Can Vitamin D Reduce Glucocorticoid-Induced Adverse Effects in Patients with Giant Cell Arteritis? Results from 1568 Patients in the Spanish ARTESER Registry †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
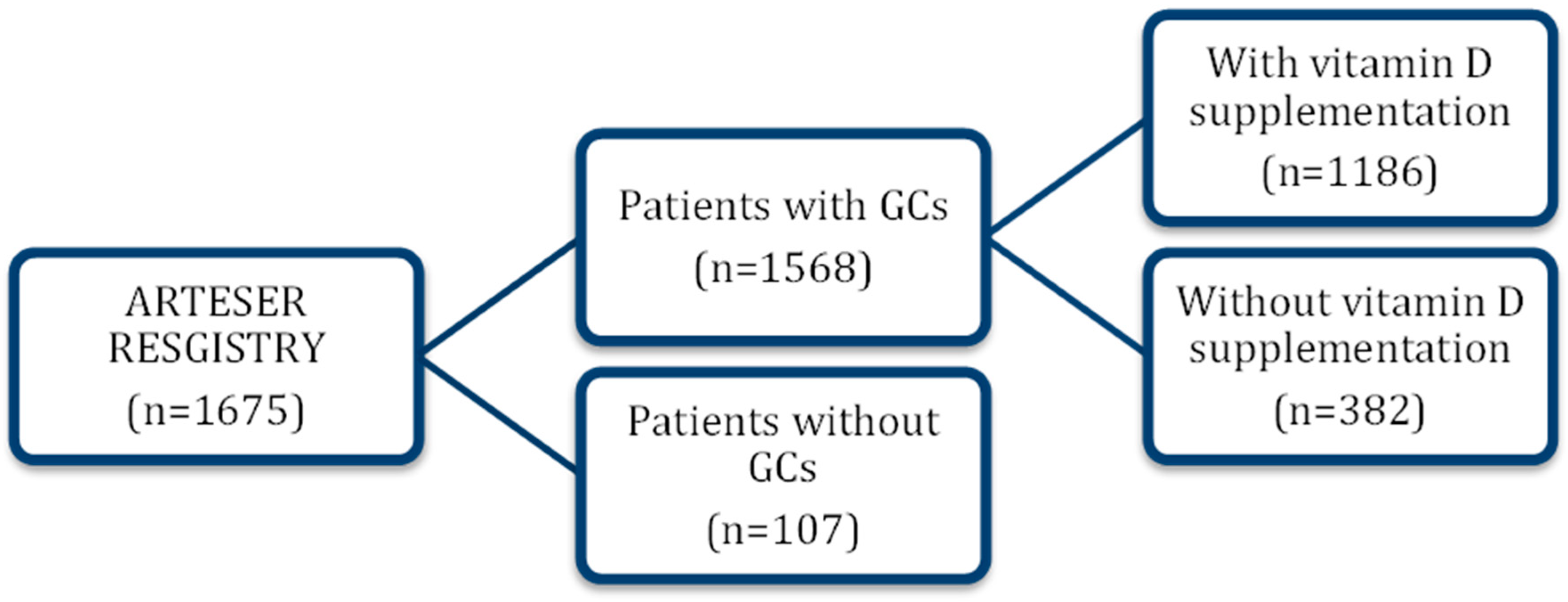
2.2. Patient Selection and Recruitment
2.3. Severe Adverse Events
2.4. Variables and Measurements
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Sociodemographic Characteristics
3.2. Incidence of Severe Adverse Events
3.3. Protective Role of Vitamin D Assessed by Cox Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schäcke, H.; Döcke, W.D.; Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002, 96, 23–46. [Google Scholar] [CrossRef]
- Buttgereit, F.; Palmowski, A. How to taper glucocorticoids in inflammatory rheumatic diseases? A narrative review of novel evidence in rheumatoid arthritis, systemic lupus erythematosus, and giant cell arteritis. Jt. Bone Spine 2022, 89, 105285. [Google Scholar] [CrossRef] [PubMed]
- Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 2018, 61, 7–16. [Google Scholar] [CrossRef]
- Alten, R.; Mischkewitz, M. New concepts to reduce glucocorticoid toxicity. Jt. Bone Spine 2019, 86, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Goglin, S.; Chung, S.A. New developments in treatments for systemic vasculitis. Curr. Opin. Pharmacol. 2022, 66, 102270. [Google Scholar] [CrossRef]
- Uppal, S.; Hadi, M.; Chhaya, S. Updates in the diagnosis and management of giant cell arteritis. Curr. Neurol. Neurosci. Rep. 2019, 19, 61. [Google Scholar] [CrossRef]
- Gérard, A.L.; Simon-Tillaux, N.; Yordanov, Y. Efficacy and safety of steroid-sparing treatments in giant cell arteritis according to the glucocorticoids tapering regimen: A systematic review and meta-analysis. Eur. J. Intern. Med. 2021, 88, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Sandovici, M.; Van der Geest, N.; Van Sleen, Y.; Brouwer, E. Need and value of targeted immunosuppressive therapy in giant cell arteritis. RMD Open 2022, 8, e001652. [Google Scholar] [CrossRef]
- Palmowski, A.; Buttgereit, F. Reducing the toxicity of long-term glucocorticoid treatment in large vessel vasculitis. Curr. Rheumatol. Rep. 2020, 22, 61. [Google Scholar] [CrossRef]
- Hellmich, B.; Águeda, A.F.; Monti, S.; Luqmani, R. Treatment of giant cell arteritis and Takayasu arteritis—Current and future. Curr. Rheumatol. Rep. 2020, 22, 84. [Google Scholar] [CrossRef]
- Hall, J.K.; Balcer, L.J. Giant cell arteritis. Curr. Treat. Options Neurol. 2004, 6, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Gysemans, C.; Korf, H.; Mathieu, C. Vitamin D insufficiency: Implications for the immune system. Pediatr. Nephrol. 2010, 25, 1597–1606. [Google Scholar] [CrossRef]
- Skversky, A.L.; Kumar, J.; Abramowitz, M.K.; Kaskel, F.J.; Melamed, M.L. Association of glucocorticoid use and low 25-hydroxyvitamin D levels: Results from the National Health and Nutrition Examination Survey (NHANES): 2001–2006. J. Clin. Endocrinol. Metab. 2011, 96, 3838–3845. [Google Scholar] [CrossRef]
- Altieri, B.; Muscogiuri, G.; Barrea, L. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev. Endocr. Metab. Disord. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Penna, G.; Adorini, L. 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000, 164, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Grubczak, K.; Lipinska, D.; Eljaszewicz, A. Vitamin D3 treatment decreases frequencies of CD16-positive and TNF-α-secreting monocytes in asthmatic patients. Int. Arch. Allergy Immunol. 2015, 166, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Sistanizad, M.; Kouchek, M.; Miri, M.M. High dose vitamin D improves total serum antioxidant capacity and ICU outcome in critically ill patients: A randomized, double-blind clinical trial. Eur. J. Integr. Med. 2021, 42, 101271. [Google Scholar] [CrossRef]
- Theiler-Schwetz, V.; Trummer, C.; Grübler, M.R. Effects of vitamin D supplementation on 24-hour blood pressure in patients with low 25-hydroxyvitamin D levels: A randomized controlled trial. Nutrients 2022, 14, 1360. [Google Scholar] [CrossRef]
- Karadeniz, Y.; Özpamuk-Karadeniz, F.; Ahbab, S.; Ataoğlu, E.; Can, G. Vitamin D deficiency is a potential risk for blood pressure elevation and the development of hypertension. Medicina 2021, 57, 1297. [Google Scholar] [CrossRef]
- Lee, C.J.; Hsieh, Y.J.; Lin, Y.L. Correlation between serum 25-hydroxyvitamin D level and peripheral arterial stiffness in chronic kidney disease stage 3–5 patients. Nutrients 2022, 14, 2429. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Gao, T. Is vitamin D deficiency a risk factor for all-cause mortality and rehospitalization in heart failure patients? A systematic review and meta-analysis. Medicine 2022, 101, e29507. [Google Scholar] [CrossRef] [PubMed]
- Hysa, E.; Balito, S.; Davoli, G. Vitamin D status and response to supplementation as predictive factors for early remission in polymyalgia rheumatica: A retrospective longitudinal investigation. Nutrients 2025, 17, 2839. [Google Scholar] [CrossRef]
- Agmon-Levin, N.; Theodor, E.; Segal, R.M. Vitamin D in systemic and organ-specific autoimmune diseases. Clin. Rev. Allergy Immunol. 2013, 45, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Souberbielle, J.C.; Body, J.J.; Lappe, J.M. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun. Rev. 2010, 9, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, K.; Fukasawa, H.; Fujigaki, Y. Protective effect of vitamins K2 and D3 on prednisolone-induced loss of bone mineral density in the lumbar spine. Am. J. Kidney Dis. 2004, 43, 786–795. [Google Scholar] [CrossRef]
- Ghio, G.; Domínguez-Álvaro, M.; Blanco-Alonso, R.; Castañeda, S.; Hernández-Rodríguez, I.; Fernández-Fernández, E.; Silva-Diaz, M.; María Belzunegui, J.; Moriano, C.; Sánchez-Martín, J.; et al. Vitamin D supplementation in the prevention of severe adverse effects caused by glucocorticoids: Study from the ARTESER Registry of Giant Cell Arteritis. In Proceedings of the ACR Convergence 2024, Washington, DC, USA, 14–19 November 2024; p. 1866415. [Google Scholar]
- Fernández-Lozano, D.; Hernández-Rodríguez, I.; Narvaez, J.; Domínguez-Álvaro, M.; De Miguel, E.; Silva-Díaz, M.; Belzunegui, J.M.; Morales, C.M.; Sánchez, J.; Galíndez-Agirregoikoa, E.; et al. Incidence and clinical manifestations of giant cell arteritis in Spain: Results of the ARTESER register. RMD Open 2024, 10, e003824. [Google Scholar] [CrossRef] [PubMed]
- Hunder, G.G.; Bloch, D.A.; Michel, B.A.; Stevens, M.B.; Arend, W.P.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y.; Lie, J.T.; et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990, 33, 1122–1128. [Google Scholar] [CrossRef]
- Montero-Pastor, N.; Sánchez-Costa, J.; Guerra-Rodríguez, M. Development of a web tool to calculate the cumulative dose of glucocorticoids. Reumatol. Clin. 2022, 18, 445–451. [Google Scholar] [CrossRef]
- Watts, R.A.; Hatemi, G.; Burns, J.C. Global epidemiology of vasculitis. Nat. Rev. Rheumatol. 2022, 18, 22–34. [Google Scholar] [CrossRef]
- Naranjo Hernández, A.; Díaz Del Campo Fontecha, P.; Aguado Acín, M.P. Recommendations by the Spanish Society of Rheumatology on osteoporosis. Reumatol. Clin. 2019, 15, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, M.B.; Russell, L.; Danila, M.I. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023, 75, 2088–2102. [Google Scholar] [CrossRef] [PubMed]
- Boulkrane, M.S.; Fedotova, J.; Kolodyaznaya, V. Vitamin D and depression in women: A mini-review. Curr. Neuropharmacol. 2019, 18, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Bivona, G.; Gambino, C.M.; Lo Sasso, B. Serum vitamin D as a biomarker in autoimmune, psychiatric and neurodegenerative diseases. Diagnostics 2022, 12, 130. [Google Scholar] [CrossRef] [PubMed]

| All Patients (n = 1568) | Not Taking Vitamin D (n = 382) | Taking Preventive Vitamin D (n = 1186) | p-Value | |
|---|---|---|---|---|
| Demographic data | ||||
| Women, n (%) | 1100 (70.2) | 257 (67.3) | 843 (71.1) | 0.158 |
| Age, mean (SD) | 76.9 (8.1) | 77.6 (8.2) | 76.6 (8.0) | 0.017 |
| Duration of symptoms (months), mean (SD) | 2.8 (5.5) | 2.2 (3.2) | 3.0 (6.0) | 0.044 |
| Comorbidity | ||||
| Smoking, n (%) | 123 (8.3) | 33 (9.0) | 90 (8.1) | 0.673 |
| Previous CVD, n (%) | 345 (22.0) | 110 (28.8) | 235 (19.8) | <0.001 |
| Hypertension, n (%) | 1014 (65.4) | 260 (69.3) | 754 (64.2) | 0.067 |
| Diabetes mellitus, n (%) | 333 (21.7) | 83 (22.6) | 250 (21.4) | 0.651 |
| Dyslipidemia, n (%) | 757 (49.2) | 192 (51.9) | 565 (48.3) | 0.227 |
| Osteoporosis, n (%) | 265 (16.9) | 35 (9.2) | 230 (19.4) | <0.001 |
| Obesity, n (%) | 143 (16.3) | 33 (16.3) | 110 (16.2) | 0.797 |
| Chronic kidney disease, n (%) | 158 (10.5) | 51 (11.4) | 117 (10.2) | 0.527 |
| Type of treatment | ||||
| Oral GC, n (%) | 1163 (74.2) | 306 (80.1) | 857 (72.3) | 0.001 |
| Intravenous GCs, n (%) | 3 (0.2) | 2 (0.5) | 1 (0.1) | 0.001 |
| Oral and intravenous GCs, n (%) | 402 (25.7) | 74 (19.4) | 328 (27.7) | 0.001 |
| Cumulative oral dose of GCs, mean (SD) | 7441.4 (4878.4) | 6716.1 (4784.4) | 7519.4 (4833.4) | <0.001 |
| All Patients (n = 1568) | Patients Without Vitamin D (n = 382) | Patients with Vitamin D (n = 1186) | p-Value | |
|---|---|---|---|---|
| Serious adverse events | ||||
| N (%) | 120 (7.7) | 31 (8.1) | 89 (7.5) | |
| Incidence rate (95%CI) | 0.039 (0.033–0.047) | 0.045 (0.031–0.064) | 0.038 (0.030–0.046) | 0.387 |
| Serious psychiatric adverse events | ||||
| N (%) | 23 (1.5) | 1 (0.3) | 22 (1.9) | |
| Incidence rate (95%CI) | 0.007 (0.005–0.011) | 0.0014 (0.000–0.008) | 0.009 (0.006–0.014) | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghio, G.A.; Domínguez-Álvaro, M.; Rodríguez, I.H.; Fernández-Fernández, E.; Silva-Díaz, M.; Belzunegui, J.M.; Moriano, C.; Martín, J.S.; Narváez, J.; Agirregoikoa, E.G.; et al. Can Vitamin D Reduce Glucocorticoid-Induced Adverse Effects in Patients with Giant Cell Arteritis? Results from 1568 Patients in the Spanish ARTESER Registry. Nutrients 2025, 17, 3291. https://doi.org/10.3390/nu17203291
Ghio GA, Domínguez-Álvaro M, Rodríguez IH, Fernández-Fernández E, Silva-Díaz M, Belzunegui JM, Moriano C, Martín JS, Narváez J, Agirregoikoa EG, et al. Can Vitamin D Reduce Glucocorticoid-Induced Adverse Effects in Patients with Giant Cell Arteritis? Results from 1568 Patients in the Spanish ARTESER Registry. Nutrients. 2025; 17(20):3291. https://doi.org/10.3390/nu17203291
Chicago/Turabian StyleGhio, Gastón A., Marta Domínguez-Álvaro, Iñigo Hernández Rodríguez, Elisa Fernández-Fernández, Maite Silva-Díaz, Joaquín M. Belzunegui, Clara Moriano, Julio Sánchez Martín, Javier Narváez, Eva Galíndez Agirregoikoa, and et al. 2025. "Can Vitamin D Reduce Glucocorticoid-Induced Adverse Effects in Patients with Giant Cell Arteritis? Results from 1568 Patients in the Spanish ARTESER Registry" Nutrients 17, no. 20: 3291. https://doi.org/10.3390/nu17203291
APA StyleGhio, G. A., Domínguez-Álvaro, M., Rodríguez, I. H., Fernández-Fernández, E., Silva-Díaz, M., Belzunegui, J. M., Moriano, C., Martín, J. S., Narváez, J., Agirregoikoa, E. G., Riveros Frutos, A., Ortiz Sanjuán, F., Salman Monte, T. C., Vasques Rocha, M., Iñiguez, C. L., García Dorta, A., Molina Almela, C., Alcalde Villar, M., Hernández, J. L., ... Blanco, R., on behalf of the ARTESER Project Collaborative Group. (2025). Can Vitamin D Reduce Glucocorticoid-Induced Adverse Effects in Patients with Giant Cell Arteritis? Results from 1568 Patients in the Spanish ARTESER Registry. Nutrients, 17(20), 3291. https://doi.org/10.3390/nu17203291







