Visceral Obesity and Metabolic Dysfunction in IgA Nephropathy: Nutritional and Metabolic Perspectives on Disease Progression
Abstract
1. Introduction
2. Methods
3. Results
3.1. Effect of Obesity, MetS on IgAN Progression
3.2. Pathophysiological Mechanisms
4. Discussion
4.1. Effect of Obesity and MetS on IgAN Progression
4.2. Molecular Mechanisms in the Pathophysiology of Obesity and IgAN
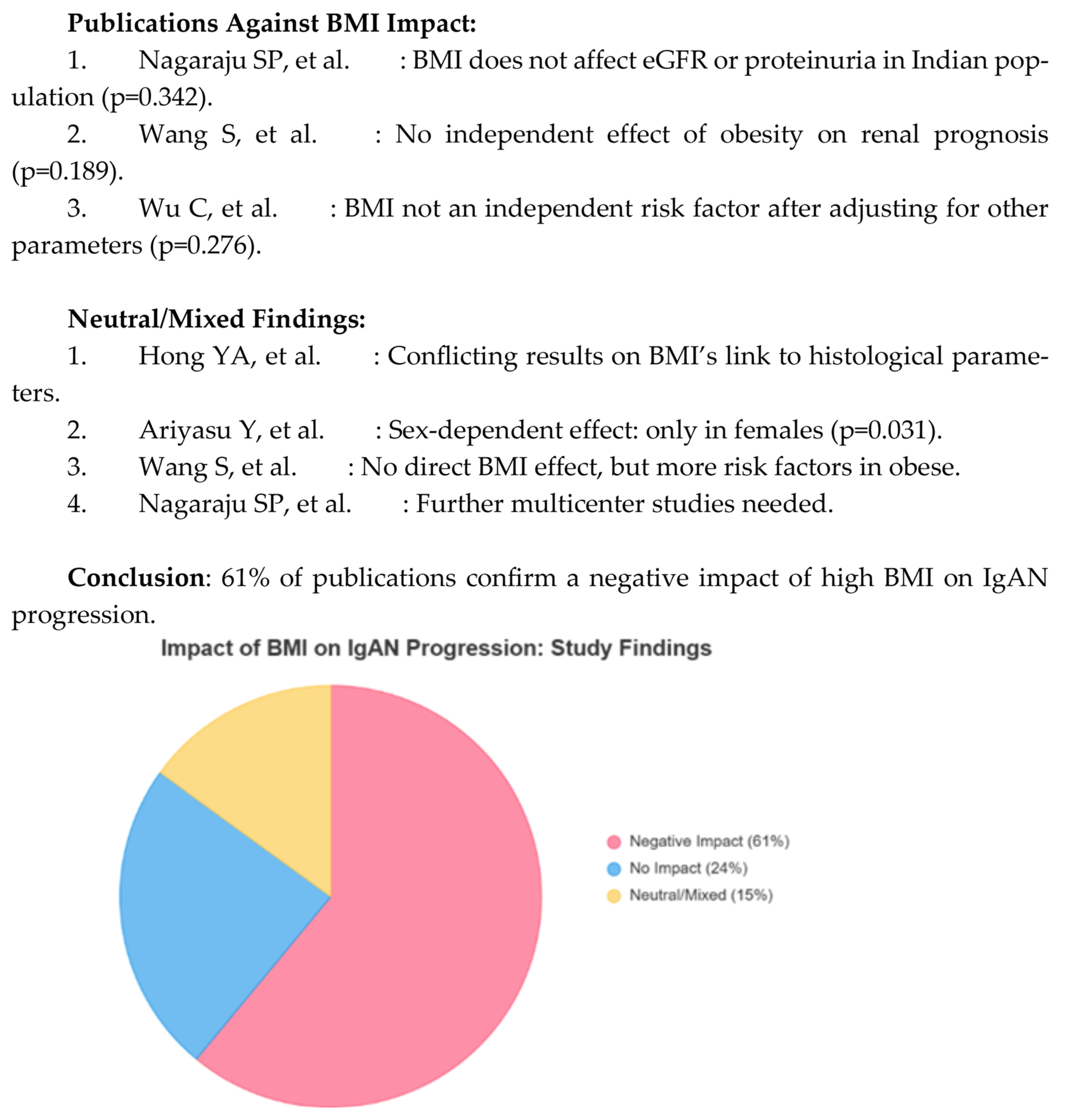
4.3. Body Mass Index
4.3.1. Limitation
4.3.2. Clinical Implications and Future Directions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wyatt, R.J.; Julian, B.A. IgA nephropathy. N. Engl. J. Med. 2013, 368, 2402–2414. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Furth, S.L.; Zoccali, C. Obesity and kidney disease: The hidden consequences of the epidemic. Physiol. Int. 2017, 104, 1–14. [Google Scholar] [CrossRef]
- Suzuki, H.; Kiryluk, K.; Novak, J.; Moldoveanu, Z.; Julian, B.A.; Wyatt, R.J.; Gharavi, A.G.; Rizk, D.V.; Tsuji, S.; Narita, I.; et al. Pathophysiology of IgA nephropathy. J. Am. Soc. Nephrol. 2011, 22, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, Z.; Wang, Y. Molecular insight into intranephritis affecting four major cell types in nephrons in IgA nephropathy. Front. Med. 2023, 10, 1128393. [Google Scholar]
- Zhu, Y.; He, H.; Sun, W.; Li, X.; Zhang, Q.; Yang, J.; Zhao, Y.; Wang, T.; Liu, L.; Chen, X.; et al. IgA nephropathy: The gut microbiome regulates the production of hypoglycosylated IgA1 through the TLR4 signaling pathway. Nephrol. Dial. Transplant. 2024, 39, 1624–1641. [Google Scholar] [CrossRef] [PubMed]
- Puthumana, J.; Thiessen-Philbrook, H.; Xu, L.; Garg, A.X.; Coca, S.G.; Parikh, C.R. Biomarkers of inflammation and repair in progression of kidney disease. J. Clin. Investig. 2021, 131, e139927. [Google Scholar] [CrossRef]
- Camilla, R.; Suzuki, H.; Daprà, V.; Loiacono, E.; Peruzzi, L.; Amore, A.; Scolari, F.; Gharavi, A.G.; Appel, G.B.; Coppo, R. Oxidative stress and galactose-deficient IgA1 as markers of IgA nephropathy progression. Clin. J. Am. Soc. Nephrol. 2011, 6, 1903–1911. [Google Scholar]
- Barratt, J.; Lafayette, R.A.; Zhang, H.; Suzuki, H.; Zhao, M.H.; Gharavi, A.G.; Julian, B.A.; Novak, J.; Rizk, D.V. IgA nephropathy: Lectin pathway and implications for targeted therapy. Kidney Int. 2023, 104, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef]
- Sagi, B.; Vas, T.; Csiky, B.; Nagy, J.; Kovács, T.J. Do metabolic syndrome and its components have prognostic significance for renal and cardiovascular outcomes in IgA nephropathy? Biomedicines 2024, 12, 1250. [Google Scholar] [CrossRef]
- Ma, H.; Lei, C.; Zhao, B.; Zhou, Y.; Liu, L.; Chen, J.; Wu, J.; Wang, C. The influence of the number of metabolic components on the prognosis of IgA nephropathy. Sci. Rep. 2024, 14, 30996. [Google Scholar] [CrossRef]
- Ouyang, Y.; Xie, J.; Yang, M.; Zhou, Q.; Ren, H.; Chen, N. Underweight is an independent risk factor for deterioration of renal function in patients with IgA nephropathy. PLoS ONE 2016, 11, e0162044. [Google Scholar]
- Wang, Q.; Zhang, J.J.; Dou, W.J.; Yang, X.; Xu, J.; Zhao, Y.; Zhang, J.; Wang, H. Effect of body mass index on the prognosis of primary immunoglobulin A nephropathy: A systematic review and meta-analysis. Int. Urol. Nephrol. 2022, 54, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Tucker, P.S.; Scanlan, A.T.; Dalbo, V.J. Chronic kidney disease affects many systems: Describing the relationship between oxidative stress, inflammation, kidney damage and concomitant disease. Oxid. Med. Cell. Longev. 2015, 2015, 806358. [Google Scholar]
- Ahmed, N.; Dalmasso, C.; Turner, M.B.; Prunotto, M.; Martín, F.; López-Giacoman, S.; Rodríguez-Iturbe, B.; Martín-Lorenzo, M.; Latosinska, A.; Mischak, H. From fat to filter: The effect of signals coming from adipose tissue on kidney function. Nat. Rev. Nephrol. 2025, 21, 417–434. [Google Scholar] [CrossRef]
- Kataoka, H.; Ohara, M.; Shibui, K.; Sato, M.; Suzuki, T.; Amemiya, N.; Taguma, Y. Overweight and obesity accelerate the progression of IgA nephropathy: Prognostic utility of a combination of BMI and histopathological parameters. Clin. Exp. Nephrol. 2012, 16, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, M.; Ohsawa, I.; Suzuki, H.; Sato, N.; Takahashi, K.; Aoki, T.; Nagase, S.; Tomino, Y. Effect of body mass index on IgA nephropathy progression among Japanese patients. J. Clin. Lab. Anal. 2015, 29, 353–360. [Google Scholar] [CrossRef]
- Wu, C.; Wang, A.Y.; Li, G.; Xu, T.; Zhang, H.; Zhao, Y.; Zhang, J.; Zhang, J.; Lin, J.; Wang, H. Association of high body mass index with the development of interstitial fibrosis in patients with IgA nephropathy. BMC Nephrol. 2018, 19, 381. [Google Scholar] [CrossRef]
- Berthoux, F.; Mariat, C.; Maillard, N. Overweight/obesity re-examined as a predictive risk factor in primary IgA nephropathy. Nephrol. Dial. Transplant. 2013, 28 (Suppl. S4), iv160–iv166. [Google Scholar] [CrossRef]
- Hong, Y.A.; Min, J.W.; Ha, M.A.; Kim, J.; Lee, M.J.; Han, S.H.; Kim, H.J.; Kang, S.W.; Choi, S.R.; Kim, C.H. The effect of obesity on the severity of clinicopathological parameters in patients with IgA nephropathy. J. Clin. Med. 2020, 9, 2824. [Google Scholar] [CrossRef]
- Wang, S.; Qin, A.; Dong, L.; Liu, X.; Xu, J.; Yang, D.; Zhang, J.; Zhao, Y. Association of obesity with the development of end-stage renal failure in patients with IgA nephropathy. Front. Endocrinol. 2023, 14, 1094534. [Google Scholar]
- Ariyasu, Y.; Torikoshi, K.; Tsukamoto, T.; Kinoshita, H.; Nakagawa, N.; Fujita, T.; Okada, H.; Ishida, T.; Kobayashi, S.; Moriyama, T. Analysis of the effect of obesity on the prognosis of IgA nephropathy depending on renal function and gender. Clin. Exp. Nephrol. 2024, 28, 1155–1167. [Google Scholar] [CrossRef]
- Arrizabalaga, P.; Solé, M.; Abellana, R.; Carrera, M.; Marti, J.; Botey, A. Renal expression of intercellular adhesion molecule-1 (ICAM-1) in mesangial nephropathy of IgA deposit. Med. Clin. 2001, 117, 321–325. [Google Scholar] [CrossRef]
- Nagaraju, S.P.; Rangaswamy, D.; Mareddy, A.S.; Reddy, K.S.; Prasad, N.S.; Raju, R.V. Effect of body mass index on the progression of primary immunoglobulin A nephropathy. Saudi J. Kidney Dis. Transplant. 2018, 29, 318–325. [Google Scholar] [CrossRef]
- Nakayamada, S.; Tanaka, Y. Therapy targeting BAFF and APRIL in systemic autoimmune diseases. Regen. Inflamm. 2016, 36, 6. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Do, M.S. Knockout BAFF improves systemic inflammation by regulating the distribution of body fat in obesity induced by a high-fat diet. Exp. Mol. Med. 2015, 47, e129. [Google Scholar] [CrossRef] [PubMed]
- Flower, V.R.; Reis, G.; Valera, I.C.; Santos, M.C.; Oliveira, R.A.; Silva, P.L.; Gomes, A.M.; Ferreira, J.R.; Lima, L.L.; Alves, L.F. Autoimmunity as a consequence of obesity and systemic inflammation. Front. Physiol. 2022, 13, 887702. [Google Scholar]
- Versini, M.; Jeandel, P.Y.; Rosenthal, E.; Shoenfeld, Y. Obesity in autoimmune diseases: Not a passive observer. Autoimmun. Rev. 2014, 13, 981–1000. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Chonchol, M. Renal disease associated with metabolic dysfunction: Pathogenesis and clinical signs. Kidney Int. 2025, 108, 194–200. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Liu, Z.; Wang, H.; Chen, X.; Zhang, W.; Li, G.; Zhang, J. Overlapping obesity-associated glomerulopathy and immunoglobulin A nephropathy: Clinical and pathological characteristics and prognosis. Clin. Exp. Nephrol. 2021, 25, 865–874. [Google Scholar] [PubMed]
- Koskinas, K.C.; Van Craenenbroeck, E.M.; Antoniades, C.; Chirinos, J.A.; Jankowski, P.; Jankowski, V.; Lavie, C.J.; Maloberti, A.; Mancia, G.; Nilsson, P.M.; et al. Obesity and cardiovascular disease: An ESC clinical consensus statement. Eur. Heart J. 2024, 45, 4063–4409. [Google Scholar]
- Yoshida, Y.; Hagiwara, Y.; Ito, M.; Tanaka, H.; Takahashi, K.; Saito, T.; Nakamura, H. Association of obesity, visceral fat accumulation and dyslipidemia with the risk of chronic kidney disease. Intern. Med. 2025, 64, 2823–2828. [Google Scholar] [CrossRef]
- Garofalo, C.; Borrelli, S.; Minutolo, R.; Chiodini, P.; De Nicola, L.; Conte, G. A systematic review and meta-analysis suggest that obesity predicts the onset of chronic kidney disease in the general population. Kidney Int. 2017, 91, 1224–1235. [Google Scholar] [CrossRef]
- Kotsis, V.; Martinez, F.; Trakatelli, C.; Rodriguez, P.; Mancia, G.; Redon, J.; Burnier, M. Effect of obesity on kidney disease. Nutrients 2021, 13, 4482. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Nagasawa, Y.; Yamamoto, R.; Shoji, T.; Hasuike, Y.; Yamamoto, S.; Inaba, M.; Nishizawa, Y. Serum uric acid levels predict the progression of IgA nephropathy in women, but not in men. PLoS ONE 2016, 11, e0160828. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yi, C.; Chang, T.; Zhang, H.; Li, X.; Yang, J.; Zhao, Y.; Wang, L. Weight gain promotes the progression of IgA nephropathy in Asians: A protocol for a systematic review and meta-analysis. Medicine 2022, 101, e31824. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Yamada, S.; Iwasaki, Y.; Takahashi, H.; Fukudome, K.; Tokumoto, M.; Tsuruya, K.; Hirakata, H. Effect of obesity on IgA nephropathy: A comparative ultrastructural study between obese and non-obese patients. Nephron Clin. Pract. 2009, 112, c71–c78. [Google Scholar] [CrossRef]
- Othman, M.; Kawar, B.; El Nahas, A.M. Effect of obesity on the progression of chronic kidney disease not associated with diabetes: A retrospective cohort study. Nephron Clin. Pract. 2009, 113, c16–c23. [Google Scholar] [CrossRef]
- Jv, M.; Zhang, J.; Han, Y.; Liu, X.; Zhao, D.; Chen, S.; Wang, L. The association of triglyceride–glucose index and body mass with left ventricular hypertrophy in patients with IgA nephropathy: A retrospective study. Eur. J. Med. Res. 2024, 29, 627. [Google Scholar] [PubMed]
- Qin, A.; Liu, X.; Yang, D.; Zhang, J.; Xu, J.; Wang, S.; Dong, L.; Zhao, Y. Triglyceride–glucose index can predict kidney survival in patients with IgA nephropathy. J. Clin. Med. 2022, 11, 5386. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Fernández-Real, J.M. The complement system is dysfunctional in metabolic disease: Evidence in plasma and adipose tissue from obese and insulin-resistant individuals. Semin. Cell Dev. Biol. 2019, 85, 164–172. [Google Scholar] [CrossRef]
- Cheung, C.K.; Barratt, J.; Liew, A.; Suzuki, H.; Zhao, M.H.; Novak, J.; Julian, B.A.; Wyatt, R.J.; Gharavi, A.G.; Rizk, D.V. The role of BAFF and APRIL in IgA nephropathy: Pathogenic mechanisms and targeted therapies. Front. Nephrol. 2023, 3, 1346769. [Google Scholar]
- Francisco, V.; Pino, J.; Campos-Cabaleiro, V.; Ruiz-Fernández, C.; Mera, A.; González-Gay, M.A.; Gómez, R.; Gualillo, O. Obesity, fat mass, and the immune system: The role of leptin. Front. Physiol. 2018, 9, 640. [Google Scholar] [CrossRef]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Polito, R.; Schettino, P.; Grandone, A.; Perrone, L.; Miraglia del Giudice, E.; Daniele, A. A new look at the role of adiponectin in obesity and obesity-related diseases. Biomed. Res. Int. 2014, 2014, 658913. [Google Scholar] [CrossRef]
- Forte, N.; Fernández-Rilo, A.C.; Palomba, L.; Di Marzo, V.; Cristino, L. Obesity affects the microbiota–gut–brain axis and its regulation by endocannabinoids and related mediators. Int. J. Mol. Sci. 2020, 21, 1554. [Google Scholar] [CrossRef]
- Shim, K.; Begum, R.; Yang, C.; Wang, H.; Kim, Y.; Hwang, H. Complement activation in obesity, insulin resistance, and type 2 diabetes. World J. Diabetes 2020, 11, 1–12. [Google Scholar]
- D’Agati, V.D.; Chagnac, A.; de Vries, A.P.; Levi, M.; Porrini, E.; Herman-Edelstein, M.; Praga, M. Obesity-associated glomerulopathy: Clinical and pathological characteristics and pathogenesis. Nat. Rev. Nephrol. 2016, 12, 453–471. [Google Scholar] [CrossRef]
- Yang, S.; Cao, C.; Deng, T.; Tan, Y.; Ding, L.; Yang, J.; Wang, H.; Zhang, J.; Liu, Z. Obesity-related glomerulopathy: A latent lesion in obesity requiring more attention. Ren. Blood Press. Res. 2020, 45, 510–522. [Google Scholar]
- Thomsen, S.B.; Gjesing, A.P.; Rathcke, C.N.; Vestergaard, H.; Pedersen, O.; Hansen, T.; Linneberg, A.; Jørgensen, T.; Christiansen, M.; Jeppesen, J.L. Association of the YKL-40 inflammatory marker with measures of obesity and dyslipidemia in individuals at high risk of type 2 diabetes. PLoS ONE 2015, 10, e0133672. [Google Scholar] [CrossRef]
- Ma, X.; Liang, Y.; Chen, W.; Zheng, L.; Lin, H.; Zhou, T. The role of endothelin receptor antagonists in kidney diseases. Ren. Fail. 2025, 47, 2465810. [Google Scholar] [CrossRef]
- Çebeci, E.; Çakan, C.; Gursu, M.; Uzun, S.; Karadag, S.; Koldas, M.; Calhan, T.; Helvaci, S.A.; Ozturk, S. The main determinants of serum resistin concentration in patients with type 2 diabetes are kidney function and inflammation, rather than the presence of microvascular complications, obesity and insulin resistance. Exp. Clin. Endocrinol. Diabetes 2019, 127, 189–194. [Google Scholar] [PubMed]
- Bae, E.H.; Oh, T.R.; Suh, S.H.; Kim, C.S.; Ma, S.K.; Kim, S.W. Underweight and weight change increases the risk of end-stage renal failure in patients with diabetes: A nationwide population-based cohort study. Nutrients 2021, 14, 154. [Google Scholar] [PubMed]
- Engelsen, S.J.; van Greevenbroek, M.M.; Stehouwer, C.D.; Schalkwijk, C.G.; Henry, R.M.; Ferreira, I. High-sensitivity C-reactive protein for detecting metabolic syndrome in centrally obese populations: A cross-sectional analysis. Cardiovasc. Diabetol. 2012, 11, 25. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Zhou, H.; Zhang, L.; Chen, R.; Liu, Y.; Wu, J.; Huang, Z.; Zhang, Y.; Sun, H. Association between weight-adjusted waist index and chronic kidney disease: A cross-sectional study. BMC Nephrol. 2023, 24, 266. [Google Scholar]

| Reference | Type of Study | Conclusion |
|---|---|---|
| Ouyang Y, et al. [12] | Cohort | Underweight increases the risk of kidney failure by 17.3% compared to 9.5% in obese people |
| Wang Q i wsp. [13] | Meta-Analyze | A high BMI increases the risk of kidney side effects (OR 2.43) |
| Kataoka H, et al. [17] | Cohort | BMI ≥ 25 kg/m2: independent predictor of creatinine growth (OR 7.4) |
| Shimamoto M, et al. [18] | Cohort | Obesity delays proteinuria remission by >5 years |
| Wu C, et al. [19] | Cohort | Overweight/obesity increases the risk of interstitial fibrosis (OR 2.28–3.43) |
| Berthoux F, et al. [20] | Cohort | High BMI: worse long-term outcomes |
| Hong YA, et al. [21] | Cohort | Obesity independently associated with severe mesangial matrix expansion |
| Wang S, et al. [22] | Cohort | High BMI: 6.01 mL/min/1.73 m2 lower eGFR |
| Ariyasu Y, et al. [23] | Cohort | Obesity contributes to the atrophy of the ear canals |
| Nagasawa Y, et al. [37] | Cohort | Uric acid in women increases the risk of disease progression (OR 1.33) |
| Liu M, i wsp. [38] | Meta-Analyze | Hyperuricemia + Vascular Damage: Synergistic Effects on Progression |
| Combination | Quantitative Data | Source |
|---|---|---|
| BMI + mesangial matrix expansion | Obesity independently associated (p = 0.020) | [21] |
| BMI + interstitial fibrosis | OR 2.28 (overweight) OR 3.43 (obesity) | [19] |
| BMI + metabolic factors | Remission of proteinuria delayed >5 years | [18] |
| BMI + histological parameters | 26.0 MATRIX for BMI ≥ 25 + M1 + Max GA ≥ 42,900 μm2 | [17] |
| BMI + Global Optical Score | WMD 1.22 (95% CI 0.70–1.74) higher in obese subjects | [13] |
| BMI + hypertension/proteinuria | Worse event-free survival (p < 0.0001) | [20] |
| BMI + GBM thickness | Significantly increased in obese subjects (p < 0.001) | [39] |
| BMI + hyperuricemia | 64.4% vs. 37% and freezes compared to normal | [22] |
| BMI + hypertriglyceridemia | 71.3% vs. 32.5% and freezes compared to normal | [22] |
| BMI + atrophy of the combat ducts | Obesity contributes to the decline of kidney function | [23] |
| BMI + progression of CKD | 79.5% vs. 44.7% progression in obesity vs. normal | [40] |
| BMI + TyG-BMI | OR 8.39 (95% CI 1.66–42.39) for LVH | [41] |
| Parameter | MetS (+) | MetS (−) | Difference | Source |
|---|---|---|---|---|
| Age (years) | 38–42 | 32–35 | +5–7 years | [10,11] |
| BMI (kg/m2) | 26–30 | 22–24 | +15–25% | [10,11] |
| eGFR (mL/min/1.73 m2) | 70–80 | 85–95 | −15–20% | [10,11] |
| Hypertension (%) | 70–80% | 30–40% | +40% | [10,11] |
| Proteinuria (g/day) | 1.5–2.0 | 0.8–1.0 | +50–100% | [10,11] |
| Renal survival | Worse | Better | HR 2.0–3.0 (ESRD risk) | [10,11,42] |
| Cardiovascular events | 23/65 | 15/60 | +8 events | [10] |
| Number of metabolic components | Higher | Dolny | Increased risk of events | [12] |
| Dyslipidemia (%) | 71.3% | 32.5% | +38.8% | [22] |
| Diabetes (%) | Greater prevalence | Lower prevalence | Increased risk of events | [10] |
| Albuminuria | Higher | Lower | Increased | [10] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skibicka, A.; Małgorzewicz, S. Visceral Obesity and Metabolic Dysfunction in IgA Nephropathy: Nutritional and Metabolic Perspectives on Disease Progression. Nutrients 2025, 17, 3307. https://doi.org/10.3390/nu17203307
Skibicka A, Małgorzewicz S. Visceral Obesity and Metabolic Dysfunction in IgA Nephropathy: Nutritional and Metabolic Perspectives on Disease Progression. Nutrients. 2025; 17(20):3307. https://doi.org/10.3390/nu17203307
Chicago/Turabian StyleSkibicka, Agnieszka, and Sylwia Małgorzewicz. 2025. "Visceral Obesity and Metabolic Dysfunction in IgA Nephropathy: Nutritional and Metabolic Perspectives on Disease Progression" Nutrients 17, no. 20: 3307. https://doi.org/10.3390/nu17203307
APA StyleSkibicka, A., & Małgorzewicz, S. (2025). Visceral Obesity and Metabolic Dysfunction in IgA Nephropathy: Nutritional and Metabolic Perspectives on Disease Progression. Nutrients, 17(20), 3307. https://doi.org/10.3390/nu17203307