Impact of Nutritional Status on Severe Radiation-Induced Mucositis in Oropharyngeal Cancer Patients Undergoing Chemo-Radiotherapy
Abstract
1. Introduction
2. Materials and Methods
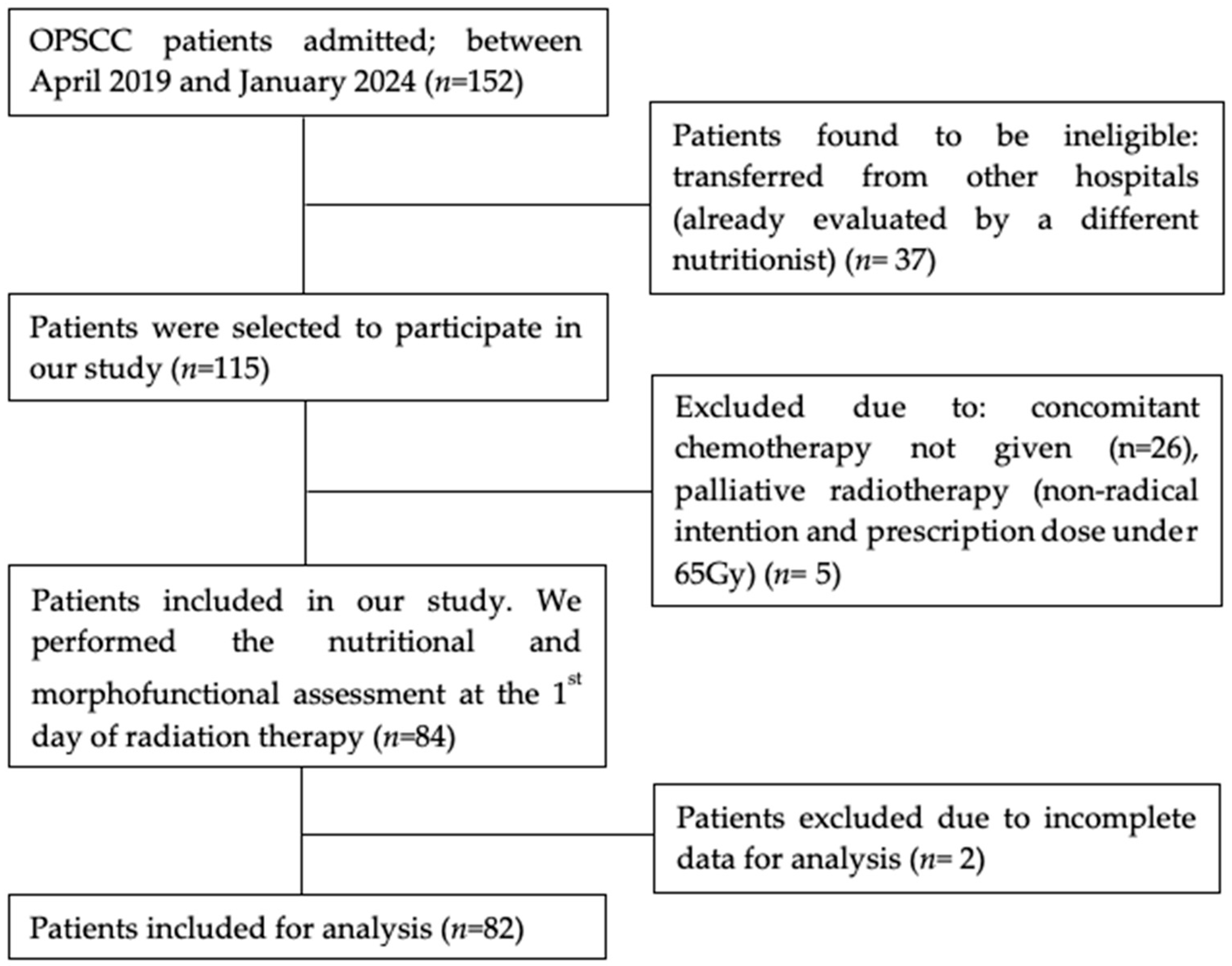
2.1. Participants
2.2. Study Design
2.3. Nutritional Assessment
2.3.1. Bioimpedance Assessments
Bioelectrical Impedance Analysis (BIA)
2.3.2. Functional Assesments
Hand Grip Strengh (HGS)
Timed up and Go (TUG)
Rectus Femoris Quadriceps Evaluation
2.3.3. Global Leadership Initiative on Malnutrition (GLIM) Criteria
2.4. Radiotherapy and Chemotherapy
2.4.1. Radiotherapy
2.4.2. Chemotherapy
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Nutritional and Morphofunctional Assessment
3.3. Association of Baseline Nutritional and Morphofunctional Status with Severe RIM
3.4. Association Between Baseline Characteristics and Severe Malnutrition
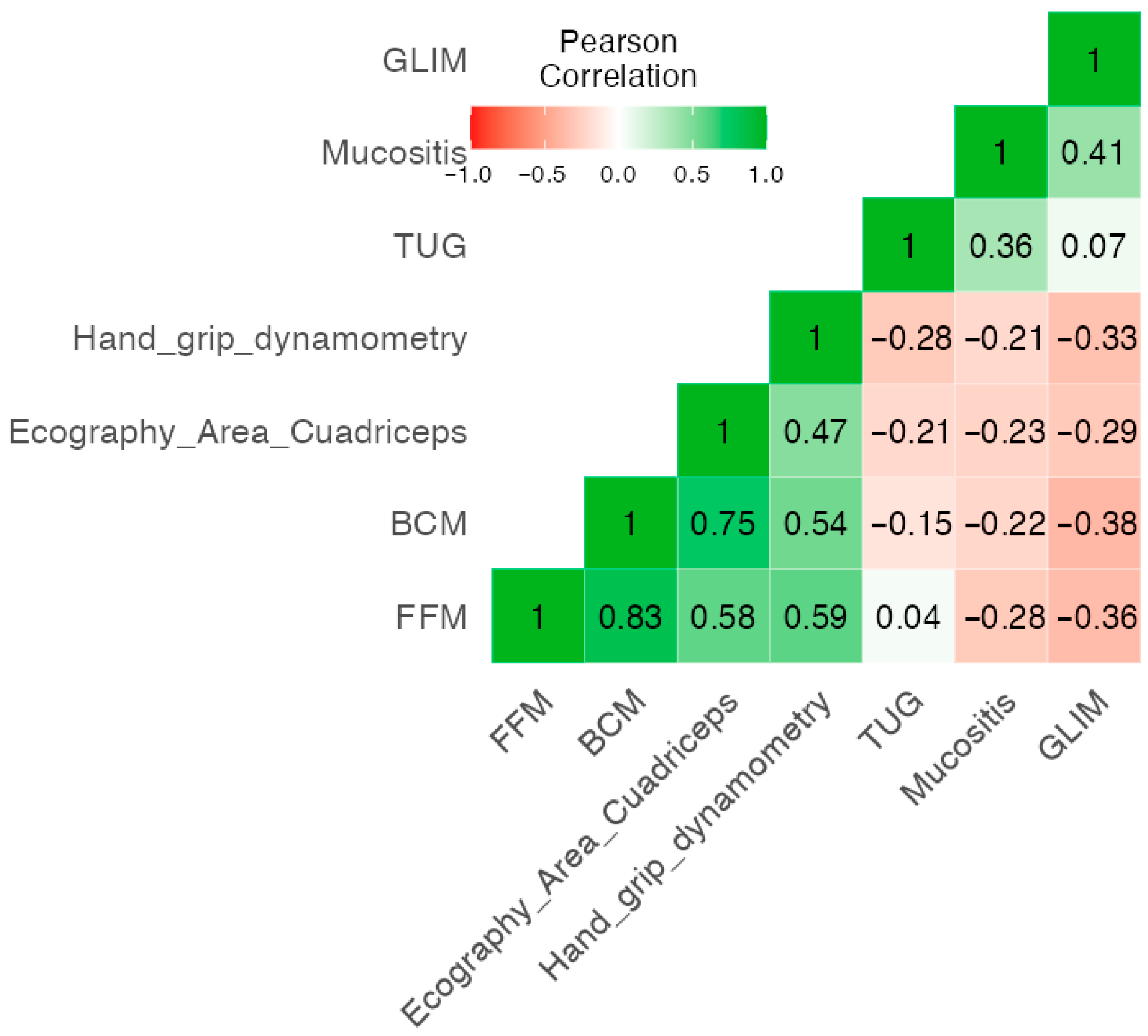
3.5. Correlation of GLIM, BIVA Parameters, Functional Status and RIM
3.6. Performance of Dosimetric Analysis, GLIM Criteria, BIVA Parameters and Functional Status to Predict Severe RIM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OPSCC | Oropharyngeal squamous cell carcinoma |
| HPV | Human papillomavirus |
| CRT | Chemo-radiotherapy |
| RIM | Radiation-induced mucositis |
| ROS | Reactive oxygen species |
| QoL | Quality of life |
| LAHNC | Locally advanced head and neck cancer |
| GLIM | Global Leadership Initiative on Malnutrition |
| HNC | Head and neck cancer |
| BIVA | Bioelectrical Impedance Vector Analysis |
| TUG | Timed up and go |
| HGS | Handgrip strength |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| FFM | Fat-free mass |
| BMI | Body mass index |
| FFMI | Fat-free mass index |
| NGT | Nasogastric tube |
| PEG | Percutaneous endoscopic gastrostomy. |
| CTCAE | Common Terminology Criteria for Adverse Events |
| SF-BIA | Single-frequency bioelectrical impedance |
| PhA | Phase angle |
| SPhA | Standardized phase angle |
| SD | Standard deviation |
| BIA | Bioelectrical impedance analysis |
| FM | Fat mass |
| BCM | Body cell mass |
| TBW | Total body water |
| ECW | Extracellular water |
| RF-CSA | Rectus femoris cross-sectional area |
| RF-CIR | Rectus femoris circumference |
| L-SAT | Subcutaneous fat of the leg |
| IMRT | Intensity-modulated radiation therapy |
| Gy | Gray |
| CDDP | Cisplatin |
| PTV | Planning tumor volume |
| ANOVA | Analysis of variance |
| ECOG | Eastern Cooperative Oncology Group |
| ROC | Receiver operating characteristic |
| PYI | Pack year index |
| CRP | C-reactive protein |
| SGA | Subjective global assessment |
References
- Zumsteg, Z.S.; Luu, M.; Rosenberg, P.S.; Elrod, J.K.; Bray, F.; Vaccarella, S.; Gay, C.; Lu, D.J.; Chen, M.M.; Chaturvedi, A.K.; et al. Global epidemiologic patterns of oropharyngeal cancer incidence trends. J. Natl. Cancer Inst. 2023, 115, 1544–1554. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Chaturvedi, A.K.; Alemany, L.; Anantharaman, D.; Bray, F.; Carrington, M.; Doorbar, J.; D’Souza, G.; Fakhry, C.; Ferris, R.L.; et al. Summary from an international seminar on HPV-positive oropharynx cancer. Oral Oncol. 2020, 108, 104736. [Google Scholar] [CrossRef]
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; I Haddad, R.; Saba, N.F. Head and neck cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; A Bhide, S.; Clark, C.; A Miles, E.; Miah, A.B.; Newbold, K.; Tanay, M.; et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011, 12, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef]
- Machtay, M.; Moughan, J.; Trotti, A.; Garden, A.S.; Weber, R.S.; Cooper, J.S.; Forastiere, A.; Ang, K.K. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J. Clin. Oncol. 2008, 26, 3582–3589. [Google Scholar] [CrossRef]
- Sankar, V.; Xu, Y. Oral complications from oropharyngeal cancer therapy. Cancers 2023, 15, 4548. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, H.R.; Overgaard, J.; Specht, L.; Overgaard, M.; Johansen, J.; Evensen, J.F.; Andersen, L.J.; Andersen, E.; Grau, C. Acute and late toxicity in accelerated radiotherapy: DAHANCA 6&7. Radiother. Oncol. 2012, 103, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Nishii, M.; Soutome, S.; Kawakita, A.; Yutori, H.; Iwata, E.; Akashi, M.; Hasegawa, T.; Kojima, Y.; Funahara, M.; Umeda, M.; et al. Factors associated with severe oral mucositis and candidiasis in patients undergoing radiotherapy for oral and oropharyngeal carcinomas: A retrospective multicenter study of 326 patients. Support. Care Cancer 2020, 28, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Maria, O.M.; Eliopoulos, N.; Muanza, T. Radiation-induced oral mucositis. Front. Oncol. 2017, 7, 89. [Google Scholar] [CrossRef]
- Forné, Á.F.; Anaya, M.J.G.; Guillot, S.J.S.; Andrade, I.P.; Fernández, L.d.l.P.; Ocón, M.J.L.; Pérez, Y.L.; Queipo-Ortuño, M.I.; Gómez-Millán, J. Influence of the microbiome on radiotherapy-induced oral mucositis and its management: A comprehensive review. Oral Oncol. 2023, 144, 106488. [Google Scholar] [CrossRef]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef]
- Liao, C.-T.; Chang, J.T.-C.; Wang, H.-M.; Ng, S.-H.; Hsueh, C.; Lee, L.-Y.; Lin, C.-H.; Chen, I.-H.; Huang, S.-F.; Cheng, A.-J.; et al. Early mucosal reactions during and after head-and-neck radiotherapy: Dependence of treatment tolerance on radiation dose and schedule duration. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 625–634. [Google Scholar] [CrossRef]
- Graboyes, E.M.; Kompelli, A.R.; Neskey, D.M.; Brennan, E.; Nguyen, S.; Sterba, K.R.; Warren, G.W.; Hughes-Halbert, C.; Nussenbaum, B.; Day, T.A. Association of treatment delays with survival for patients with head and neck cancer: A systematic review. JAMA Otolaryngol. Neck Surg. 2019, 145, 166–177. [Google Scholar] [CrossRef]
- Xiang, M.; Holsinger, F.C.; Colevas, A.D.; Chen, M.M.; Le, Q.; Beadle, B.M. Survival of patients with head and neck cancer treated with definitive radiotherapy and concurrent cisplatin or concurrent cetuximab: A Surveillance, Epidemiology, and End Results-Medicare analysis. Cancer 2018, 124, 4486–4494. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Su, L.; Lin, Q.; Liu, S.; Zhang, W.; Hong, J. Effect of nutritional status before radiotherapy on radiation-induced acute toxicities in patients with nasopharyngeal carcinoma. Head Neck 2023, 45, 620–628. [Google Scholar] [CrossRef]
- Ghaly, P.; Iliopoulos, J.; Ahmad, M. The role of nutrition in wound healing: An overview. Br. J. Nurs. 2021, 30, S38–S42. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, B.; Vadalà, M.; Laurino, C. Nutrition in wound healing: Investigation of the molecular mechanisms, a narrative review. J. Wound Care 2019, 28, 683–693. [Google Scholar] [CrossRef]
- Nazari, V.; Pashaki, A.S.; Hasanzadeh, E. The reliable predictors of severe weight loss during the radiotherapy of Head and Neck Cancer. Cancer Treat. Res. Commun. 2021, 26, 100281. [Google Scholar] [CrossRef]
- Steer, B.; Loeliger, J.; Edbrooke, L.; Deftereos, I.; Laing, E.; Kiss, N. Malnutrition prevalence according to the GLIM criteria in head and neck cancer patients undergoing cancer treatment. Nutrients 2020, 12, 3493. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Lu, T.; Liao, Z.; Rich, E.; Gong, X.; Lv, Q.; Li, J. Nutritional status and incidence of radiation-induced oral mucositis in nasopharyngeal carcinoma patients. Nutr. Cancer 2024, 76, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C.; Garcia-Almeida, J.M. Phase angle in applications of bioimpedance in health and disease. Rev. Endocr. Metab. Disord. 2023, 24, 367–370. [Google Scholar] [CrossRef]
- Garlini, L.M.; Alves, F.D.; Ceretta, L.B.; Perry, I.S.; Souza, G.C.; Clausell, N.O. Phase angle and mortality: A systematic review. Eur. J. Clin. Nutr. 2019, 73, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, S.; Huisman, M.G.; Ghignone, F.; Vigano, A.; Carino, N.d.L.; Farinella, E.; Girocchi, R.; Audisio, R.A.; van Munster, B.; de Bock, G.H.; et al. Timed up and go test and long-term survival in older adults after oncologic surgery. BMC Geriatr. 2022, 22, 934. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Gonzalez, M.C.; Schulzke, J.-D.; Pirlich, M. Hand grip strength: Outcome predictor and marker of nutritional status. Clin. Nutr. 2011, 30, 135–142. [Google Scholar] [CrossRef]
- Torralvo, F.J.S.; Porras, N.; Fernandez, J.A.; Torres, F.G.; Tapia, M.J.; Lima, F.; Soriguer, F.; Gonzalo, M.; Martínez, G.R.; Olveira, G.; et al. Normative reference values for hand grip dynamometry in Spain. Association with lean mass. Nutr. Hosp. 2018, 35, 98–103. [Google Scholar] [CrossRef]
- García-Almeida, J.M.; García-García, C.; Vegas-Aguilar, I.M.; Pomar, M.D.B.; Cornejo-Pareja, I.M.; Medina, B.F.; Román, D.A.d.L.; Guerrero, D.B.; Lesmes, I.B.; Madueño, F.J.T. Nutritional ultrasound®: Conceptualisation, technical considerations and standardisation. Endocrinol. Diabetes Nutr. 2023, 70, 74–84. [Google Scholar] [CrossRef]
- Sarhill, N.; Mahmoud, F.A.; Christie, R.; Tahir, A. Assessment of nutritional status and fluid deficits in advanced cancer. Am. J. Hosp. Palliat. Care 2003, 20, 465–473. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- García-Almeida, J.M.; García-García, C.; Ballesteros-Pomar, M.D.; Olveira, G.; Lopez-Gomez, J.J.; Bellido, V.; Lesmes, I.B.; Burgos, R.; Sanz-Paris, A.; Matia-Martin, P.; et al. Expert consensus on morphofunctional assessment in disease-related malnutrition. Nutrients 2023, 15, 612. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Zeng, Z.; Yu, B.; Huang, S.; Hua, Y.; Jin, T.; Tao, C.; Wang, L.; Cao, C.; Xu, Z.; et al. Nutritional status and its association with radiation-induced oral mucositis in patients with nasopharyngeal carcinoma during radiotherapy: A prospective study. Front. Oncol. 2020, 10, 594687. [Google Scholar] [CrossRef]
- Herrera-Martínez, A.D.; Prior-Sánchez, I.; Fernández-Soto, M.L.; García-Olivares, M.; Novo-Rodríguez, C.; González-Pacheco, M.; Martínez-Ramirez, M.J.; Carmona-Llanos, A.; Jiménez-Sánchez, A.; Muñoz-Jiménez, C.; et al. Improving the nutritional evaluation in head and neck cancer using bioelectrical impedance analysis: Not only the phase angle matters. J. Cachexia Sarcopenia Muscle 2024, 15, 2426–2436. [Google Scholar] [CrossRef]
- Celis-Morales, C.A.; Welsh, P.; Lyall, D.M.; Steell, L.; Petermann, F.; Anderson, J.; Iliodromiti, S.; Sillars, A.; Graham, N.; Mackay, D.F.; et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: Prospective cohort study of half a million UK Biobank participants. BMJ 2018, 361, k1651. [Google Scholar] [CrossRef]
- Lapornik, N.; Brumen, B.A.; Plavc, G.; Strojan, P.; Kozjek, N.R. Influence of fat-free mass index on survival in head and neck cancer. Eur. Arch. Otorhinolaryngol. 2023, 280, 1909–1917. [Google Scholar] [CrossRef]
- Löser, A.; Avanesov, M.; Thieme, A.; Gargioni, E.; Baehr, A.; Hintelmann, K.; Tribius, S.; Krüll, A.; Petersen, C. Nutritional status impacts quality of life in head and neck cancer patients: HEADNUT Trial. Nutr. Cancer 2022, 74, 2887–2895. [Google Scholar] [CrossRef]
- Liu, M.; Gao, F.; An, R.; Wu, Z.; Chen, W. Correlation analysis between prognostic nutritional index trajectory categories and radiotherapy-induced severe oral mucositis in head and neck cancer patients undergoing radiotherapy. Oral. Health Prev. Dent. 2024, 22, 671–680. [Google Scholar] [CrossRef]
- Fanetti, G.; Polesel, J.; Fratta, E.; Muraro, E.; Lupato, V.; Alfieri, S.; Gobitti, C.; Minatel, E.; Matrone, F.; Caroli, A.; et al. Prognostic nutritional index predicts toxicity in head and neck cancer patients treated with definitive radiotherapy in association with chemotherapy. Nutrients 2021, 13, 1277. [Google Scholar] [CrossRef] [PubMed]
- Alhambra Expósito, M.R.; Herrera-Martínez, A.D.; Manzano García, G.; Serrano, B. Early nutrition support therapy in patients with head-neck cancer. Nutr. Hosp. 2018, 35, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, Y.; Su, J.; Zhao, Q.; Wang, H.; Zheng, Z.; Wu, J.; Jiang, X. Effects of early nutritional intervention on oral mucositis and basic conditions in patients receiving radiotherapy for head and neck cancer: Randomized controlled trial (ChiCTR2000031418). Clin. Nutr. 2024, 43, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Cereda, E.; Caccialanza, R.; Pedrazzoli, P.; Tarricone, R.; Ciani, O. Cost-effectiveness of oral nutritional supplements with counselling in head and neck cancer. Cost. Eff. Resour. Alloc. 2021, 19, 35. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- National Cancer Institute (NCI); U.S. Department of Health & Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; National Cancer Institute: Bethesda, MD, USA, 2017. [Google Scholar]
- Sardinha, L.B.; Rosa, G.B.; Hetherington-Rauth, M.; Correia, I.R.; Magalhães, J.P.; Silva, A.M.; Lukaski, H. Development and validation of bioelectrical impedance prediction equations estimating regional lean soft tissue mass in middle-aged adults. Eur. J. Clin. Nutr. 2023, 77, 202–211. [Google Scholar] [CrossRef]
- Cardinal, T.R.; Wazlawik, E.; Bastos, J.L.; Nakazora, L.M.; Scheunemann, L. Standardized phase angle indicates nutritional status in hospitalized preoperative patients. Nutr. Res. 2010, 30, 594–600. [Google Scholar] [CrossRef]
- Piccoli, A.; Rossi, B.; Pillon, L.; Bucciante, G. A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney Int. 1994, 46, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A.; Nigrelli, S.; Caberlotto, A.; Bottazzo, S.; Rossi, B.; Pillon, L.; Maggiore, Q. Bivariate normal values of the bioelectrical impedance vector in adult and elderly populations. Am. J. Clin. Nutr. 1995, 61, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Montero-Benitez, M.Z.; Carmona-Llanos, A.; Fernández-Jiménez, R.; Román-Jobacho, A.; Gómez-Millán, J.; Modamio-Molina, J.; Cabrera-Cesar, E.; Vegas-Aguilar, I.; Amaya-Campos, M.d.M.; Tinahones, F.J.; et al. AI-assistance body composition CT at T12 and T4 in lung cancer: Diagnosing sarcopenia, and its correlation with morphofunctional assessment techniques. Cancers 2025, 17, 3255. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Bolonchuk, W.W.; Hall, C.B.; Siders, W.A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J. Appl. Physiol. 1986, 60, 1327–1332. [Google Scholar] [CrossRef]
- Dunbar, C.C.; Melahrinides, E.; Michielli, D.W.; Kalinski, M.I. Effects of small errors in electrode placement on body composition assessment by bioelectrical impedance. Res. Q. Exerc. Sport 1994, 65, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Jiménez, R.; García-Rey, S.; Roque-Cuéllar, M.C.; Fernández-Soto, M.L.; García-Olivares, M.; Novo-Rodríguez, M.; González-Pacheco, M.; Prior-Sánchez, I.; Carmona-Llanos, A.; Muñoz-Jiménez, C.; et al. Ultrasound muscle evaluation for predicting prognosis in head and neck cancer: A large-scale multicenter study. Nutrients 2024, 16, 387. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Brouwer, C.L.; Steenbakkers, R.J.; Bourhis, J.; Budach, W.; Grau, C.; Grégoire, V.; van Herk, M.; Lee, A.; Maingon, P.; Nutting, C.; et al. CT-based delineation of organs at risk in the head and neck region: Consensus guidelines. Radiother. Oncol. 2015, 117, 83–90. [Google Scholar] [CrossRef]
- Grégoire, V.; Evans, M.; Le, Q.-T.; Bourhis, J.; Budach, V.; Chen, A.; Eisbruch, A.; Feng, M.; Giralt, J.; Gupta, T.; et al. Delineation of primary tumour clinical target volumes in head and neck cancers: Consensus guidelines. Radiother. Oncol. 2018, 126, 3–24. [Google Scholar] [CrossRef]
- Biau, J.; Lapeyre, M.; Troussier, I.; Budach, W.; Giralt, J.; Grau, C.; Kazmierska, J.; Langendijk, J.A.; Ozsahin, M.; O’Sullivan, B.; et al. Selection of lymph node target volumes for definitive head and neck radiation therapy: A 2019 update. Radiother. Oncol. 2019, 134, 1–9. [Google Scholar] [CrossRef]
- Lee, N.; Harris, J.; Garden, A.S.; Straube, W.; Glisson, B.; Xia, P.; Bosch, W.; Morrison, W.H.; Quivey, J.; Thorstad, W.; et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: Radiation therapy oncology group phase II trial 0225. J. Clin. Oncol. 2009, 27, 3684–3690. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Zhang, Q.; Pfister, D.G.; Kim, J.; Garden, A.S.; Mechalakos, J.; Hu, K.; Le, Q.T.; Colevas, A.D.; Glisson, B.S.; et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): A phase 2 multi-institutional trial. Lancet Oncol. 2012, 13, 172–180. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, S.; Liu, T.; Sun, Y.; Li, S. Diagnostic performance of GLIM and PG-SGA for malnutrition assessment in adult cancer patients: A systematic review and meta-analysis. BMC Cancer 2025, 25, 765. [Google Scholar] [CrossRef]
- Wan, M.; Zhang, L.; Chen, C.; Zhao, D.; Zheng, B.; Xiao, S.; Liu, W.; Xu, X.; Wang, Y.; Zhuang, B.; et al. GLIM criteria-defined malnutrition informs on survival of nasopharyngeal carcinoma patients undergoing radiotherapy. Nutr. Cancer 2022, 74, 2920–2929. [Google Scholar] [CrossRef]
- Yip, C.; Dinkel, C.; Mahajan, A.; Siddique, M.; Cook, G.; Goh, V. Imaging body composition in cancer patients: Visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging 2015, 6, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Middelburg, J.G.; Mast, M.E.; de Kroon, M.; Jobsen, J.J.; Rozema, T.; Maas, H.; Baartman, E.A.; Geijsen, D.; van der Leest, A.H.; Bongard, D.J.v.D.; et al. G8 and TUG tests in relation to radiotherapy compliance and toxicity in elderly patients. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 843–849. [Google Scholar] [CrossRef] [PubMed]
- García-Anaya, M.; Segado-Guillot, S.; Cabrera-Rodríguez, J.; Serrano, T.; Medina-Carmona, J.; Gómez-Millán, J. Dose and volume de-escalation of radiotherapy in head and neck cancer. Crit. Rev. Oncol. Hematol. 2023, 186, 103994. [Google Scholar] [CrossRef] [PubMed]

| Variable | Category | N = 82 | (%) | |
|---|---|---|---|---|
|
Demographic characteristics | Sex | Male | 64 | (78) |
| Female | 18 | (22) | ||
| Age | <70 years | 62 | (75.6) | |
| ≥70 years | 20 | (24.4) | ||
| ECOG | 0 | 43 | (52.4) | |
| 1 | 39 | (47.6) | ||
| Smoking | No/stopped | 56 | (68.3) | |
| Yes | 26 | (31.7) | ||
| Cumulative smoking | <10 PYI | 14 | (17.1) | |
| ≥10 PYI | 68 | (82.9) | ||
| Alcohol | No/stopped | 40 | (48.7) | |
| Yes | 42 | (51.2) | ||
| Tumor characteristics | HPV status (p16) | No/Unknown | 45 | (54.9) |
| Yes | 37 | (45.1) | ||
| Primary stage | Tx | 1 | (1.2) | |
| T1 | 10 | (12.2) | ||
| T2 | 35 | (42.7) | ||
| T3 | 19 | (23.2) | ||
| T4 | 17 | (20.7) | ||
| Nodal stage | N0 | 9 | (11) | |
| N1 | 34 | (41.5) | ||
| N2 | 35 | (42.7) | ||
| N3 | 4 | (4.9) | ||
| TNM stage (AJCC 8°Ed) | I | 14 | (17.1) | |
| II | 17 | (20.7) | ||
| III | 23 | (28) | ||
| IV | 28 | (34.1) | ||
| Differentiation | I | 4 | (4.9) | |
| II | 50 | (61) | ||
| III | 28 | (34.1) | ||
| Treatment characteristics | Treatment option | CRT | 80 | (97.6) |
| Adjuvant CRT | 2 | (2.4) | ||
| Systemic therapy | Every 3 weeks | 66 | (80.5) | |
| Every week | 16 | (19.5) | ||
| Nutritional status and radiation therapy toxicity | GLIM status | Normonutrition | 51 | (62.2%) |
| Moderate malnutrition | 19 | (23.2%) | ||
| Severe malnutrition | 12 | (14.6%) | ||
| RIM (CTCAE V5.0) | Grade 0 | 0 | (0%) | |
| Grade 1 | 11 | (13.4%) | ||
| Grade 2 | 33 | (40.2%) | ||
| Grade 3 | 38 | (46.3%) |
| Nutritional Assessment | Variable | Mean (N = 82) | SD |
|---|---|---|---|
| Classic parameters | BMI (kg/m2) | 26.89 | 4.63 |
| Glucose (mg/dL) | 106.6 | 29.68 | |
| Albumin (g/dL) | 4 | 0.39 | |
| Prealbumin (mg/dL) | 27.89 | 5.97 | |
| CRP (mg/L) | 7.35 | 13.3 | |
| C-peptide (ng/mL) | 1.95 | 0.95 | |
| Bioimpedance analysis | PhA (°) | 5.33 | 1.02 |
| SPhA | −0.25 | 2.08 | |
| FFM (kg) | 53.78 | 8.04 | |
| FFMI (kg/m2) | 18.61 | 2.44 | |
| BCM (kg) | 26.89 | 5.66 | |
| Functional measurement | HGS (kg) | 35.62 | 9.38 |
| RF-CSA (cm2) | 3.86 | 1.01 | |
| TUG (seconds) | 7.7 | 2.84 |
| Variable | Category | Mild-Moderate RIM N = 44 (54%) | Severe RIM N = 38 (46%) | p Value |
|---|---|---|---|---|
| Active smoker | No | 33 (58.9) | 23 (41.1) | 0.16 |
| Yes | 11 (42.3) | 15 (57.7) | ||
| Cumulative smoking (PYI) | <10 | 10 (71.4) | 4 (28.6) | 0.14 |
| ≥10 | 34 (50) | 34 (50) | ||
| GLIM status | Normonutrition/ moderate malnutrition | 43 (61.4) | 27 (38.6) | 0.001 |
| Severe malnutrition | 1 (8.3) | 11 (91.7) | ||
| PTV65 (cm3) | 143.9 ± 77.2 | 181.6 ± 87.4 | 0.042 | |
| PTV54 (cm3) | 388.2 ± 112.4 | 448.3 ± 119.3 | 0.022 | |
| FFM (kg) | 55.9 ± 6.2 | 51.3 ± 9.2 | 0.047 | |
| FFMI (kg/m2) | 19.2 ± 1.4 | 17.9 ± 3.1 | 0.07 | |
| BCM (kg) | 28 ± 4.4 | 4.8 ± 6.6 | 0.04 | |
| RF-CSA (cm2) | 4.08 ± 0.94 | 3.45 ± 1.07 | 0.02 | |
| TUG (s) | 6.73 ± 1.63 | 8.73 ± 3.45 | 0.014 |
| Model 1 | |||
| Variable | p Value | OR | CI 95% |
| Age | 0.704 | 1.01 | 0.94–1.09 |
| Sex | 0.717 | 1.26 | 0.35–4.47 |
| PTV54 | 0.049 | 1.01 | 1.00–1.01 |
| GLIM | 0.011 | 16.79 | 2.76–328 |
| Model 2 | |||
| Variable | p Value | OR | CI 95% |
| Age | 0.54 | 1.04 | 0.91–1.21 |
| Sex | 0.34 | 0.28 | 0.01–3.42 |
| PTV54 | 0.031 | 1.01 | 1.00–1.03 |
| FFM | 0.006 | 0.74 | 0.58–0.89 |
| TUG | 0.025 | 1.83 | 1.20–3.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Forné, Á.; Fernández-Jiménez, R.; Toledo-Serrano, M.D.; Jiménez-Rodríguez, H.; Muñoz-Lupiáñez, M.; Ruiz-López, M.A.; García-Almeida, J.M.; De la Peña-Fernández, L.; Queipo-Ortuño, M.I.; Gómez-Millán, J. Impact of Nutritional Status on Severe Radiation-Induced Mucositis in Oropharyngeal Cancer Patients Undergoing Chemo-Radiotherapy. Nutrients 2025, 17, 3301. https://doi.org/10.3390/nu17203301
Fernández-Forné Á, Fernández-Jiménez R, Toledo-Serrano MD, Jiménez-Rodríguez H, Muñoz-Lupiáñez M, Ruiz-López MA, García-Almeida JM, De la Peña-Fernández L, Queipo-Ortuño MI, Gómez-Millán J. Impact of Nutritional Status on Severe Radiation-Induced Mucositis in Oropharyngeal Cancer Patients Undergoing Chemo-Radiotherapy. Nutrients. 2025; 17(20):3301. https://doi.org/10.3390/nu17203301
Chicago/Turabian StyleFernández-Forné, África, Rocío Fernández-Jiménez, María Dolores Toledo-Serrano, Herminda Jiménez-Rodríguez, Marina Muñoz-Lupiáñez, María Asunción Ruiz-López, José Manuel García-Almeida, Lourdes De la Peña-Fernández, María Isabel Queipo-Ortuño, and Jaime Gómez-Millán. 2025. "Impact of Nutritional Status on Severe Radiation-Induced Mucositis in Oropharyngeal Cancer Patients Undergoing Chemo-Radiotherapy" Nutrients 17, no. 20: 3301. https://doi.org/10.3390/nu17203301
APA StyleFernández-Forné, Á., Fernández-Jiménez, R., Toledo-Serrano, M. D., Jiménez-Rodríguez, H., Muñoz-Lupiáñez, M., Ruiz-López, M. A., García-Almeida, J. M., De la Peña-Fernández, L., Queipo-Ortuño, M. I., & Gómez-Millán, J. (2025). Impact of Nutritional Status on Severe Radiation-Induced Mucositis in Oropharyngeal Cancer Patients Undergoing Chemo-Radiotherapy. Nutrients, 17(20), 3301. https://doi.org/10.3390/nu17203301







