; Orgel, K.A.; Dave, M.; Mackay, S.; Good, M. Necrotizing enterocolitis: Bench to bedside approaches and advancing our understanding of disease pathogenesis. Front. Pediatr. 2022, 10, 1107404. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef]
- Walsh, M.C.; Kliegman, R.M. Necrotizing enterocolitis: Treatment based on staging criteria. Pediatr. Clin. N. Am. 1986, 33, 179–201. [Google Scholar] [CrossRef]
- Parker, M.G.; Stellwagen, L.M.; Noble, L.; Kim, J.H.; Poindexter, B.B.; Puopolo, K.M.; Section on Breastfeeding; Committee on Nutrition; Committee on Fetus and Newborn. Promoting Human Milk and Breastfeeding for the Very Low Birth Weight Infant. Pediatrics 2021, 148, e2021054272. [Google Scholar] [CrossRef] [PubMed]
- Autran, C.A.; Kellman, B.P.; Kim, J.H.; Asztalos, E.; Blood, A.B.; Spence, E.C.H.; Patel, A.L.; Hou, J.; Lewis, N.E.; Bode, L. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut 2018, 67, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human Milk Oligosaccharides in the Prevention of Necrotizing Enterocolitis: A Journey From in vitro and in vivo Models to Mother-Infant Cohort Studies. Front. Pediatr. 2018, 6, 385. [Google Scholar] [CrossRef]
- Kleinman, R.E.; Greer, F.R. Pediatric Nutrition, 8th ed.; American Academy of Pediatrics: Itasca, IL, USA, 2019. [Google Scholar]
- Meek, J.Y.; Noble, L.; Section on Breastfeeding. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics 2022, 150, e2022057988. [Google Scholar] [CrossRef]
- Cristofalo, E.A.; Schanler, R.J.; Blanco, C.L.; Sullivan, S.; Trawoeger, R.; Kiechl-Kohlendorfer, U.; Dudell, G.; Rechtman, D.J.; Lee, M.L.; Lucas, A.; et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J. Pediatr. 2013, 163, 1592–1595.e1591. [Google Scholar] [CrossRef]
- Sullivan, S.; Schanler, R.J.; Kim, J.H.; Patel, A.L.; Trawöger, R.; Kiechl-Kohlendorfer, U.; Chan, G.M.; Blanco, C.L.; Abrams, S.; Cotten, C.M.; et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J. Pediatr. 2010, 156, 562–567.e561. [Google Scholar] [CrossRef]
- Assad, M.; Elliott, M.J.; Abraham, J.H. Decreased cost and improved feeding tolerance in VLBW infants fed an exclusive human milk diet. J. Perinatol. 2016, 36, 216–220. [Google Scholar] [CrossRef]
- Swanson, J.R.; Becker, A.; Fox, J.; Horgan, M.; Moores, R.; Pardalos, J.; Pinheiro, J.; Stewart, D.; Robinson, T. Implementing an exclusive human milk diet for preterm infants: Real-world experience in diverse NICUs. BMC Pediatr. 2023, 23, 237. [Google Scholar] [CrossRef]
- O’Connor, D.L.; Kiss, A.; Tomlinson, C.; Bando, N.; Bayliss, A.; Campbell, D.M.; Daneman, A.; Francis, J.; Kotsopoulos, K.; Shah, P.S.; et al. Nutrient enrichment of human milk with human and bovine milk-based fortifiers for infants born weighing <1250 g: A randomized clinical trial. Am. J. Clin. Nutr. 2018, 108, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.B.; Domellof, M.; Ahlsson, F.; Elfvin, A.; Naver, L.; Abrahamsson, T. Effect of human milk-based fortification in extremely preterm infants fed exclusively with breast milk: A randomised controlled trial. EClinicalMedicine 2024, 68, 102375. [Google Scholar] [CrossRef]
- Brownell, E.A.; Matson, A.P.; Smith, K.C.; Moore, J.E.; Esposito, P.A.; Lussier, M.M.; Lerer, T.J.; Hagadorn, J.I. Dose-response Relationship Between Donor Human Milk, Mother’s Own Milk, Preterm Formula, and Neonatal Growth Outcomes. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xiu, W.; Dai, Y.; Yang, C. Protective effects of different doses of human milk on neonatal necrotizing enterocolitis. Medicine 2020, 99, e22166. [Google Scholar] [CrossRef] [PubMed]
- Galis, R.; Trif, P.; Mudura, D.; Mazela, J.; Daly, M.C.; Kramer, B.W.; Diggikar, S. Association of Fortification with Human Milk versus Bovine Milk-Based Fortifiers on Short-Term Outcomes in Preterm Infants-A Meta-Analysis. Nutrients 2024, 16, 910. [Google Scholar] [CrossRef]
- Premkumar, M.H.; Pammi, M.; Suresh, G. Human milk-derived fortifier versus bovine milk-derived fortifier for prevention of mortality and morbidity in preterm neonates. Cochrane Database Syst. Rev. 2019, 11, CD013145. [Google Scholar] [CrossRef] [PubMed]
- Thokagevistk, K.; Coppo, C.; Rey, L.; Carelli, A.; Diez, V.; Vaselenak, S.; Oliveira, L.; Patel, A.; Sicari, E.; Ramos, T.; et al. Real-World Evidence to Reinforce Clinical Trial Evidence in Health Technology Assessment: A Critical Review of Real-World Evidence Requirements from Seven Countries and Recommendations to Improve Acceptance. J. Mark. Access Health Policy 2024, 12, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Nutrition Evidence Systematic Review Team (U.S. Department of Agriculture). Risk of Bias for Nutrition Observational Studies (RoB-NObs) Tool; U.S. Department of Agriculture: Washington, DC, USA, 2019. [Google Scholar]
- 2025 Dietary Guidelines Advisory Committee (U.S. Department of Health and Human Services). Scientific Report of the 2025 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and Secretary of Agriculture; U.S. Department of Health and Human Services: Washington, DC, USA, 2024. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sidik, K.; Jonkman, J.N. Simple Heterogeneity Variance Estimation for Meta-Analysis. J. R. Stat. Soc. Ser. C Appl. Stat. 2005, 54, 367–384. [Google Scholar] [CrossRef]
- Bushati, C.; Chan, B.; Harmeson Owen, A.; Woodbury, A.; Yang, M.; Fung, C.; Lechtenberg, E.; Rigby, M.; Baserga, M. Challenges in Implementing Exclusive Human Milk Diet to Extremely Low-Birth-Weight Infants in a Level III Neonatal Intensive Care Unit. Nutr. Clin. Pract. 2021, 36, 1198–1206. [Google Scholar] [CrossRef]
- Carome, K.; Rahman, A.; Parvez, B. Exclusive human milk diet reduces incidence of severe intraventricular hemorrhage in extremely low birth weight infants. J. Perinatol. 2021, 41, 535–543. [Google Scholar] [CrossRef]
- Colacci, M.; Murthy, K.; DeRegnier, R.O.; Khan, J.Y.; Robinson, D.T. Growth and Development in Extremely Low Birth Weight Infants After the Introduction of Exclusive Human Milk Feedings. Am. J. Perinatol. 2017, 34, 130–137. [Google Scholar] [CrossRef]
- Eibensteiner, F.; Auer-Hackenberg, L.; Jilma, B.; Thanhaeuser, M.; Wald, M.; Haiden, N. Growth, Feeding Tolerance and Metabolism in Extreme Preterm Infants under an Exclusive Human Milk Diet. Nutrients 2019, 11, 1443. [Google Scholar] [CrossRef] [PubMed]
- El-Fadeel, H.; Velumula, P.; Lulic-Botica, M.; Natarajan, G.; Thomas, R.; Botica, G.; Bajaj, M. Effect of an exclusive human milk diet on feeding tolerance in preterm infants. J. Perinatol. 2022, 42, 1070–1075. [Google Scholar] [CrossRef]
- Embleton, N.D.; Sproat, T.; Uthaya, S.; Young, G.R.; Garg, S.; Vasu, V.; Masi, A.C.; Beck, L.; Modi, N.; Stewart, C.J.; et al. Effect of an Exclusive Human Milk Diet on the Gut Microbiome in Preterm Infants: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e231165. [Google Scholar] [CrossRef]
- Hair, A.B.; Peluso, A.M.; Hawthorne, K.M.; Perez, J.; Smith, D.P.; Khan, J.Y.; O’Donnell, A.; Powers, R.J.; Lee, M.L.; Abrams, S.A. Beyond Necrotizing Enterocolitis Prevention: Improving Outcomes with an Exclusive Human Milk-Based Diet. Breastfeed. Med. 2016, 11, 70–74. [Google Scholar] [CrossRef]
- Hanford, J.; Mannebach, K.; Ohler, A.; Patten, M.; Pardalos, J. Rates of Comorbidities in Very Low Birth Weight Infants Fed an Exclusive Human Milk Diet Versus a Bovine Supplemented Diet. Breastfeed. Med. 2021, 16, 814–820. [Google Scholar] [CrossRef]
- Harris, L.; Lewis, S.; Vardaman, S. Exclusive Human Milk Diets and the Reduction of Necrotizing Enterocolitis. Adv. Neonatal Care 2024, 24, 400–407. [Google Scholar] [CrossRef]
- Herrmann, K.; Carroll, K. An exclusively human milk diet reduces necrotizing enterocolitis. Breastfeed. Med. 2014, 9, 184–190. [Google Scholar] [CrossRef]
- Huston, R.K.; Markell, A.M.; McCulley, E.A.; Gardiner, S.K.; Sweeney, S.L. Improving Growth for Infants </=1250 Grams Receiving an Exclusive Human Milk Diet. Nutr. Clin. Pract. 2018, 33, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Malai, S.; Razmjouy, B. Necrotizing Enterocolitis Reduction Using an Exclusive Human-Milk Diet and Probiotic Supplementation in Infants With 1000-1499 Gram Birth Weight. Nutr Clin Pract 2020, 35, 331–334. [Google Scholar] [CrossRef]
- Tetarbe, M.; Chang, M.R.; Barton, L.; Cayabyab, R.; Ramanathan, R. Economic and Clinical Impact of Using Human Milk-Derived Fortifier in Very Low Birth Weight Infants. Breastfeed. Med. 2024, 19, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Wickland, J.; Wade, C.; Micetic, B.; Meredith, K.; Martin, G. A Retrospective Analysis of the Effects of an Exclusively Human Milk Protein Diet on Neonatal Feeding Tolerance. Am. J. Perinatol. 2022, 39, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Boscardin, J.; Abrams, S.A. Preterm Infants Fed Cow’s Milk-Derived Fortifier Had Adverse Outcomes Despite a Base Diet of Only Mother’s Own Milk. Breastfeed. Med. 2020, 15, 297–303. [Google Scholar] [CrossRef]
- Hair, A.B.; Bergner, E.M.; Lee, M.L.; Moreira, A.G.; Hawthorne, K.M.; Rechtman, D.J.; Abrams, S.A.; Blanco, C.L. Premature Infants 750-1,250 g Birth Weight Supplemented with a Novel Human Milk-Derived Cream Are Discharged Sooner. Breastfeed. Med. 2016, 11, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Huston, R.K.; Markell, A.M.; McCulley, E.A.; Pathak, M.; Rogers, S.P.; Sweeney, S.L.; Dolphin, N.G.; Gardiner, S.K. Decreasing Necrotizing Enterocolitis and Gastrointestinal Bleeding in the Neonatal Intensive Care Unit. ICAN Infant Child Adolesc. Nutr. 2014, 6, 86–93. [Google Scholar] [CrossRef]
- Mitani, A.A.; Haneuse, S. Small Data Challenges of Studying Rare Diseases. JAMA Netw. Open 2020, 3, e201965. [Google Scholar] [CrossRef]
- Baker, M. Statisticians issue warning over misuse of P values. Nature 2016, 531, 151. [Google Scholar] [CrossRef]
- Parker, M.G.; Greenberg, L.T.; Edwards, E.M.; Ehret, D.; Belfort, M.B.; Horbar, J.D. National Trends in the Provision of Human Milk at Hospital Discharge Among Very Low-Birth-Weight Infants. JAMA Pediatr. 2019, 173, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; Angeletti, P.M.; Verrotti, A.; Petrocelli, R. The Impact of Human Milk on Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1322. [Google Scholar] [CrossRef] [PubMed]
- Schanler, R.J.; Shulman, R.J.; Lau, C. Feeding strategies for premature infants: Beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 1999, 103, 1150–1157. [Google Scholar] [CrossRef]
- Ananthan, A.; Balasubramanian, H.; Rao, S.; Patole, S. Human Milk-Derived Fortifiers Compared with Bovine Milk-Derived Fortifiers in Preterm Infants: A Systematic Review and Meta-Analysis. Adv. Nutr. 2020, 11, 1325–1333. [Google Scholar] [CrossRef]
- Grace, E.; Hilditch, C.; Gomersall, J.; Collins, C.T.; Rumbold, A.; Keir, A.K. Safety and efficacy of human milk-based fortifier in enterally fed preterm and/or low birthweight infants: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V.; Hay, J.W.; Kim, J.H. Costs of necrotizing enterocolitis and cost-effectiveness of exclusively human milk-based products in feeding extremely premature infants. Breastfeed. Med. 2012, 7, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hampson, G.; Roberts, S.L.E.; Lucas, A.; Parkin, D. An economic analysis of human milk supplementation for very low birth weight babies in the USA. BMC Pediatr. 2019, 19, 337. [Google Scholar] [CrossRef]
- Scholz, S.M.; Greiner, W. An exclusive human milk diet for very low birth weight newborns-A cost-effectiveness and EVPI study for Germany. PLoS ONE 2019, 14, e0226496. [Google Scholar] [CrossRef]
- Igarashi, A.; Reyes, S.M.; Mizuno, K. Cost-utility analysis of an exclusive human milk diet for very low birthweight infants in Japan. J. Med. Econ. 2025, 1–27. [Google Scholar] [CrossRef]
- Battersby, C.; Santhalingam, T.; Costeloe, K.; Modi, N. Incidence of neonatal necrotising enterocolitis in high-income countries: A systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F182–F189. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.H. Equipoise in Clinical Trials: Enough Uncertainty in Whose Opinion? Circulation 2022, 145, 943–945. [Google Scholar] [CrossRef]
- Henderson, G.; Craig, S.; Brocklehurst, P.; McGuire, W. Enteral feeding regimens and necrotising enterocolitis in preterm infants: A multicentre case-control study. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F120–F123. [Google Scholar] [CrossRef]
- McKeown, R.E.; Marsh, T.D.; Amarnath, U.; Garrison, C.Z.; Addy, C.L.; Thompson, S.J.; Austin, J.L. Role of delayed feeding and of feeding increments in necrotizing enterocolitis. J. Pediatr. 1992, 121, 764–770. [Google Scholar] [CrossRef]
- Berseth, C.L.; Bisquera, J.A.; Paje, V.U. Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2003, 111, 529–534. [Google Scholar] [CrossRef]
- Fenin, A.; Newman, J.C.; Taylor, S.N. Very low birth weight infants receive full enteral nutrition within 2 postnatal weeks. J. Perinatol. 2020, 40, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.A.; Gunawan, E.; Nguyen, K.; Reeves, A.; Argent, V.; Finck, A.; Carlo, W.A. Early Human Milk Fortification in Infants Born Extremely Preterm: A Randomized Trial. Pediatrics 2023, 152, e2023061603. [Google Scholar] [CrossRef]
- Huston, R.; Lee, M.; Rider, E.; Stawarz, M.; Hedstrom, D.; Pence, M.; Chan, V.; Chambers, J.; Rogers, S.; Sager, N.; et al. Early fortification of enteral feedings for infants <1250 grams birth weight receiving a human milk diet including human milk based fortifier. J. Neonatal Perinatal Med. 2020, 13, 215–221. [Google Scholar] [CrossRef]
- Mathes, T.; Buehn, S.; Prengel, P.; Pieper, D. Registry-based randomized controlled trials merged the strength of randomized controlled trails and observational studies and give rise to more pragmatic trials. J. Clin. Epidemiol. 2018, 93, 120–127. [Google Scholar] [CrossRef]
- Zuidgeest, M.G.P.; Goetz, I.; Groenwold, R.H.H.; Irving, E.; van Thiel, G.J.M.W.; Grobbee, D.E.; GetReal Work Package 3. Series: Pragmatic trials and real world evidence: Paper 1. Introduction. J. Clin. Epidemiol. 2017, 88, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.M.; Margolis, P.A.; Miller, M. Collaborative networks for both improvement and research. Pediatrics 2013, 131, S210–S214. [Google Scholar] [CrossRef]
- Rouse, B.; Chaimani, A.; Li, T. Network meta-analysis: An introduction for clinicians. Intern. Emerg. Med. 2017, 12, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Hair, A.B.; Blanco, C.L.; Moreira, A.G.; Hawthorne, K.M.; Lee, M.L.; Rechtman, D.J.; Abrams, S.A. Randomized trial of human milk cream as a supplement to standard fortification of an exclusive human milk-based diet in infants 750-1250 g birth weight. J. Pediatr. 2014, 165, 915–920. [Google Scholar] [CrossRef]
- Knake, L.A.; King, B.C.; Gollins, L.A.; Hurst, N.M.; Hagan, J.; Ford, S.L.; Hair, A.B. Optimizing the Use of Human Milk Cream Supplement in Very Preterm Infants: Growth and Cost Outcomes. Nutr. Clin. Pract. 2020, 35, 689–696. [Google Scholar] [CrossRef]
- Fanaro, S. Feeding intolerance in the preterm infant. Early Hum. Dev. 2013, 89 (Suppl. 2), S13–S20. [Google Scholar] [CrossRef]
- Reyes, S.M.; Moore, J.B.; Lee, M.L.; Ferry, J.; Elliott, M.J. Associations of an Exclusive Human Milk Diet with Morbidity and Mortality in ELBW Infants Born Weighing <750 Grams: An Individual Participant Data Meta-analysis. Neonatol. Today 2023, 18, 3–23. [Google Scholar]
| Variable | Definition | Base Diet | Fortifier |
|---|---|---|---|
| EHMD | Exclusive human milk diet | Human milk | Vat-pasteurized human milk-derived fortifier |
| CMD+F | Cow milk-containing diet that may include formula | Varied by study, including formula or human milk as base diet | Cow milk-derived fortifier or infant formula |
| CMD-F | Cow milk-containing diet excluding formula | Human milk | Cow milk-derived fortifier |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes, S.M.; Paul, T.L.; Ferry, J. Human Milk Fortification and Necrotizing Enterocolitis in Very Low Birthweight Infants: State of Evidence and Systematic Review with Meta-Analysis. Nutrients 2025, 17, 3384. https://doi.org/10.3390/nu17213384
Reyes SM, Paul TL, Ferry J. Human Milk Fortification and Necrotizing Enterocolitis in Very Low Birthweight Infants: State of Evidence and Systematic Review with Meta-Analysis. Nutrients. 2025; 17(21):3384. https://doi.org/10.3390/nu17213384
Chicago/Turabian StyleReyes, Sarah M., Tristen L. Paul, and Jenelle Ferry. 2025. "Human Milk Fortification and Necrotizing Enterocolitis in Very Low Birthweight Infants: State of Evidence and Systematic Review with Meta-Analysis" Nutrients 17, no. 21: 3384. https://doi.org/10.3390/nu17213384
APA StyleReyes, S. M., Paul, T. L., & Ferry, J. (2025). Human Milk Fortification and Necrotizing Enterocolitis in Very Low Birthweight Infants: State of Evidence and Systematic Review with Meta-Analysis. Nutrients, 17(21), 3384. https://doi.org/10.3390/nu17213384






