Dietary Vitamin Intake and Blood Biomarkers in Relation to Muscle Activation in Amyotrophic Lateral Sclerosis: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Selection and Study Design
2.2. Measures
2.2.1. Vitamins
2.2.2. Diet and Eating Habits Assessment
2.2.3. Biomarker Determination
2.2.4. Muscle Activation
2.3. Data Analysis
3. Results
3.1. Descriptive Statistics and Correlations
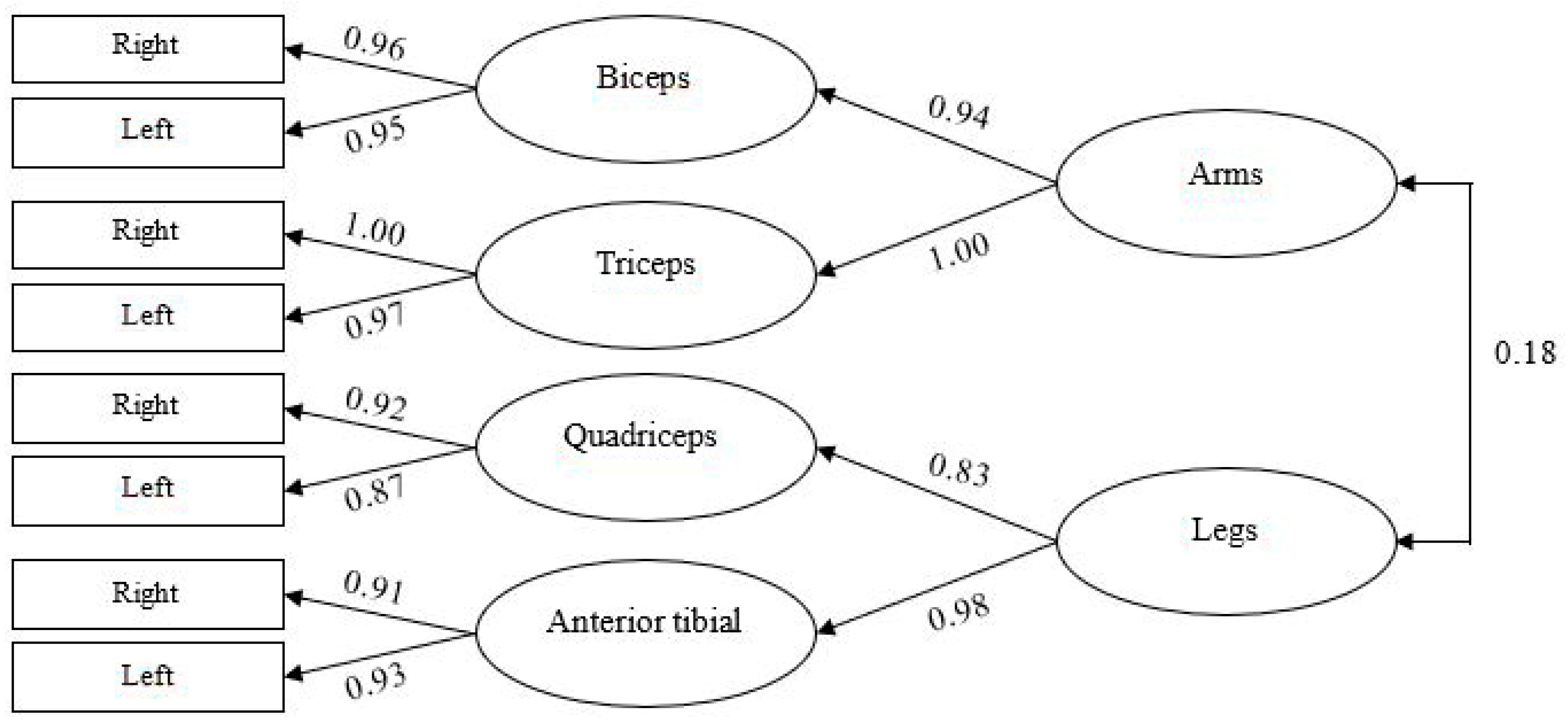
3.2. Confirmatory Factor Analysis
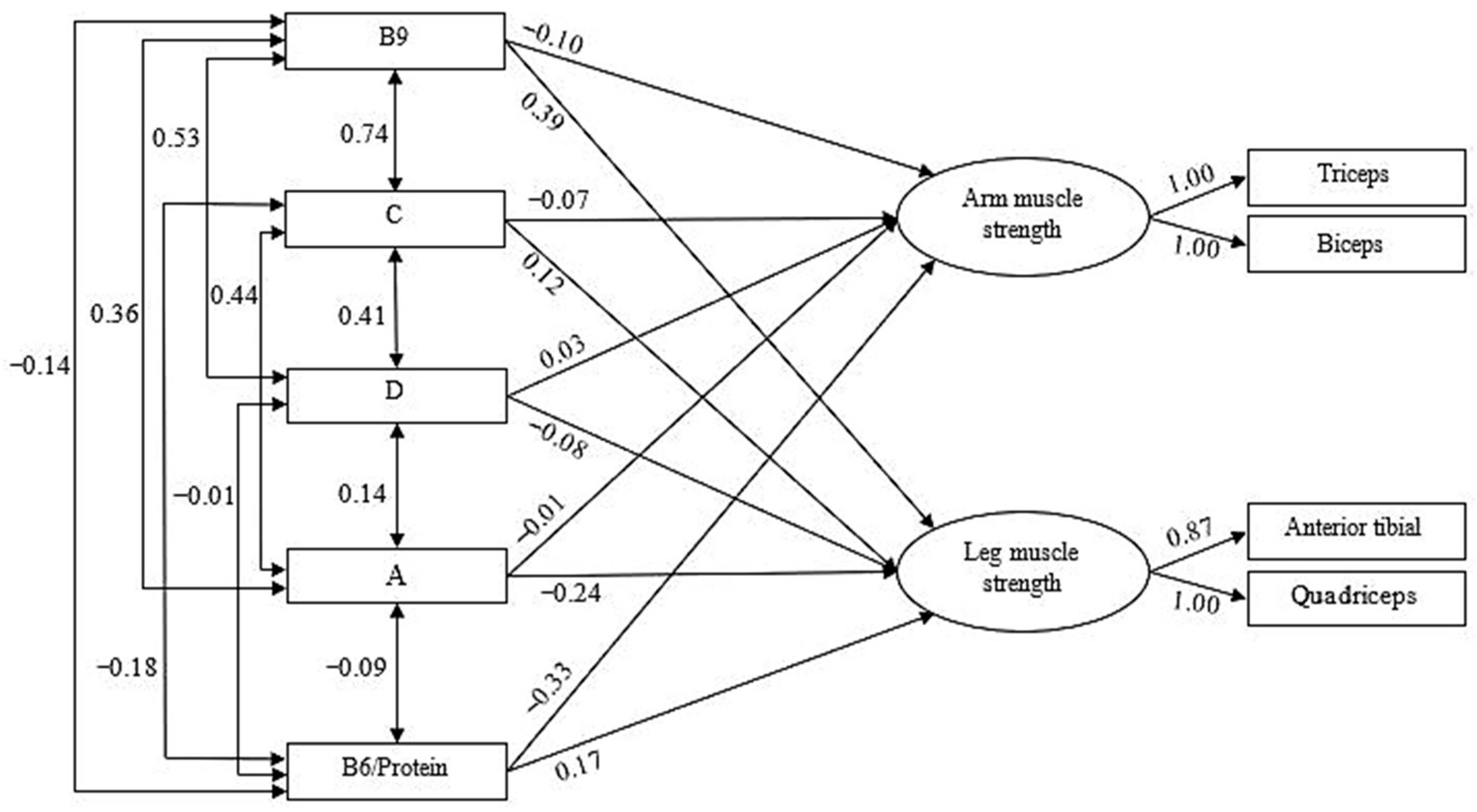
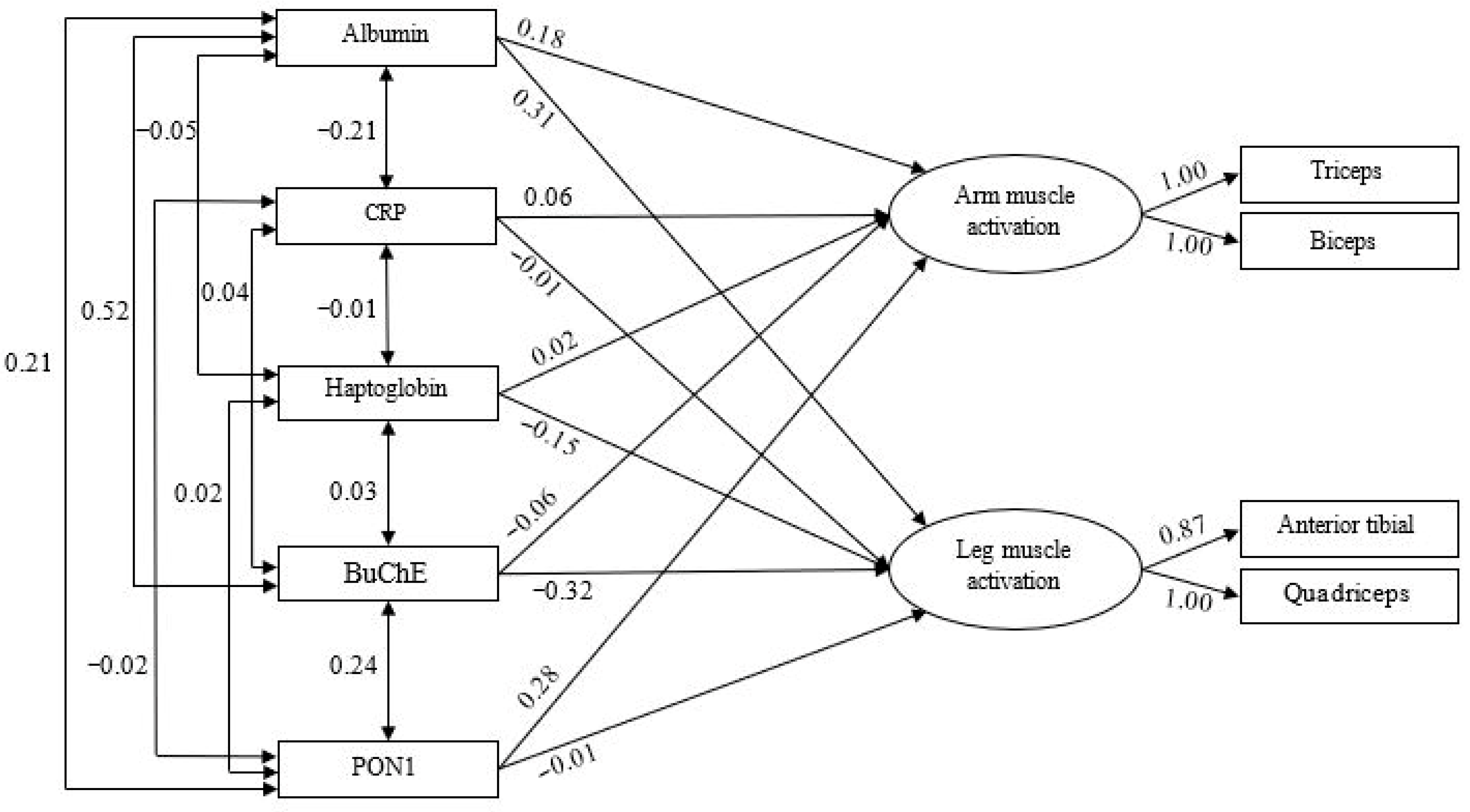
3.3. Predictive Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALS | Amyotrophic Lateral Sclerosis |
| BuChE | Butyrylcholinesterase |
| CFI | Parsimony Comparative Fit Index |
| CRP | C-Reactive Protein |
| GFI | Goodness-of-Fit Index |
| NFI | Normed Fit Index |
| PON1 | Paraoxonase 1 |
| RMS | Root Mean Square |
| RMSEA | Root Mean Squared Errors |
| SRMR | Standardized Root Mean Square |
| TLI | Tuker-Lewiss Index |
References
- Oskarsson, B.; Gendron, T.F.; Staff, N.P. Amyotrophic lateral sclerosis: An update for 2018. Mayo Clin. Proc. 2018, 93, P1617–P1628. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Kim, J.A. Supportive care needs of patients with amyotrophic lateral sclerosis/motor neuron disease and their caregivers: A scoping review. J. Clin. Nurs. 2017, 26, 4129–4152. [Google Scholar] [CrossRef]
- Morris, J. Amyotrophic Lateral Sclerosis (ALS) and Related Motor Neuron Diseases: An Overview. Neurodiagn. J. 2015, 55, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.C.; Carter, G.T. Electrodiagnosis in persons with amyotrophic lateral sclerosis. PM R 2013, 5, S89–S95. [Google Scholar] [CrossRef]
- Hardiman, O.; van den Berg, L.H.; Kiernan, M.C. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011, 7, 639–649. [Google Scholar] [CrossRef]
- Stavroulakis, T.; Baxter, S.K.; Walsh, T.; Shaw, P.J.; McDermott, C.J. The impact of gastrostomy in motor neurone disease: Challenges and benefits from a patient and carer perspective. BMJ Support. Palliat. Care 2016, 6, 52–59. [Google Scholar] [CrossRef]
- Nelson, A.T.; Trotti, D. Altered Bioenergetics and Metabolic Homeostasis in Amyotrophic Lateral Sclerosis. Neurotherapeutics 2022, 19, 1102–1118. [Google Scholar] [CrossRef]
- Hannaford, A.; Byth, K.; Pavey, N.; Henderson, R.D.; Mathers, S.; Needham, M.; Schultz, D.; Menon, P.; Kiernan, M.C.; Vucic, S. Clinical and neurophysiological biomarkers of disease progression in amyotrophic lateral sclerosis. Muscle Nerve 2023, 67, 17–24. [Google Scholar] [CrossRef]
- Wills, A.M.; Hubbard, J.; Macklin, E.A.; Glass, J.; Tandan, R.; Simpson, E.P.; Brooks, B.; Gelinas, D.; Mitsumoto, H.; Mozaffar, T.; et al. Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled phase 2 trial. Lancet 2014, 383, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Birsa, N.; Bentham, M.P.; Fratta, P. Cytoplasmic functions of TDP-43 and FUS and their role in ALS. Semin. Cell Dev. Biol. 2020, 99, 193–201. [Google Scholar] [CrossRef]
- Funalot, B.; Desport, J.C.; Sturtz, F.; Camu, W.; Couratier, P. High metabolic level in patients with familial amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2009, 10, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Barr, S.I.; McNulty, H.; Li, D.; Blumberg, J.B. Health effects of vitamin and mineral supplements. BMJ 2020, 369, m2511. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. (Eds.) Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. In Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Oki, R.; Izumi, Y.; Fujita, K.; Miyamoto, R.; Nodera, H.; Sato, Y.; Sakaguchi, S.; Nokihara, H.; Kanai, K.; Tsunemi, T.; et al. Efficacy and safety of ultrahigh-dose methylcobalamin in early-stage amyotrophic lateral sclerosis: A randomized clinical trial. JAMA Neurol. 2022, 79, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Kaji, R.; Imai, T.; Iwasaki, Y.; Okamoto, K.; Nakagawa, M.; Ohashi, Y.; Takase, T.; Hanada, T.; Shimizu, H.; Tashiro, K.; et al. Ultra-high-dose methylcobalamin in amyotrophic lateral sclerosis: A long-term phase II/III randomised controlled study. J. Neurol. Neurosurg. Psychiatry 2019, 90, 451–457. [Google Scholar] [CrossRef]
- Camu, W.; Tremblier, B.; Plassot, C.; Alphandery, S.; Salsac, C.; Pageot, N.; Juntas-Morales, R.; Scamps, F.; Daures, J.-P.; Raoul, C. Vitamin D confers protection to motoneurons and is a prognostic factor of amyotrophic lateral sclerosis. Neurobiol. Aging 2014, 35, 1198–1205. [Google Scholar] [CrossRef]
- Wang, H.; O’Reilly, É.J.; Weisskopf, M.G.; Logroscino, G.; McCullough, M.L.; Thun, M.J.; Schatzkin, A.; Kolonel, L.N.; Ascherio, A. Vitamin E intake and risk of amyotrophic lateral sclerosis: A pooled analysis of data from 5 prospective cohort studies. Am. J. Epidemiol. 2011, 173, 595–602. [Google Scholar] [CrossRef]
- Levin, J.; Bötzel, K.; Giese, A.; Vogeser, M.; Lorenzl, S. Elevated levels of methylmalonate and homocysteine in Parkinson’s disease, progressive supranuclear palsy and amyotrophic lateral sclerosis. Dement. Geriatr. Cogn. Disord. 2010, 29, 553–559. [Google Scholar] [CrossRef]
- Kumar, R.R.; Singh, L.; Thakur, A.; Singh, S.; Kumar, B. Role of Vitamins in Neurodegenerative Diseases: A Review. CNS Neurol. Disord. Drug Targets 2022, 21, 766–773. [Google Scholar] [CrossRef]
- Hu, N.; Ding, J.; Tian, H.; Shen, D.; Yang, X.; Niu, J.; Liu, M.; Cui, L. Impacts of oral supplementation of vitamin B12 and plasma levels of homocysteine on progression and survival in a Chinese ALS cohort. Neurol. Res. 2025, 28, 1–9. [Google Scholar] [CrossRef]
- Amirkhizi, F.; Ghoreishy, S.M.; Baker, E.; Hamedi-Shahraki, S.; Asghari, S. The association of vitamin D status with oxidative stress biomarkers and matrix metalloproteinases in patients with knee osteoarthritis. Front. Nutr. 2023, 10, 1101516. [Google Scholar] [CrossRef]
- Blum, S.; Vardi, M.; Brown, J.B.; Russell, A.; Milman, U.; Shapira, C.; Levy, N.S.; Miller-Lotan, R.; Asleh, R.; Levy, A.P. Vitamin E reduces cardiovascular disease in individuals with diabetes mellitus and the haptoglobin 2-2 genotype. Pharmacogenomics 2010, 11, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Kumrungsee, T.; Onishi, K.; Komaru, T.; Yanaka, N.; Kato, N. Vitamin B6 Regulates Muscle Satellite Cell Function: A Novel Possible Role of Vitamin B6 in Muscle Regeneration. Curr. Dev. Nutr. 2020, 4, 1818. [Google Scholar] [CrossRef]
- Cabrerizo, S.; Cuadras, D.; Gomez-Busto, F.; Artaza-Artabe, I.; Marín-Ciancas, F.; Malafarina, V. Serum albumin and health in older people: Review and meta analysis. Maturitas 2015, 81, 17–27. [Google Scholar] [CrossRef]
- Bertaggia, E.; Scabia, G.; Dalise, S.; Lo Verso, F.; Santini, F.; Vitti, P.; Chisari, C.; Sandri, M.; Maffei, M. Haptoglobin is required to prevent oxidative stress and muscle atrophy. PLoS ONE 2014, 9, e100745. [Google Scholar] [CrossRef]
- Nozoe, M.; Kanai, M.; Kubo, H.; Kitamura, Y.; Yamamoto, M.; Furuichi, A.; Takashima, S.; Mase, K.; Shimada, S. Changes in Quadriceps Muscle Thickness, Disease Severity, Nutritional Status, and C-Reactive Protein after Acute Stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 2470–2474. [Google Scholar] [CrossRef]
- Delacour, H.; Dedome, E.; Courcelle, S.; Hary, B.; Ceppa, F. Butyrylcholinesterase deficiency. Ann. Biol. Clin. 2016, 74, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.S.; Shahbazian, A.; Wang, J.; Golub, I.; Oganesian, B.; Dowd, T.; Vayngortin, B.; Wang, R.; Elashoff, D.; Reddy, S.T.; et al. Abnormal paraoxonase-1 (PON1) enzyme activity in idiopathic inflammatory myopathies. Rheumatology 2022, 61, 2512–2523. [Google Scholar] [CrossRef]
- Ortega, R.M.; Pérez-Rodrigo, C.; López-Sobaler, A.M. Métodos de evaluación de la ingesta actual: Registro o diario dietético. Nutr. Hosp. 2015, 31, 38–45. [Google Scholar] [CrossRef]
- Trinidad Rodríguez, I.; Fernández Ballart, J.; Cucó Pastor, G.; BiarnésJordà, E.; Arija Val, V. Validación de un cuestionario de frecuencia de consumo alimentario corto: Reproducibilidad y validez. Nutr. Hosp. 2008, 23, 242–252. [Google Scholar]
- Dapcich, V.; Salvador, G.; Ribas, L.; Pérez, C.; Aranceta, J.; Serra, L.L. Guía de Alimentación Saludable; Sociedad Española de Nutrición Comunitaria: Barcelona, Spain, 2004. [Google Scholar]
- Tecles, F.; Martínez Subiela, S.; Bernal, L.J.; Cerón, J.J. Use of Whole Blood for Spectrophotometric Determination of Cholinesterase Activity in Dogs. Vet. J. 2000, 160, 242–249. [Google Scholar] [CrossRef]
- Cerón, J.J.; Tecles, F.; Tvarijonaviciute, A. Serum paraoxonase 1 (PON1) measurement: An update. BMC Vet. Res. 2014, 10, 74. [Google Scholar] [CrossRef]
- SENIAM. Sensor Locations. Available online: http://seniam.org/sensor_location.htm (accessed on 26 December 2021).
- Criswell, E. Cram’s Introduction to Surface Electromyography; Jones & Bartlett Publishers: Boston, MA, USA, 2010. [Google Scholar]
- Tayashiki, K.; Maeo, S.; Usui, S.; Miyamoto, N.; Kanehisa, H. Effect of abdominal bracing training on strength and power of trunk and lower limb muscles. Eur. J. Appl. Physiol. 2016, 116, 1703–1713. [Google Scholar] [CrossRef]
- Arbuckle, J.L. Amos 7.0 User’s Guide; SPSS: Chicago, IL, USA, 2006. [Google Scholar]
- Bentler, P.M.; Bonett, D.G. Significance tests and goodness of fit in the analysis of covariance structures. Psychol. Bull. 1980, 88, 588–606. [Google Scholar] [CrossRef]
- Jöreskog, K.G.; Sörbom, D. LISREL 8: User’s Guide; Scientific Software International: Chicago, IL, USA, 1993. [Google Scholar]
- Hu, L.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Steiger, J.H. Structural model evaluation modification: An interval estimation approach. Multivar. Behav. Res. 1990, 25, 173–180. [Google Scholar] [CrossRef]
- Hair, J.F.; Anderson, R.E.; Tatham, R.L.; Black, W.C. Análisis Multivariante, 5th ed.; Prentice Hall: Madrid, Spain, 1999. [Google Scholar]
- Bentler, P.M. Comparative fit indexes in structural models. Psychol. Bull. 1990, 107, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Byrne, B.M. Structural Equation Modeling with AMOS Basic Concepts, Applications, and Programming; Lawrence Erlbaum: Mahwah, NJ, USA, 2001. [Google Scholar]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Bollen, K.A.; Stine, R.A. Bootstrapping goodness-of-fit measures in structural equation models. In Testing Structural Equation Models; Bollen, K.A., Long, J.S., Eds.; Sage: Newbury Park, CA, USA, 1993; pp. 111–135. [Google Scholar]
- Milisav, I.; Ribarič, S.; Poljsak, B. Antioxidant Vitamins and Ageing. In Biochemistry and Cell Biology of Ageing: Part I Biomedical Science. Subcellular Biochemistry; Springer: Singapore, 2018; Volume 90, pp. 1–23. [Google Scholar] [CrossRef]
- Sturman, J.A.; Rivlin, R.S. Pathogenesis of brain dysfunction in deficiency of thiamine, riboflavin, pantothenic acid, or vitamin B6. In Biology of Brain Dysfunction; Springer: Berlin/Heidelberg, Germany, 1975; pp. 425–475. [Google Scholar]
- Sechi, G.; Sechi, E.; Fois, C.; Kumar, N. Advances in clinical determinants and neurological manifestations of B vitamin deficiency in adults. Nutr. Rev. 2016, 74, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Skulstad Johanson, G.A.; Tysnes, O.B.; Bjerknes, T.L. Use of Off-Label Drugs and Nutrition Supplements among Patients with Amyotrophic Lateral Sclerosis in Norway. Neurol. Res. Int. 2022, 2022, 1789946. [Google Scholar] [CrossRef]
- Romero, S.A.; Gagnon, D.; Adams, A.N.; Moralez, G.; Kouda, K.; Jaffery, M.F.; Cramer, M.N.; Crandall, C.G. Folic acid ingestion improves skeletal muscle blood flow during graded handgrip and plantar flexion exercise in aged humans. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H658–H666. [Google Scholar] [CrossRef]
- Lee, M.R.; Jung, S.M. Folic Acid Is Related to Muscle Strength and Vitamin A Is Related to Health-Related Quality of Life: Results of the Korea National Health and Nutrition Examination Survey (KNHANES VII 2016–2018). Nutrients 2021, 13, 3618. [Google Scholar] [CrossRef]
- Miller, L.T.; Leklem, J.E.; Shultz, T.D. The effect of dietary protein on the metabolism of vitamin B-6 in humans. J. Nutr. 1985, 115, 1663–1673. [Google Scholar] [CrossRef]
- Pannemans, D.L.; van den Berg, H.; Westerterp, K.R. The influence of protein intake on vitamin B-6 metabolism differs in young and elderly humans. J. Nutr. 1994, 124, 1207–1214. [Google Scholar] [CrossRef]
- Scott, K.; Zeris, S.; Kothari, M.J. Elevated B6 levels and peripheral neuropathies. Electromyogr. Clin. Neurophysiol. 2008, 48, 219–223. [Google Scholar]
- van Hunsel, F.; van de Koppel, S.; van Puijenbroek, E.; Kant, A. Vitamin B6 in Health Supplements and Neuropathy: Case Series Assessment of Spontaneously Reported Cases. Drug Saf. 2018, 41, 859–869. [Google Scholar] [CrossRef]
- Hadtstein, F.; Vrolijk, M. Vitamin B-6-Induced Neuropathy: Exploring the Mechanisms of Pyridoxine Toxicity. Adv. Nutr. 2021, 12, 1911–1929. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.J.; Lee, C.Y. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J. Agric. Food Chem. 2004, 52, 7514–7517. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Bozonet, S.M.; Pullar, J.M.; Simcock, J.W.; Vissers, M.C. Human skeletal muscle ascorbate is highly responsive to changes in vitamin C intake and plasma concentrations. Am. J. Clin. Nutr. 2013, 97, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.C.; O’Reilly, É.J.; Fondell, E.; Falcone, G.J.; McCullough, M.L.; Park, Y.; Kolonel, L.N.; Ascherio, A. Intakes of vitamin C and carotenoids and risk of amyotrophic lateral sclerosis: Pooled results from 5 cohort studies. Ann. Neurol. 2013, 73, 236–245. [Google Scholar] [CrossRef]
- Goncharova, P.S.; Davydova, T.K.; Popova, T.E.; Novitsky, M.A.; Petrova, M.M.; Gavrilyuk, O.A.; Al-Zamil, M.; Zhukova, N.G.; Nasyrova, R.F.; Shnayder, N.A. Nutrient Effects on Motor Neurons and the Risk of Amyotrophic Lateral Sclerosis. Nutrients 2021, 13, 3804. [Google Scholar] [CrossRef]
- Karam, C.; Barrett, M.J.; Imperato, T.; MacGowan, D.J.; Scelsa, S. Vitamin D deficiency and its supplementation in patients with amyotrophic lateral sclerosis. J. Clin. Neurosci. 2013, 20, 1550–1553. [Google Scholar] [CrossRef]
- De Marchi, F.; Saraceno, M.; Sarnelli, M.F.; Virgilio, E.; Cantello, R.; Mazzini, L. Potential role of vitamin D levels in amyotrophic lateral sclerosis cognitive impairment. Neurol. Sci. 2023, 44, 2795–2802. [Google Scholar] [CrossRef]
- Hillgartner, F.B.; Morin, D.; Hansen, R.J. Effect of excessive vitamin A intake on muscle protein turnover in the rat. Biochem. J. 1982, 202, 499–508. [Google Scholar] [CrossRef]
- Petiz, L.L.; Girardi, C.S.; Bortolin, R.C.; Kunzler, A.; Gasparotto, J.; Rabelo, T.K.; Matté, C.; Moreira, J.C.; Gelain, D.P. Vitamin A Oral Supplementation Induces Oxidative Stress and Suppresses IL-10 and HSP70 in Skeletal Muscle of Trained Rats. Nutrients 2017, 9, 353. [Google Scholar] [CrossRef]
- Silva-Fhon, J.R.; Rojas-Huayta, V.M.; Aparco-Balboa, J.P.; Céspedes-Panduro, B.; Partezani-Rodrigues, R.A. Sarcopenia and blood albumin: A systematic review with meta-analysis. Biomedica 2021, 41, 590–603. [Google Scholar] [CrossRef]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tataka, Y.; Sakazaki, M.; Kamemoto, K.; Someya, Y.; Takemiya, T.; Kondo, T. Acute effects of exercise intensity on butyrylcholinesterase and ghrelin in young men: A randomized controlled study. J. Exerc. Sci. Fit. 2024, 22, 39–50. [Google Scholar] [CrossRef] [PubMed]
- de la Rubia Ortí, J.E.; Platero, J.L.; Yang, I.H.; Cerón, J.J.; Tvarijonaviciute, A.; Sabater, P.S.; Benlloch, M.; Sancho-Cantus, D.; Sancho, S. Possible Role of Butyrylcholinesterase in Fat Loss and Decreases in Inflammatory Levels in Patients with Multiple Sclerosis after Treatment with Epigallocatechin Gallate and Coconut Oil: A Pilot Study. Nutrients 2021, 13, 3230. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Zweyer, M.; Mundegar, R.R.; Swandulla, D.; Ohlendieck, K. Proteomic serum biomarkers for neuromuscular diseases. Expert Rev. Proteo. 2018, 15, 277–291. [Google Scholar] [CrossRef]
- Tuttle, C.S.L.; Thang, L.A.N.; Maier, A.B. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res. Rev. 2020, 64, 101185. [Google Scholar] [CrossRef]
- Lunetta, C.; Lizio, A.; Maestri, E.; Sansone, V.A.; Mora, G.; Miller, R.G.; Appel, S.H.; Chiò, A. Serum C-Reactive Protein as a Prognostic Biomarker in Amyotrophic Lateral Sclerosis. JAMA Neurol. 2017, 74, 660–667. [Google Scholar] [CrossRef] [PubMed]
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Vitamin B1 (mg) | 1.00 | |||||||||||||||||||||||
| 2. Vitamin B2 (mg) | 0.99 | 1.00 | ||||||||||||||||||||||
| 3. Vitamin B6 (mg) | 0.75 | 0.75 | 1.00 | |||||||||||||||||||||
| 4. Vitamin B12 (µg) | 0.99 | 0.98 | 0.78 | 1.00 | ||||||||||||||||||||
| 5. Vitamin B9 (µg) | 0.53 | 0.53 | 0.40 | 0.55 | 1.00 | |||||||||||||||||||
| 6. Vitamin B3 (mg) | 0.17 | 0.18 | 0.78 | 0.24 | 0.12 | 1.00 | ||||||||||||||||||
| 7. Vitamin C (mg) | 0.40 | 0.40 | 0.18 | 0.41 | 0.74 | −0.10 | 1.00 | |||||||||||||||||
| 8. Vitamin A (µg) | 0.10 | 0.12 | −0.01 | 0.14 | 0.36 | −0.10 | 0.44 | 1.00 | ||||||||||||||||
| 9. Vitamin D (µg) | 0.98 | 0.97 | 0.71 | 0.98 | 0.53 | 0.14 | 0.41 | 0.14 | 1.00 | |||||||||||||||
| 10. Vitamin E (mg) | 0.98 | 0.98 | 0.71 | 0.98 | 0.61 | 0.13 | 0.48 | 0.17 | 0.97 | 1.00 | ||||||||||||||
| 11. Vitamin B6/Protein | −0.02 | −0.02 | −0.03 | −0.02 | −0.14 | −0.03 | −0.18 | −0.09 | −0.01 | −0.04 | 1.00 | |||||||||||||
| 12. Albumin (g/dL) | −0.20 | −0.20 | −0.12 | −0.21 | 0.06 | 0.00 | 0.06 | 0.07 | −0.18 | −0.18 | −0.15 | 1.00 | ||||||||||||
| 13. CRP (mg/L) | −0.08 | −0.07 | −0.02 | −0.09 | −0.07 | 0.05 | −0.06 | −0.02 | −0.06 | −0.11 | −0.05 | −0.21 | 1.00 | |||||||||||
| 14. Haptoglobin (mg/dL) | −0.09 | −0.08 | −0.34 | −0.12 | 0.02 | −0.42 | 0.22 | 0.10 | −0.05 | −0.03 | −0.02 | −0.05 | −0.01 | 1.00 | ||||||||||
| 15. BuChE (UI/L) | −0.11 | −0.12 | 0.04 | −0.15 | −0.04 | 0.17 | −0.18 | −0.15 | −0.14 | −0.14 | −0.14 | 0.52 | 0.04 | 0.03 | 1.00 | |||||||||
| 16. PON 1 (UI/L) | −0.22 | −0.22 | −0.18 | −0.21 | −0.25 | −0.08 | −0.17 | −0.14 | −0.19 | −0.20 | 0.01 | 0.21 | −0.02 | 0.02 | 0.24 | 1.00 | ||||||||
| 17. Right Biceps Muscle Activation | −0.13 | −0.12 | −0.12 | −0.12 | −0.10 | −0.06 | −0.10 | −0.10 | −0.08 | −0.13 | −0.32 | 0.10 | −0.03 | 0.03 | 0.00 | 0.24 | 1.00 | |||||||
| 18. Left Biceps Muscle Activation | −0.13 | −0.12 | −0.12 | −0.12 | −0.14 | −0.06 | −0.11 | −0.01 | −0.08 | −0.13 | −0.32 | 0.22 | 0.02 | 0.03 | 0.14 | 0.29 | 0.91 | 1.00 | ||||||
| 19. Right Triceps Muscle Activation | −0.05 | −0.05 | −0.07 | −0.05 | −0.04 | −0.06 | 0.02 | −0.10 | 0.00 | −0.05 | −0.26 | 0.17 | −0.01 | 0.00 | 0.06 | 0.28 | 0.92 | 0.86 | 1.00 | |||||
| 20. Left Triceps Muscle Activation | −0.04 | −0.04 | −0.05 | −0.03 | −0.04 | −0.05 | −0.05 | −0.03 | 0.02 | −0.03 | −0.24 | 0.21 | 0.03 | −0.02 | 0.12 | 0.33 | 0.86 | 0.94 | 0.90 | 1.00 | ||||
| 21. Right Rectus Femoris Muscle Activation | 0.10 | 0.07 | 0.09 | 0.11 | 0.32 | 0.03 | 0.25 | −0.14 | 0.12 | 0.13 | 0.10 | 0.10 | −0.06 | −0.17 | −0.21 | −0.04 | 0.11 | 0.03 | 0.22 | 0.12 | 1.00 | |||
| 22. Left Rectus Femoris Muscle Activation | 0.11 | 0.09 | 0.09 | 0.13 | 0.27 | 0.03 | 0.16 | −0.02 | 0.14 | 0.14 | 0.11 | 0.14 | −0.08 | −0.21 | −0.14 | −0.02 | 0.13 | 0.07 | 0.23 | 0.13 | 0.80 | 1.00 | ||
| 23. Right Anterior Tibialis Muscle Activation | 0.09 | 0.06 | 0.02 | 0.07 | 0.23 | −0.06 | 0.18 | −0.05 | 0.09 | 0.10 | 0.09 | 0.15 | −0.10 | −0.07 | −0.06 | −0.03 | 0.16 | 0.11 | 0.25 | 0.16 | 0.70 | 0.59 | 1.00 | |
| 24. Left Anterior Tibialis Muscle Activation | 0.10 | 0.08 | −0.01 | 0.10 | 0.36 | −0.12 | 0.31 | 0.11 | 0.13 | 0.13 | 0.10 | 0.26 | −0.10 | 0.00 | −0.07 | 0.03 | 0.11 | 0.09 | 0.21 | 0.14 | 0.68 | 0.70 | 0.85 | 1.00 |
| Mean | 7.99 | 9.08 | 14.26 | 16.04 | 318.03 | 75.49 | 174.55 | 1133.13 | 14.59 | 20.04 | 5.7 | 4.21 | 6.58 | 371.53 | 4.66 | 3.08 | 3.34 | 3.31 | 3.48 | 3.38 | 3.87 | 3.89 | 2.92 | 2.75 |
| SD | 48.33 | 48.55 | 64.3 | 54.83 | 157.51 | 277.95 | 114.23 | 1013.89 | 48.1 | 50.02 | 41.09 | 0.24 | 5.93 | 118.01 | 1.41 | 0.81 | 1.35 | 1.35 | 1.25 | 1.29 | 1.48 | 1.32 | 1.65 | 1.66 |
| Models | χ2/df | GFI | NFI | CFI | TLI | RMSEA | SRMR | Residues ≥ |±2.58| |
|---|---|---|---|---|---|---|---|---|
| Confirmatory Factor Analysis | 0.81 | 0.996 | 0.993 | 0.043 | 0.00% | |||
| Predictive Model: Vitamins | 1.58 | 0.929 | 0.955 | 0.982 | 0.954 | 0.099 | 0.072 | 0.00% |
| Predictive Model: Muscle Damage | 1.04 | 0.952 | 0.966 | 0.999 | 0.997 | 0.025 | 0.047 | 0.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Rubia Ortí, J.E.; Bargues-Navarro, G.; Privado, J.; Menarques-Ramírez, R.; Sanchis-Sanchis, C.E.; Sancho-Castillo, S.; Rubio, C.P.; Pardo-Marin, L.; Benlloch, M.; Martín-Ruiz, J. Dietary Vitamin Intake and Blood Biomarkers in Relation to Muscle Activation in Amyotrophic Lateral Sclerosis: A Cross-Sectional Study. Nutrients 2025, 17, 3345. https://doi.org/10.3390/nu17213345
de la Rubia Ortí JE, Bargues-Navarro G, Privado J, Menarques-Ramírez R, Sanchis-Sanchis CE, Sancho-Castillo S, Rubio CP, Pardo-Marin L, Benlloch M, Martín-Ruiz J. Dietary Vitamin Intake and Blood Biomarkers in Relation to Muscle Activation in Amyotrophic Lateral Sclerosis: A Cross-Sectional Study. Nutrients. 2025; 17(21):3345. https://doi.org/10.3390/nu17213345
Chicago/Turabian Stylede la Rubia Ortí, Jose Enrique, Guillermo Bargues-Navarro, Jesús Privado, Rubén Menarques-Ramírez, Claudia Emmanuela Sanchis-Sanchis, Sandra Sancho-Castillo, Camila Peres Rubio, Luis Pardo-Marin, María Benlloch, and Julio Martín-Ruiz. 2025. "Dietary Vitamin Intake and Blood Biomarkers in Relation to Muscle Activation in Amyotrophic Lateral Sclerosis: A Cross-Sectional Study" Nutrients 17, no. 21: 3345. https://doi.org/10.3390/nu17213345
APA Stylede la Rubia Ortí, J. E., Bargues-Navarro, G., Privado, J., Menarques-Ramírez, R., Sanchis-Sanchis, C. E., Sancho-Castillo, S., Rubio, C. P., Pardo-Marin, L., Benlloch, M., & Martín-Ruiz, J. (2025). Dietary Vitamin Intake and Blood Biomarkers in Relation to Muscle Activation in Amyotrophic Lateral Sclerosis: A Cross-Sectional Study. Nutrients, 17(21), 3345. https://doi.org/10.3390/nu17213345









