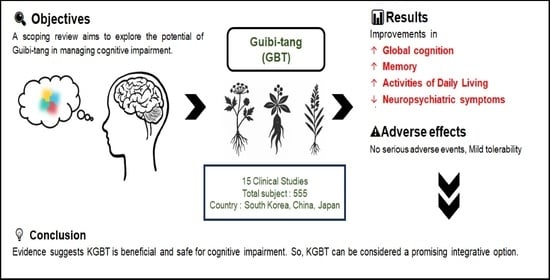
Current Utilization and Research Status of the Herbal Medicine Guibi-Tang and Its Variants for Cognitive Impairment: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Formulation of the research questions;
- (2)
- Identification of relevant literature;
- (3)
- Selection of eligible studies;
- (4)
- Data charting and organization;
- (5)
- Synthesis and reporting of findings.
2.1. Identifying the Research Questions
- (1)
- What types of studies have been conducted?
- (2)
- What types of cognitive impairment or dementia have been treated with GBT?
- (3)
- What improvements have been observed following the use of GBT?
- (4)
- What is the level of evidence supporting the efficacy of GBT or its variants in this context?
2.2. Literature Search
2.3. Literature Selection
2.4. Data Extraction and Organization
3. Results
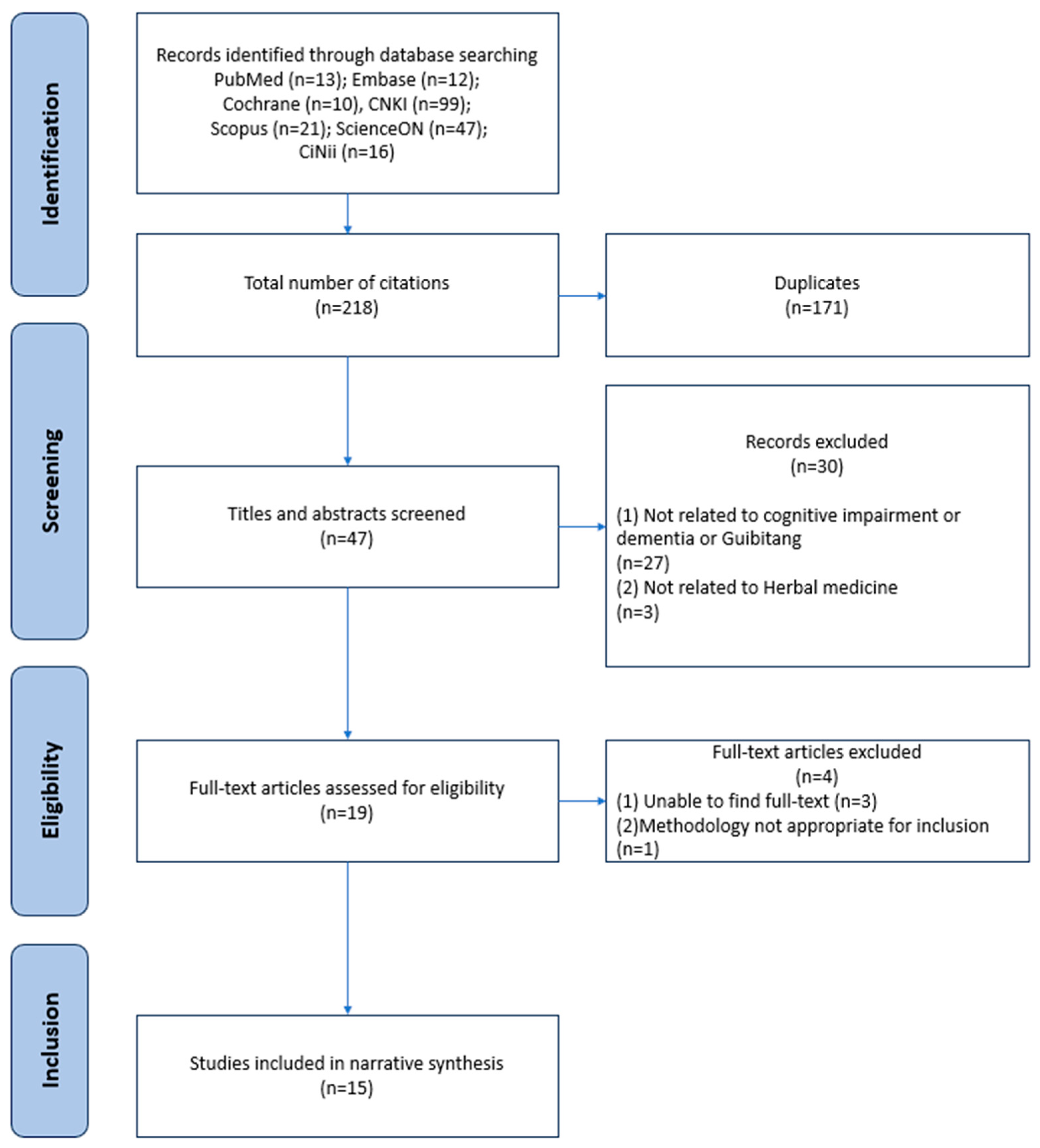
3.1. Literature Search and Selection Process
3.2. Study Designs and Regions
3.3. Demographic Characteristics of Study Participants
3.4. Details of Intervention
3.4.1. Dosage, Frequency, and Treatment Period of Herbal Medicine
3.4.2. Composition of GBT, KGBT, and Herbal Medicines Included in Their Variants
3.4.3. Types of Concomitant Medication
3.5. Evaluation Methods
3.6. Treatment Outcomes by Endpoints
3.7. Treatment Outcomes by Type of Disease
3.8. Safety
4. Discussion
4.1. Research Status
4.2. Types of Cognitive Impairment
4.3. Significance of GBT Treatment
4.4. Proposed Treatment Mechanisms for GBT in Cognitive Impairment
4.5. Strength of the Study and Clinical Suggestions
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCD | Subjective Cognitive Decline |
| MCI | Mild Cognitive Impairment |
| AD | Alzheimer’s Disease |
| VD | Vascular Dementia |
| PSCI | Post-Stroke Cognitive Impairment |
| GBT | Guibi-tang |
| KGBT | Kami-guibi-tang |
| TEAM | Traditional East Asian Medicine |
| RCT | Randomized Controlled Trial |
| MMSE | Mini-Mental State Examination |
| CREB | cAMP Response Element-Binding Protein |
| ERK | Extracellular Signal-Regulated Kinase |
| MRI | Magnetic Resonance Imaging |
| ASL-MRI | Arterial Spin Labeling Magnetic Resonance Imaging |
| SPECT | Single-Photon Emission Computed Tomography |
| CBF | Cerebral Blood Flow |
| MoCA | Montreal Cognitive Assessment |
| RBMT | Rivermead Behavioural Memory Test |
| TMT-B | Trail Making Test Part B |
| SNSB-D | Seoul Neuropsychological Screening Battery—Dementia |
| MAS | Memory Assessment Scales |
| GDS | Geriatric Depression Scale |
| NPI | Neuropsychiatric Inventory |
| MMT | Manual Muscle Testing |
| VFSS | Videofluoroscopic Swallowing Study |
| ADL | Activities of Daily Living |
| K-MBI | Korean Modified Barthel Index |
| CDR | Clinical Dementia Rating |
| CDR-SB | Clinical Dementia Rating—Sum of Boxes |
| SVLT | Seoul Verbal Learning Test |
| RCFT | Rey–Osterrieth Complex Figure Test |
| K-IADL | Korean Instrumental Activities of Daily Living |
| SGDS | Short Geriatric Depression Scale |
| NPI-NH | Neuropsychiatric Inventory—Nursing Home version |
| DEI | Dementia Elderly Integration Scale |
| MMSE-J | Japanese Mini-Mental State Examination |
| RBANS-J | Repeatable Battery for the Assessment of Neuropsychological Status—Japanese version |
| S-GDS | Short Geriatric Depression Scale |
| ChEI(s) | Cholinesterase Inhibitor(s) |
| ACh | Acetylcholine |
| Hcy | Homocysteine |
| NAA | N-acetylaspartate |
| Cr | Creatine |
| Cho | Choline |
| Glx | Glutamate + Glutamine |
| mI | Myo-inositol |
| GABA | Gamma-aminobutyric acid |
| MRS | Magnetic Resonance Spectroscopy |
| 8-OHDG | 8-hydroxy-2′-deoxyguanosine |
| ox-LDL | Oxidized Low-Density Lipoprotein |
| MDA | Malondialdehyde |
| SOD | Superoxide Dismutase |
| IL | Interleukin |
| CRP | C-Reactive Protein |
| FIB | Fibrinogen |
| CGA-NPI | Comprehensive Geriatric Assessment—Neuropsychiatric Inventory |
References
- van Harten, A.C.; Mielke, M.M.; Swenson-Dravis, D.M.; Hagen, C.E.; Edwards, K.K.; Roberts, R.O.; Geda, Y.E.; Knopman, D.S.; Petersen, R.C. Subjective cognitive decline and risk of MCI: The Mayo Clinic Study of Aging. Neurology 2018, 91, e300–e312. [Google Scholar] [CrossRef] [PubMed]
- Luis, C.A.; Loewenstein, D.A.; Acevedo, A.; Barker, W.W.; Duara, R. Mild cognitive impairment: Directions for future research. Neurology 2003, 61, 438–444. [Google Scholar] [CrossRef]
- Wilbur, J. Dementia: Dementia Types. FP Essent 2023, 534, 7–11. [Google Scholar] [PubMed]
- Rost, N.S.; Brodtmann, A.; Pase, M.P.; van Veluw, S.J.; Biffi, A.; Duering, M.; Hinman, J.D.; Dichgans, M. Post-Stroke Cognitive Impairment and Dementia. Circ. Res. 2022, 130, 1252–1271. [Google Scholar] [CrossRef]
- World Health Organization. Dementia: Number of People Affected to Triple in Next 30 Years. Available online: https://www.who.int/news/item/07-12-2017-dementia-number-of-people-affected-to-triple-in-next-30-years (accessed on 20 May 2025).
- Saunders, N.L.J.; Summers, M.J. Attention and working memory deficits in mild cognitive impairment. J. Clin. Exp. Neuropsychol. 2010, 32, 350–357. [Google Scholar] [CrossRef]
- Tayeb, H.O.; Yang, H.D.; Price, B.H.; Tarazi, F.I. Pharmacotherapies for Alzheimer’s disease: Beyond cholinesterase inhibitors. Pharmacol. Ther. 2012, 134, 8–25. [Google Scholar] [CrossRef]
- Yamada, K.; Hayashi, T.; Hasegawa, T.; Ishihara, S.; Kameyama, T.; Morimasa, T.; Kaneyuki, T.; Shohmori, T.; Nabeshima, T. Effects of Kamikihito, a Traditional Chinese Medicine, on Neurotransmitter Receptor Binding in the Aged Rat Brain Determined by In Vitro Autoradiography (2): Changes in GABA A and Benzodiazepine Receptor Binding. Jpn. J. Pharmacol. 1994, 66, 53–58. [Google Scholar] [CrossRef]
- Watari, H.; Shimada, Y.; Tohda, C. New Treatment for Alzheimer’s Disease, Kamikihito, Reverses Amyloid-β-Induced Progression of Tau Phosphorylation and Axonal Atrophy. Evid. Based Complement. Altern. Med. 2014, 2014, 706487. [Google Scholar] [CrossRef]
- Tohda, C.; Nakada, R.; Urano, T.; Okonogi, A.; Kuboyama, T. Kamikihi-to (KKT) rescues axonal and synaptic degeneration associated with memory impairment in a mouse model of Alzheimer’s disease, 5XFAD. Int. J. Neurosci. 2011, 121, 641–648. [Google Scholar] [CrossRef]
- Oizumi, H.; Miyazaki, S.; Tabuchi, M.; Endo, T.; Omiya, Y.; Mizoguchi, K. Kamikihito Enhances Cognitive Functions and Reward-Related Behaviors of Aged C57BL/6J Mice in an Automated Behavioral Assay System. Front. Pharmacol. 2020, 11, 1037. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-Y.; Kim, H.-R.; Jahng, G.-H.; Jin, C.; Kwon, S.; Cho, S.-Y.; Park, S.-U.; Jung, W.-S.; Moon, S.-K.; Ko, C.-N.; et al. Efficacy and safety of Kami-guibi-tang for mild cognitive impairment: A pilot, randomized, double-blind, placebo-controlled trial. Bmc Complement. Med. Ther. 2021, 21, 251. [Google Scholar] [CrossRef]
- Kim, H.-R.; Shin, H.-Y.; Yim, T.-B.; Jahng, G.-H.; Jin, C.; Kwon, S.; Cho, S.-Y.; Park, S.-U.; Jung, W.-S.; Moon, S.-K.; et al. Efficacy of Kami Guibi-tang as an Add-On Therapy to Acetylcholinesterase Inhibitor for Cognitive Function in Mild Alzheimer’s Disease: A Pilot Study. Evid.-Based Complement. Altern. Med. Ecam 2023, 2023, 4846770. [Google Scholar] [CrossRef]
- Higashi, K.; Rakugi, H.; Yu, H.; Moriguchi, A.; Shintani, T.; Ogihara, T. Effect of kihito extract granules on cognitive function in patients with Alzheimer’s-type dementia. Geriatr. Gerontol. Int. 2007, 7, 245–251. [Google Scholar] [CrossRef]
- Watari, H.; Shimada, Y.; Matsui, M.; Tohda, C. Kihito, a Traditional Japanese Kampo Medicine, Improves Cognitive Function in Alzheimer’s Disease Patients. Evid. Based Complement. Altern. Med. 2019, 2019, 4086749. [Google Scholar] [CrossRef]
- Nogami, T.; Iwasaki, K.; Kimura, H.; Higashi, T.; Arai, M.; Butler, J.P.; Fujii, M.; Sasaki, H. Traditional Chinese medicine Jia Wei Gui Pi Tang improves behavioural and psychological symptoms of dementia and favourable positive emotions in patients. Psychogeriatrics 2023, 23, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Kwon, S.; Shin, H.Y.; Kim, H.R.; Kim, J.H.; Park, S.; Ryu, C.W.; Park, J.M.; Edden, R.A.E.; Jahng, G.H. Treatment evaluation of Kami Guibi-tang on participants with amnestic mild cognitive impairment using magnetic resonance imaging on brain metabolites, gamma-aminobutyric acid, and cerebral blood flow. J. Appl. Clin. Med. Phys. 2021, 22, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- NICE. Methods for the Development of NICE Public Health Guidance. Available online: https://www.nice.org.uk/process/pmg4/chapter/introduction (accessed on 19 October 2025).
- Kwak, B. Memory-Improving Effect of Herbal Product IQ-One: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial. Ph.D. Thesis, Kyung Hee University, Seoul, Republic of Korea, 2008. [Google Scholar]
- Liu, L. Efficacy of Guipi Decoction in the Treatment of Cognitive Impairment After Stroke: A Clinical Study. Chin. J. Pract. Nerv. Dis. 2012, 15, 31–32. [Google Scholar] [CrossRef]
- Lee, H.-m.; Kim, J.-h.; Yang, S.-b.; Lee, H.-j.; Cho, S.-y.; Park, S.-y.; Ko, C.-n.; Park, J.-m. A Case Report of Alcohol-Related Dementia Treated with Korean Medicine, Including Gwibi-Tang-Gami. J. Intern. Korean Medicine 2016, 37, 678–684. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, H.-m.; Shin, H.-y.; Kim, H.; Yang, S.-B.; Cho, S.-Y.; Park, S.-U.; Ko, C.-N.; Park, J.-M. Two Cases of Gami-Guibitang on Cognitive Impairment after Stroke with Improved Recall Memory including Korean Medicine. J. Soc. Stroke Korean Med. 2018, 19, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Tamano, M.; Kato, S.; Okamura, A.; Hoshino, T.; Takahashi, S. A case of dementia with high anxiety and insomnia successfully treated with kamikihito. J. Neurosurg. Kampo Med. 2018, 4, 28–33. [Google Scholar] [CrossRef]
- Feng, T.; Sun, D. Effect of Modified Guipi Decoction on Homocysteine Levels in Mild Cognitive Impairment with Heart and Spleen Deficiency Syndrome. Med. Diet Health 2021, 19, 31+88. [Google Scholar]
- Li, W.-h.; Wu, Z.-f.; Wang, K.; Zhang, Y.-y.; Sun, X.-s.; Kuang, X.-y. Clinical Study on Modified Guipitang Combined with Xuefu Zhuyutang in Treating Mild Cognitive Impairment After Cerebral Infarction. Chin. J. Exp. Tradit. Med. Formulae 2022, 28, 147–153. [Google Scholar]
- Youn, H.-s.; Lee, E.-c.; Son, J.-m.; Kwon, S.-w.; Park, C.-h.; Kwon, Y.-j.; Lee, H.-j.; Lee, J.-e. A Case Report of a Patient with Vascular Dementia Caused by Intracerebral Hemorrhage Treated with Modified Guibi-tang. J. Soc. Stroke Korean Med. 2023, 44, 1033–1040. [Google Scholar] [CrossRef]
- Yim, T.-B.; Jeon, G.-R.; Lee, H.-J.; Lee, K.-H.; Heo, H.-M.; Lee, H.-G.; Kwon, S.; Cho, S.-Y.; Park, S.-U.; Jung, W.-S.; et al. The effectiveness of Kami Guibi-tang for cognitive impairment patients: A retrospective chart review. Heliyon 2024, 10, e23615. [Google Scholar] [CrossRef] [PubMed]
- Oken, B.S. Placebo effects: Clinical aspects and neurobiology. Brain 2008, 131, 2812–2823. [Google Scholar] [CrossRef]
- Park, H.; Hwang, Y.-H.; Yang, H.J.; Kim, H.-K.; Song, K.S.; Ma, J.Y. Acute toxicity and genotoxicity study of fermented traditional herb formula Guibi-tang. J. Ethnopharmacol. 2014, 156, 182–189. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Seo, C.-S.; Kim, Y.-B.; Shin, H.-K. Safety assessment of Guibi-tang: Subchronic toxicity study in Crl:CD SD rats. Regul. Toxicol. Pharmacol. 2015, 73, 485–493. [Google Scholar] [CrossRef]
- Rong, Y.; Wu, D.; Li, M.; Teng, J. Efficacy and safety of Guipi Decoction in the treatment of chronic heart failure: A systematic review and meta-analysis of randomized controlled trials. Medicine 2023, 102, e33181. [Google Scholar] [CrossRef]
- Chen, H.; Dou, Z.; Liu, S.; Dong, H.; Zhang, D.; Wang, S. Efficacy and safety of Guipi Decoction combined with conventional Western medicine in the treatment of insomnia with deficiency of heart and spleen:a meta-analysis. Asian Toxicol. Res. 2022, 4, 3. [Google Scholar] [CrossRef]
| Author | Year | Study Location | Research Design | Sample Size (Male/Female) | Age (Treatment/Control) | Herbal Medicines Added (+) or Removed (–) from the Original Composition of GBT † | Formulation | Dosage and Administration | Treatment Duration |
|---|---|---|---|---|---|---|---|---|---|
| Higashi [14] | 2007 | Japan | RCT | 64 (11/53) | Wuchasingihwan: 84.7 ± 6.5/GBT: 84.3 ± 6.1/Non-treatment: 82.8 ± 8.1 | (+): Saussureae Radix, Zingiberis Rhizoma, Zizyphi Fructus (–): Aucklandiae Radix | Extract | 7.5 g/day, p.o., TID | 3 months |
| Kwak [21] | 2008 | Republic of Korea | RCT | 63 (13/50) | 52.0 ± 7.1/50.8 ± 6.7 | (+): Acori Graminei Rhizoma, Schisandrae Fructus, Cnidii Rhizoma, Ginkgo Folium (–): Longan Arillus, Ziziphi Semen, Astragali Radix, Atractylodis Rhizoma Alba, Poria Sclerotium cum Pini Radix, Aucklandiae Radix, Glycyrrhizae Radix et Rhizoma | Extract | 8.7 g/day, p.o., BID | 6 weeks |
| Liu [22] | 2012 | China | RCT | 58 (39/19) | Not reported | (+): Codonopsis Radix (–): Atractylodis Rhizoma Alba, Poria Sclerotium cum Pini Radix | Decoction | 1 dose/day, p.o., BID | 10 weeks |
| Feng [26] | 2021 | China | RCT | 70 (38/32) | Not reported | (+): Codonopsis Radix, Poria cum Ligno Hospite, Acori Graminei Rhizoma (–): Ginseng Radix, Poria Sclerotium cum Pini Radix | Decoction | 1 dose/day, p.o., BID | 30 days |
| Cho [17] | 2021 | Republic of Korea | RCT | 30 (18/12) | Not reported | Not reported | Extract | 9 g/day, p.o., TID | 24 weeks |
| Shin [12] | 2021 | Republic of Korea | RCT | 33 (17/16) | 70.13 (Treatment: 70.2/Control: 70.1) | (+): Moutan Cortex, Zizyphi Fructus, Zingiberis Rhizoma Recens, Bupleuri Radix, Gardeniae Fructus | Extract | 9 g/day, p.o., TID | 24 weeks |
| Li [27] | 2022 | China | RCT | 114 (66/48) | 63.91 ± 7.26/63.78 ± 7.47 | (+): Persicae Semen, Carthami Flos, Cnidii Rhizoma, Ginkgo Folium, Salviae Miltiorrhizae Radix, Rehmanniae Radix Praeparata, Alpiniae Oxyphyllae Fructus (–): Ziziphi Semen, Poria Sclerotium cum Pini Radix, Aucklandiae Radix | Decoction | 1 dose/day, p.o., BID | 8 weeks |
| Kim [13] | 2023 | Republic of Korea | RCT | 14 (3/11) | 70.7 ± 6.8/75.7 ± 10.1 | (+): Moutan Cortex, Gardeniae Fructus, Zizyphi Fructus, Bupleuri Radix, Zingiberis Rhizoma Recens | Extract | 9 g/day, p.o., TID | 24 weeks |
| Nogami [16] | 2023 | Japan | RCT | 63 (18/45) | 82.5 ± 6.6/84.0 ± 5.1 | (+): Zizyphi Fructus, Zingiberis Rhizoma, Saussureae Radix, Gardeniae Fructus, Bupleuri Radix (–): Aucklandiae Radix | Extract | 7.5 g/day, p.o., TID | 28 days |
| Watari [15] | 2019 | Japan | Open-label crossover clinical trial | 10 (4/6) | 71.8 ± 6.93 | (+): Zizyphi Fructus, Zingiberis Rhizoma, Saussureae Radix (–): Aucklandiae Radix | Extract | 7.5 g/day, p.o., TID | 24 weeks |
| Yim [29] | 2024 | Republic of Korea | Retrospective chart review | 31 (9/22) | Overall: 69.6 ± 11.2; AD: 64.5; MCI: 71.2 ± 10.3; SCD: 67.5; VD: 70.5 | (+): Moutan Cortex, Zizyphi Fructus, Zingiberis Rhizoma Recens, Bupleuri Radix | Extract | 9 g/day, p.o., TID or 7.5 g/day, p.o., TID | ≥ 90 days |
| Lee [23] | 2016 | Republic of Korea | Case report | 1 (1/0) | 59 | (+): Acori Graminei Rhizoma, Zingiberis Rhizoma Recens | Decoction | 2 doses/day, p.o., TID | 29 days |
| Kim [24] | 2018 | Republic of Korea | Case report | 2 (1/1) | 83, 66 | (+): Zizyphi Fructus, Zingiberis Rhizoma Recens | Decoction | 2 doses/day, p.o., TID | (1) 87 days (2) 29 days |
| Tamano [25] | 2018 | Japan | Case report | 1 (0/1) | 82 | Not reported | Extract | 7.5 g/day, p.o., TID | 9 months |
| Youn [28] | 2023 | Republic of Korea | Case Report | 1 (0/1) | 87 | (+): Acori Graminei Rhizoma, Zingiberis Rhizoma Recens, Zingiberis Rhizoma Recens | Decoction | 2 doses/day, p.o., TID | 59 days |
| Author | Year | Target Disease | Herbal Intervention | Comparator | Concomitant Treatment | Outcome Measures | Key Efficacy Results | Safety/Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Higashi [14] | 2007 | AD | GBT | Wuchasingihwan (control 1), no treatment (control 2) | None | MMSE; Barthel ADL; regional CBF (SPECT) | MMSE score improved: +1.65 ± 0.53 (p < 0.01); MMSE orientation sub-score improved vs. non-treatment (p = 0.001); MMSE attention sub-score improved vs. non-treatment (p = 0.006). Raw MMSE sub-score data not reported. | Hypertension (GBT); diarrhea (control 1) |
| Kwak [21] | 2008 | Normal cognition | GBT | No treatment | None | K-MAS; AST, ALT, BUN, creatinine | K-MAS visual delayed recognition improved in control group: +2.4 ± 2.4 (vs. case +0.6 ± 2.7, p = 0.023); K-MAS language memory process—word clustering recall improved in case group: +0.1 ± 0.2 (vs. control +0.0 ± 0.1, p = 0.017); ALT increased in case group: +7.8 ± 7.5 (vs. control +2.6 ± 5.7, p = 0.010). | Adverse event rate: 34% (treatment), 19.5% (control) |
| Liu [22] | 2012 | PSCI | GBT | Piracetam | None | MMSE; Barthel ADL | Clinical effectiveness: 75.9% (treatment) vs. 37.9% (control), p < 0.05; MMSE improved: 22.47 ± 3.17 vs. 19.39 ± 1.44 (p < 0.05); Barthel ADL improved from 50.71 ± 8.5 to 72.47 ± 10.76 (treatment, p < 0.05); post-treatment Barthel ADL higher vs. control (p < 0.05). | Not reported |
| Feng [26] | 2021 | aMCI | GBT variant 1 | Nimodipine 30 mg | None | TCM symptom score; ACh; Hcy | TCM score improved (observation < control, p < 0.05); ACh increased (p < 0.05); Hcy decreased (p < 0.05). | Not reported |
| Cho [17] | 2021 | PSCI | KGBT | Placebo | None | MRS metabolites (NAA/Cr, Cho/Cr, Glx/Cr, mI/Cr, GABA+/Cr); CBF (ASL-MRI); K-MMSE | K-MMSE difference (F = 4.71, p = 0.039); GABA+/Cr difference (F = 5.27, p = 0.029); CBF decreased in both groups (cluster 2 p < 0.001, hippocampus p < 0.001, fusiform gyrus p = 0.003); within KGBT, similar decreases; fusiform CBF % decrease lower vs. placebo (p = 0.024); cluster 1 CBF higher vs. placebo (F = 8.71, p = 0.006). | Not reported |
| Shin [12] | 2021 | aMCI | KGBT | Placebo | None (excluded ChEIs, memantine) | s-D total; CDR-SB; SNSB-D memory; SVLT; RCFT; K-MMSE; GDS; Barthel ADL; K-IADL; SGDS | CDR-SB improved in KGBT (p = 0.010), worsened in placebo; SNSB-D total and memory improved (p < 0.001); no significant changes in K-MMSE, GDS, ADL/IADL, depression. | Adverse event rate: 11.8% (treatment), 12.5% (control) |
| Li [27] | 2022 | PSCI | GBT variant 2 | Red deer ginseng tablets | None | MoCA; RBMT; TMT-B; Barthel ADL; NPI-1/2; TCM symptom score; serum 8-OHDG, ox-LDL, MDA, SOD, Hcy, IL-8, CRP, FIB | Cognitive improvement rate: 92.98% vs. 78.95% (p < 0.05); MoCA recovery ≥ 26: 54.39% vs. 33.33% (p < 0.05); post-treatment MoCA, RBMT, ADL higher; TMT-B time shorter (p < 0.01); TCM score, NPI-1/2 lower (p < 0.01); SOD higher; 8-OHDG, ox-LDL, MDA, Hcy, IL-8, CRP, FIB lower (p < 0.01). | Not reported |
| Kim [13] | 2023 | AD | KGBT | Placebo + ChEI | ChEI | SNSB-D total; SNSB sub-scores; K-MMSE; CDR; GDS; SGDS; K-IADL; Barthel ADL; KQoL-AD; CGA-NPI | No significant change in SNSB-D total/domains (p > 0.6); K-MMSE declined in both (p = 0.80); earlier-stage aMCI data showed better effects in memory and executive function; no significant differences in SGDS, ADL/IADL, CDR, GDS. | No adverse events reported |
| Nogami [16] | 2023 | AD, PSCI | KGBT | No treatment | ChEIs, memantine, anxiolytics, hypnotics, anticonvulsants | NPI-NH; DEI; MMSE; labs | NPI-NH improved: 29.8 → 13.2 (p < 0.001); DEI improved: 24.3 → 32.5 (p = 0.001); significant between-group differences in NPI-NH subcategories (agitation, dysphoria, anxiety, disinhibition, irritability). | Aspiration pneumonia (n = 2) |
| Watari [15] | 2019 | AD | GBT | ChEI | None | MMSE-J; RBANS-J | MMSE-J improved during kihito intake vs. ChEI-only (p = 0.0039) | Not reported |
| Yim [29] | 2024 | SCD, MCI, AD, VD | KGBT | None | cognitive medications (e.g., donepezil, rivastigmine, memantine); gliatilin, gliatamine, nutritional supplements | MMSE-K (total/subdomains); S-GDS | MMSE-K improved at 3 months (p = 0.021) and 9 months (p = 0.041); time effect (p = 0.007); attention/calculation and orientation improved; VD subgroup improved at 9 months (p = 0.015); S-GDS decreased (p = 0.006) | Not reported |
| Lee [23] | 2016 | Alcoholic dementia | KGBT | None | Donepezil Thiamine | MMSE-K; CDR | MMSE-K: 20 → 25 after 28 days; CDR: 1 → 0.5; orientation, attention/calculation, memory recall improved. | Not reported |
| Kim [24] | 2018 | PSCI | KGBT | None | Case 1: Donepezil, Atenolol, Amlodipine, Acetylcysteine, Rebamipide, Aspirin, Choline Alfoscerate, Finasteride, Silodosin Case 2: Aspirin, Cilostazol, Losartan, Pravastatin | MMSE-K; CDR; GDS | Case 1 (83 M): MMSE-K 11 → 21 → 27; sub-items improved; CDR 2 → 2 → 0.5; GDS 6 → 5 → 3. Case 2 (66 F): MMSE-K unchanged, recall improved (0 → 1); CDR 2 → 1; GDS 6 → 5. | Not reported |
| Tamano [25] | 2018 | Dementia (unspecified) | KGBT | None | Donepezil Amlodipine Sitagliptin | BPSD; family burden | Week 1: calmer, better sleep; 1 month: reduced wandering, improved orientation/executive function; 2 months: improved appetite, weight gain, anemia recovery, family QOL. | Not reported |
| Youn [28] | 2023 | PSCI | GBT variant 4 | None | Levetiracetam, choline alfoscerate, carvedilol, amlodipine, metformin, famotidine, sodium alginate, quetiapine, acetaminophen/tramadol, ezetimibe, rosuvastatin, levothyroxine | K-MMSE-2; CDR; MMT; K-MBI; VFSS | K-MMSE-2: 6 → 14; CDR: 3 → 2; MMT grade 2 → 3; K-MBI: 0 → 17; VFSS PAS 8 → 5, then solid food intake; delirium resolved; improved communication/orientation after 2 weeks. | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.; Lee, H.-G.; Kwon, S. Current Utilization and Research Status of the Herbal Medicine Guibi-Tang and Its Variants for Cognitive Impairment: A Scoping Review. Nutrients 2025, 17, 3365. https://doi.org/10.3390/nu17213365
Kim G, Lee H-G, Kwon S. Current Utilization and Research Status of the Herbal Medicine Guibi-Tang and Its Variants for Cognitive Impairment: A Scoping Review. Nutrients. 2025; 17(21):3365. https://doi.org/10.3390/nu17213365
Chicago/Turabian StyleKim, Gyeongmuk, Han-Gyul Lee, and Seungwon Kwon. 2025. "Current Utilization and Research Status of the Herbal Medicine Guibi-Tang and Its Variants for Cognitive Impairment: A Scoping Review" Nutrients 17, no. 21: 3365. https://doi.org/10.3390/nu17213365
APA StyleKim, G., Lee, H.-G., & Kwon, S. (2025). Current Utilization and Research Status of the Herbal Medicine Guibi-Tang and Its Variants for Cognitive Impairment: A Scoping Review. Nutrients, 17(21), 3365. https://doi.org/10.3390/nu17213365