Development of Iron-Chelating/Antioxidant Nutraceuticals and Natural Products as Pharmaceuticals for Clinical Use in Diseases with Free Radical Pathologies
Abstract
1. Introduction
2. The Development of Nutraceuticals for Use in Antioxidant Pharmaceuticals
3. Limitations of the Use of Iron-Chelating Drugs as Antioxidants for Clinical Use
4. Repurposing of Selected Nutraceuticals as Iron-Chelating Antioxidant Drugs in Medicine
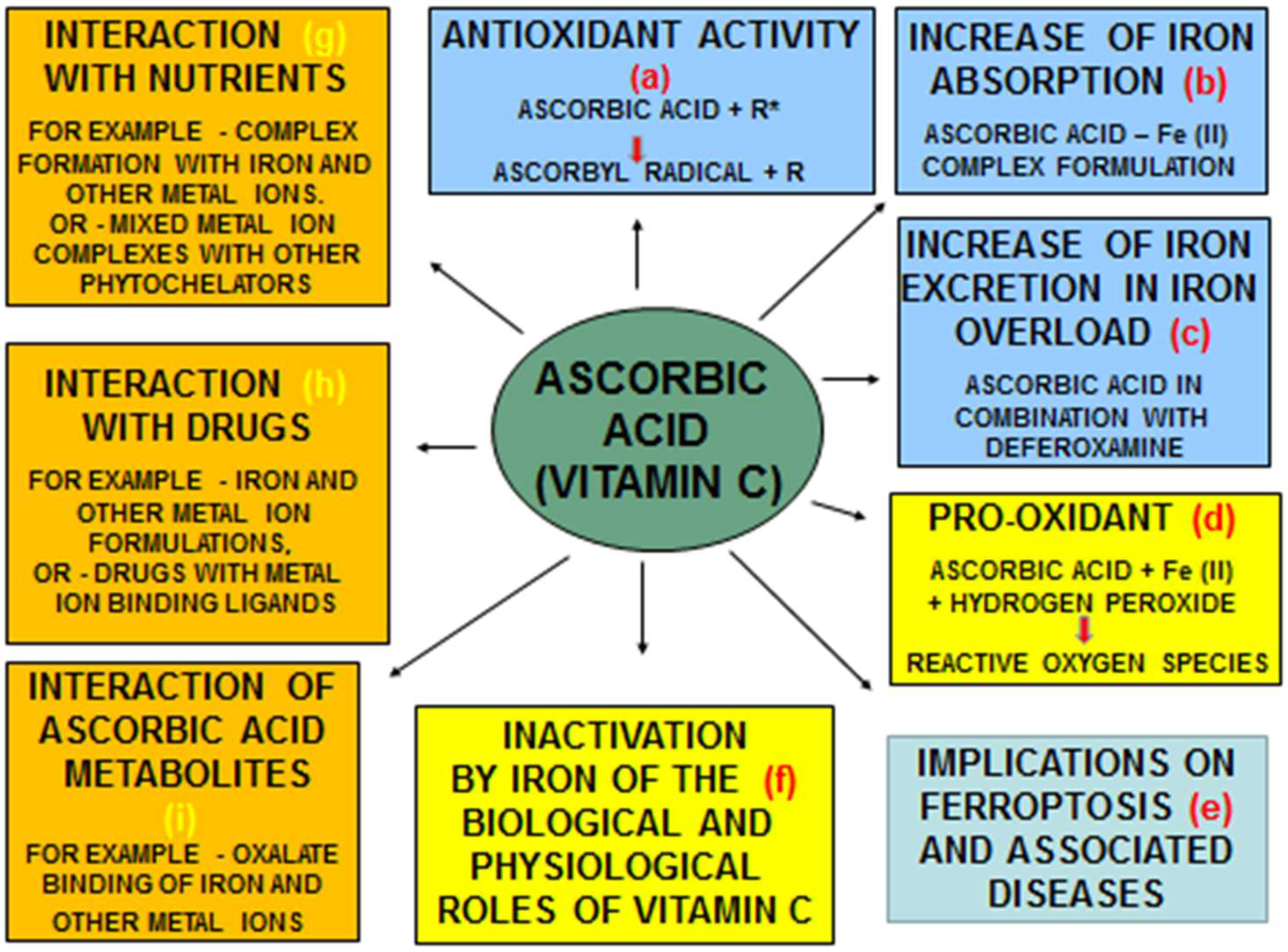
4.1. Antioxidant and Other Therapeutic Effects of Ascorbic Acid and Its Interactions with Iron
4.2. Efforts for the Development of Quercetin as Clinical Chelator/Antioxidant
4.3. Efforts for the Development of Curcumin as Clinical Chelator/Antioxidant
4.4. Clinical Advances with Maltol, Fisetin and Other Iron Chelating/Antioxidant Phytochelators
5. Future Prospects for the Clinical Development of Iron-Chelating/Antioxidant Nutraceuticals
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMET | absorption, distribution, metabolism, elimination and toxicity |
| DF | deferoxamine |
| DFRA | deferasirox |
| EDTA | ethylenediaminetetraacetic acid |
| FR | free radicals |
| iv | intravenous |
| L1 | deferiprone |
| LVEF | left-ventricular ejection fraction |
| MRI | magnetic resonance imaging |
| OST | oxidative stress toxicity |
| ROS | reactive oxygen species |
References
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar]
- AlAli, M.; Alqubaisy, M.; Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. Nutraceuticals: Transformation of Conventional Foods into Health Promoters/Disease Preventers and Safety Considerations. Molecules 2021, 26, 2540. [Google Scholar] [CrossRef]
- Chandra, S.; Saklani, S.; Kumar, P.; Kim, B.; Coutinho, H.D.M. Nutraceuticals: Pharmacologically Active Potent Dietary Supplements. Biomed. Res. Int. 2022, 2022, 2051017. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. New Approaches and Strategies for the Repurposing of Iron Chelating/Antioxidant Drugs for Diseases of Free Radical Pathology in Medicine. Antioxidants 2025, 14, 982. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Prospects for the introduction of targeted antioxidant drugs for the prevention and treatment of diseases related to free radical pathology. Expert. Opin. Investig. Drugs. 2019, 28, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.E.; Tooze, J.A.; Gahche, J.J.; AEicher-Miller, H.; Guenther, P.M.; Dwyer, J.T.; Potischman, N.; Bhadra, A.; Carroll, R.J.; Bailey, R.L. Trends in Overall and Micronutrient-Containing Dietary Supplement Use in US Adults and Children, NHANES 2007–2018. J. Nutr. 2022, 152, 2789–2801. [Google Scholar] [CrossRef]
- Imam, M.U.; Zhang, S.; Ma, J.; Wang, H.; Wang, F. Antioxidants Mediate Both Iron Homeostasis and Oxidative Stress. Nutrients 2017, 9, 671. [Google Scholar] [CrossRef]
- Sarkar, C.; Chaudhary, P.; Jamaddar, S.; Janmeda, P.; Mondal, M.; Mubarak, M.S.; Islam, M.T. Redox Activity of Flavonoids: Impact on Human Health, Therapeutics, and Chemical Safety. Chem. Res. Toxicol. 2022, 35, 140–162. [Google Scholar] [CrossRef]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef]
- Iakovou, E.; Kourti, M. A Comprehensive Overview of the Complex Role of Oxidative Stress in Aging, The Contributing Environmental Stressors and Emerging Antioxidant Therapeutic Interventions. Front. Aging Neurosci. 2022, 14, 827900. [Google Scholar] [CrossRef]
- Stangherlin, A.; Reddy, A.B. Regulation of circadian clocks by redox homeostasis. J. Biol. Chem. 2013, 288, 26505–26511. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J. Redox signaling: An evolution from free radicals to aging. Free Radic. Biol. Med. 2016, 97, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Hu, Q.; Li, X.; Wang, Z.; Xie, Y. The Influence of Circadian Rhythms on DNA Damage Repair in Skin Photoaging. Int. J. Mol. Sci. 2024, 25, 10926. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, J.A.; Vázquez-Medina, J.P. The Role of Peroxiredoxin 6 in Cell Signaling. Antioxidants 2018, 7, E172. [Google Scholar] [CrossRef]
- Pagano, G.; Talamanca, A.A.; Castello, G.; Cordero, M.D.; D’iSchia, M.; Gadaleta, M.N.; Pallardó, F.V.; Petrović, S.; Tiano, L.; Zatterale, A. Oxidative stress and mitochondrial dysfunction across broad-ranging pathologies: Toward mitochondria-targeted clinical strategies. Oxidative Med. Cell. Longev. 2014, 2014, 541230. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Black, H.S. Reassessment of a free radical theory of cancer with emphasis on ultraviolet carcinogenesis. Integr. Cancer Ther. 2004, 3, 279–293. [Google Scholar] [CrossRef]
- Flora, S.J.; Shrivastava, R.; Mittal, M. Chemistry and pharmacological properties of some natural and synthetic antioxidants for heavy metal toxicity. Curr. Med. Chem. 2013, 20, 4540–4574. [Google Scholar] [CrossRef]
- Pan, Z.; Gong, T.; Liang, P. Heavy Metal Exposure and Cardiovascular Disease. Circ. Res. 2024, 134, 1160–1178. [Google Scholar] [CrossRef]
- Marant-Micallef, C.; Shield, K.D.; Vignat, J.; Cléro, E.; Kesminiene, A.; Hill, C.; Rogel, A.; Vacquier, B.; Bray, F.; Laurier, D.; et al. The risk of cancer attributable to diagnostic medical radiation: Estimation for France in 2015. Int. J. Cancer 2019, 144, 2954–2963. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2005. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C.; Cross, C.E. Free radicals, antioxidants and human disease: Where are we now? J. Lab. Clin. Med. 1992, 119, 598–620. [Google Scholar] [PubMed]
- Chakraborty, P.; Dewanjee, S. Unrevealing the mechanisms behind the cardioprotective effect of wheat polyphenolics. Arch. Toxicol. 2024, 98, 3543–3567. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Phytochelators Intended for Clinical Use in Iron Overload, Other Diseases of Iron Imbalance and Free Radical Pathology. Molecules 2015, 20, 20841–20872. [Google Scholar] [CrossRef]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms-A Comprehensive Review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995, 41, 1819–1828. [Google Scholar] [CrossRef]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA damage and disease: Induction, repair and significance. Mutat. Res. Mol. Mech. Mutagen. 2004, 567, 1–61. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Chen, X.; Sun-Waterhouse, D.; Yao, W.; Li, X.; Zhao, M.; You, L. Free radical-mediated degradation of polysaccharides: Mechanism of free radical formation and degradation, influence factors and product properties. Food Chem. 2021, 365, 130524. [Google Scholar] [CrossRef]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Iron and Chelation in Biochemistry and Medicine: New Approaches to Controlling Iron Metabolism and Treating Related Diseases. Cells 2020, 9, 1456. [Google Scholar] [CrossRef] [PubMed]
- Pantopoulos, K.; Porwal, S.K.; Tartakoff, A.; Devireddy, L. Mechanisms of mammalian iron homeostasis. Biochemistry 2012, 51, 5705–5724. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Cao, J.Y.; Dixon, S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Li, M.; Luo, Z. Autophagy-Dependent Ferroptosis as a Therapeutic Target in Cancer. ChemMedChem 2021, 16, 2942–2950. [Google Scholar] [CrossRef]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef]
- Catapano, A.; Cimmino, F.; Petrella, L.; Pizzella, A.; D’ANgelo, M.; Ambrosio, K.; Marino, F.; Sabbatini, A.; Petrelli, M.; Paolini, B.; et al. Iron metabolism and ferroptosis in health and diseases: The crucial role of mitochondria in metabolically active tissues. J. Nutr. Biochem. 2025, 140, 109888. [Google Scholar] [CrossRef]
- Zhong, M.; Wang, Y.; Min, J.; Wang, F. Iron metabolism and ferroptosis in human health and disease. BMC Biol. 2025, 23, 263. [Google Scholar] [CrossRef]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Potential clinical applications of chelating drugs in diseases targeting transferrin-bound iron and other metals. Expert. Opin. Investig. Drugs 2013, 22, 591–618. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Iron mobilisation from lactoferrin by chelators at physiological pH. Biochim. Biophys. Acta 1986, 882, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P. New Advances in Iron Metabolism, Ferritin and Hepcidin Research. Int. J. Mol. Sci. 2022, 23, 14700. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. The Importance and Essentiality of Natural and Synthetic Chelators in Medicine: Increased Prospects for the Effective Treatment of Iron Overload and Iron Deficiency. Int. J. Mol. Sci. 2024, 25, 4654. [Google Scholar] [CrossRef]
- Wouters, O.J.; Denolle, C.; Wei, J.; Papanicolas, I. Prices and Affordability of Essential Medicines in 72 Low-, Middle-, and High-Income Markets. JAMA Health Forum 2025, 6, e252043. [Google Scholar] [CrossRef]
- Kontoghiorghe, C.N.; Andreou, N.; Constantinou, K.; Kontoghiorghes, G.J. World health dilemmas: Orphan and rare diseases, orphan drugs and orphan patients. World J. Methodol. 2014, 4, 163–188. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Drug Selection and Posology, Optimal Therapies and Risk/Benefit Assessment in Medicine: The Paradigm of Iron-Chelating Drugs. Int. J. Mol. Sci. 2023, 24, 16749. [Google Scholar] [CrossRef]
- Kontoghiorghe, C.N.; Kontoghiorghes, G.J. Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with non-transfusion-dependent thalassemia syndromes. Drug Des. Dev. Ther. 2016, 10, 465–481. [Google Scholar] [CrossRef]
- Klein, H.G.; Spahn, D.R.; Carson, J.L. Red blood cell transfusion in clinical practice. Lancet 2007, 370, 415–426. [Google Scholar] [CrossRef]
- Coates, T.D. Management of iron overload: Lessons from transfusion-dependent hemoglobinopathies. Blood 2025, 145, 359–371. [Google Scholar] [CrossRef]
- Iancu, T.C. Iron overload. Mol. Asp. Med. 1983, 6, 1–100. [Google Scholar] [CrossRef]
- Zurlo, M.; De Stefano, P.; Borgna-Pignatti, C.; Di Palma, A.; Melevendi, C.; Piga, A.; Di Gregorio, F.; Burattini, M.; Terzoli, S. Survival and causes of death in thalassaemia major. Lancet 1989, 334, 27–30. [Google Scholar] [CrossRef]
- Modell, B.; Khan, M.; Darlison, M. Survival in β-thalassaemia major in the UK: Data from the UK Thalassaemia Register. Lancet 2000, 355, 2051–2052. [Google Scholar] [CrossRef]
- Telfer, P.T.; Warburton, F.; Christou, S.; Hadjigavriel, M.; Sitarou, M.; Kolnagou, A.; Angastiniotis, M. Improved survival in thalassemia major patients on switching from desferrioxamine to combined chelation therapy with desferrioxamine and deferiprone. Haematologica 2009, 94, 1777–1778. [Google Scholar] [CrossRef] [PubMed]
- Kolnagou, A.; Kontoghiorghes, G.J. Advances in the prevention and treatment are changing thalassemia from a fatal to a chronic disease. experience from a Cyprus model and its use as a paradigm for future applications. Hemoglobin 2009, 33, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Modell, B.; Khan, M.; Darlison, M.; Westwood, M.A.; Ingram, D.; Pennell, D.J. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2008, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.; Filosa, A.; Vitrano, A.; Aloj, G.; Kattamis, A.; Ceci, A.; Fucharoen, S.; Cianciulli, P.; Grady, R.W.; Prossomariti, L.; et al. Iron chelation therapy in thalassemia major: A systematic review with meta-analyses of 1520 patients included on randomized clinical trials. Blood Cells Mol. Dis. 2011, 47, 166–175. [Google Scholar] [CrossRef]
- Au, W.Y.; Lee, V.; Lau, C.W.; Yau, J.; Chan, D.; Chan, E.Y.T.; Cheung, W.W.W.; Ha, S.Y.; Kho, B.; Lee, C.Y.; et al. A synopsis of current care of thalassaemia major patients in Hong Kong. Hong Kong Med. J. 2011, 17, 261–266. [Google Scholar]
- US Food and Drug Administration. Clinical Review: Exjade (Deferasirox, ICL-670). 2005. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021882_s000_Exjade_BioPharmr.pdf (accessed on 6 August 2025).
- Exjade (Deferasirox) Tablets for Oral Suspension [Prescribing Information]; Novartis Pharmaceutical Corporation: East Hanover, NJ, USA, 2011. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021882s010lbl.pdf (accessed on 1 December 2015).
- Kontoghiorghes, G.J.; Kolnagou, A.; Peng, C.T.; Shah, S.V.; Aessopos, A. Safety issues of iron chelation therapy in patients with normal range iron stores including thalassaemia, neurodegenerative, renal and infectious diseases. Expert. Opin. Drug Saf. 2010, 9, 201–206. [Google Scholar] [CrossRef]
- Chuang, G.T.; Tsai, I.J.; Tsau, Y.K.; Lu, M.Y. Transfusion-dependent thalassaemic patients with renal Fanconi syndrome due to deferasirox use. Nephrology 2015, 20, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Al-Khabori, M.; Bhandari, S.; Al-Huneini, M.; Al-Farsi, K.; Panjwani, V.; Daar, S. Side effects of Deferasirox Iron Chelation in Patients with Beta Thalassemia Major or Intermedia. Oman Med. J. 2013, 28, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Maximova, N.; Gregori, M.; Simeone, R.; Sonzogni, A.; Zanon, D.; Boz, G.; D’Antiga, L. Total body irradiation and iron chelation treatment are associated with pancreatic injury following pediatric hematopoietic stem cell transplantation. Oncotarget 2018, 9, 19543–19554. [Google Scholar] [CrossRef] [PubMed]
- Fucile, C.; Mattioli, F.; Marini, V.; Gregori, M.; Sonzogni, A.; Martelli, A.; Maximova, N. What is known about deferasirox chelation therapy in pediatric HSCT recipients: Two case reports of metabolic acidosis. Ther. Clin. Risk Manag. 2018, 14, 1649–1655. [Google Scholar] [CrossRef]
- Boelaert, J.R.; Fenves, A.Z.; Coburn, J.W. Deferoxamine therapy and mucormycosis in dialysis patients: Report of an international registry. Am. J. Kidney Dis. 1991, 18, 660–667. [Google Scholar] [CrossRef]
- Cases, A.; Kelly, J.; Sabater, F.; Torras, A.; Griño, M.C.; Lopez-Pedret, J.; Revert, L. Ocular and auditory toxicity in hemodialyzed patients receiving desferrioxamine. Nephron 1990, 56, 19–23. [Google Scholar] [CrossRef]
- Ioannides, A.S.; Panisello, J.M. Acute respiratory distress syndrome in children with acute iron poisoning: The role of intravenous desferrioxamine. Eur. J. Pediatr. 2000, 159, 158–159. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. The Vital Role Played by Deferiprone in the Transition of Thalassaemia from a Fatal to a Chronic Disease and Challenges in Its Repurposing for Use in Non-Iron-Loaded Diseases. Pharmaceuticals 2023, 16, 1016. [Google Scholar] [CrossRef]
- Chan, S.; Lian, Q.; Chen, M.P.; Jiang, D.; Ho, J.T.K.; Cheung, Y.F.; Chan, G.C. Deferiprone inhibits iron overload-induced tissue factor bearing endothelial microparticle generation by inhibition oxidative stress induced mitochondrial injury, and apoptosis. Toxicol. Appl. Pharmacol. 2018, 338, 148–158. [Google Scholar] [CrossRef]
- Eybl, V.; Caisová, D.; Koutenský, J.; Kontoghiorghes, G.J. Influence of iron chelators, 1,2-dialkyl-3-hydroxypyridin-4-ones, on the lipid peroxidation and glutathione level in the liver of mice. Arch. Toxicol. Suppl. 1991, 14, 185–187. [Google Scholar] [CrossRef]
- Sadrzadeh, S.M.; Nanji, A.A.; Price, P.L. The oral iron chelator, 1,2-dimethyl-3-hydroxypyrid-4-one reduces hepatic-free iron, lipid peroxidation and fat accumulation in chronically ethanol-fed rats. J. Pharmacol. Exp. Ther. 1994, 269, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Jackson, M.J.; Lunec, J. In vitro screening of iron chelators using models of free radical damage. Free. Radic. Res. Commun. 1986, 2, 115–124. [Google Scholar] [CrossRef]
- Seddiek, H.; Hanna, M.; Hamoud, A.E.M.; Elbaset, M.A.; Akabawy, A.M.A.; Kotb, M.Z.; Khalifa, M.M. Deferiprone ameliorates cisplatin induced peripheral neurotoxicity via ferritinophagy adjustment. Sci. Rep. 2025, 15, 4485. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.R.; Galanello, R.; Piga, A.; De Sanctis, V.; Tricta, F. Safety and effectiveness of long-term therapy with the oral iron chelator deferiprone. Blood 2003, 102, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Ceci, A.; Baiardi, P.; Felisi, M.; Cappellini, M.D.; Carnelli, V.; De Sanctis, V.; Galanello, R.; Maggio, A.; Masera, G.; Piga, A.; et al. The safety and effectiveness of deferiprone in a large-scale, 3-year study in Italian patients. Br. J. Haematol. 2002, 118, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Galanello, R. Deferiprone in the treatment of transfusion-dependent thalassemia: A review and perspective. Ther. Clin. Risk Manag. 2007, 3, 795–805. [Google Scholar] [PubMed]
- Mazza, P.; Amurri, B.; Lazzari, G.; Masi, C.; Palazzo, G.; Spartera, M.A.; Giua, R.; Sebastio, A.M.; Suma, V.; De Marco, S.; et al. Oral iron chelating therapy. A single center interim report on deferiprone (L1) in thalassemia. Haematologica 1998, 83, 496–501. [Google Scholar] [PubMed]
- Calvaruso, G.; Vitrano, A.; Di Maggio, R.; Lai, E.; Colletta, G.; Quota, A.; Gerardi, C.; Rigoli, L.C.; Sacco, M.; Pitrolo, L.; et al. Deferiprone versus deferoxamine in thalassemia intermedia: Results from a 5-year long-term Italian multicenter randomized clinical trial. Am. J. Hematol. 2015, 90, 634–638. [Google Scholar] [CrossRef]
- Agarwal, M.B. L1 arthropathy syndrome. J. Assoc. Physicians India 1994, 42, 929–930. [Google Scholar] [PubMed]
- Kolnagou, A.; Kleanthous, M.; Kontoghiorghes, G.J. Benefits and Risks in Polypathology and Polypharmacotherapy Challenges in the Era of the Transition of Thalassaemia from a Fatal to a Chronic or Curable Disease. Front. Biosci. 2022, 14, 18. [Google Scholar] [CrossRef]
- Varoni, E.M.; Lodi, G.; Iriti, M. Efficacy behind activity-phytotherapeutics are not different from pharmaceuticals. Pharm. Biol. 2015, 53, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, Á.; Briones, L.; Andrews, M.; Arredondo, M.; Olivares, M.; Brito, A.; Pizarro, F. Effect of phytic acid, tannic acid and pectin on fasting iron bioavailability both in the presence and absence of calcium. J. Trace Elem. Med. Biol. 2015, 30, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Moridani, M.Y.; O’Brien, P.J. Iron complexes of deferiprone and dietary plant catechols as cytoprotective superoxide radical scavengers. Biochem. Pharmacol. 2001, 62, 1579–1585. [Google Scholar] [CrossRef]
- Korkina, L.G.; Afanas’ev, I.B. Antioxidant and chelating properties of flavonoids. Adv. Pharmacol. 1997, 38, 151–163. [Google Scholar]
- Nkhili, E.; Loonis, M.; Mihai, S.; El Hajji, H.; Dangles, O. Reactivity of food phenols with iron and copper ions: Binding, dioxygen activation and oxidation mechanisms. Food Funct. 2014, 5, 1186–1202. [Google Scholar] [CrossRef]
- Fowler, A.A.; Truwit, J.D.; Hite, R.D.; Morris, P.E.; Dewilde, C.; Priday, A.; Fisher, B.; Thacker, L.R.; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients with Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2019, 322, 1261–1270. [Google Scholar] [CrossRef]
- Carr, A.C.; Rosengrave, P.C.; Bayer, S.; Chambers, S.; Mehrtens, J.; Shaw, G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care 2017, 21, 300. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jo, E.J.; Eom, J.S.; Mok, J.; Kim, M.H.; Kim, K.U.; Park, H.K.; Lee, M.K.; Lee, K. Combined vitamin C, hydrocortisone, and thiamine therapy for patients with severe pneumonia who were admitted to the intensive care unit: Propensity score-based analysis of a before-after cohort study. J. Crit. Care 2018, 47, 211–218. [Google Scholar] [CrossRef]
- Berenson, J.R.; Matous, J.; Swift, R.A.; Mapes, R.; Morrison, B.; Yeh, H.S. A phase I/II study of arsenic trioxide/bortezomib/ascorbic acid combination therapy for the treatment of relapsed or refractory multiple myeloma. Clin. Cancer Res. 2007, 13, 1762–1768. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Kolnagou, A.; Kontoghiorghe, C.N.; Mourouzidis, L.; Timoshnikov, V.A.; Polyakov, N.E. Trying to Solve the Puzzle of the Interaction of Ascorbic Acid and Iron: Redox, Chelation and Therapeutic Implications. Medicines 2020, 7, 45. [Google Scholar] [CrossRef]
- Weber, G.; von Wirén, N.; Hayen, H. Investigation of ascorbate-mediated iron release from ferric phytosiderophores in the presence of nicotianamine. Biometals 2008, 21, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.R.; Hasinoff, B.B. Iron supplements: A common cause of drug interactions. Br. J. Clin. Pharmacol. 1991, 31, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, V.; Pullakhandam, R.; Nair, K.M. Dietary ligands as determinants of iron-zinc interactions at the absorptive enterocyte. J. Food Sci. 2010, 75, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Petry, N.; Egli, I.; Zeder, C.; Walczyk, T.; Hurrell, R. Polyphenols and phytic acid contribute to the low iron bioavailability from common beans in young women. J. Nutr. 2010, 140, 1977–1982. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Redox Interactions of Vitamin C and Iron: Inhibition of the Pro-Oxidant Activity by Deferiprone. Int. J. Mol. Sci. 2020, 21, 3967. [Google Scholar] [CrossRef]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic Metals, Ascorbate and Free Radicals: Combinations to Avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef]
- Zümreoglu-Karan, B. The coordination chemistry of Vitamin C: An overview. Coord. Chem. Rev. 2006, 250, 2295–2307. [Google Scholar] [CrossRef]
- Macan, A.M.; Kraljević, T.G.; Raić-Malić, S. Therapeutic perspective of vitamin C and its derivatives. Antioxidants 2019, 8, 247. [Google Scholar] [CrossRef]
- Naidu, K.A. Vitamin C in human health and disease is still a mystery? An overview. Nutr. J. 2003, 2, 7. [Google Scholar] [CrossRef]
- Sharma, D.C.; Mathur, R. Correction of anemia and iron deficiency in vegetarians by administration of ascorbic acid. Indian J. Physiol. Pharmacol. 1995, 39, 403–406. [Google Scholar] [PubMed]
- Marik, P.E. Hydrocortisone, ascorbic acid and thiamine (HAT therapy) for the treatment of sepsis. focus on ascorbic acid. Nutrients 2018, 10, 1762. [Google Scholar] [CrossRef] [PubMed]
- Hager, D.N.; Hinson, J.S.; Rothman, R.E. Vitamin C for Sepsis and Acute Respiratory Failure. JAMA J. Am. Med. Assoc. 2020, 323, 791–792. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Sabri, S.M.; Hadi, R.W.; Prémusz, V.; Beregi, T. Melatonin and Vitamins: A Promising Combination to Augment Conventional Anticancer Therapies. Nutrients 2025, 17, 3120. [Google Scholar] [CrossRef]
- Loft, F.C.; Holse, C.; Aasvang, E.K.; Vester-Andersen, M.; Rasmussen, L.S.; Jørgensen, L.N.; Meyhoff, C.S. Effects of Hyperoxia and Antioxidants on Mortality, Hospital Admissions, and Myocardial Infarction After Noncardiac Surgery: 1-Year Follow-Up of a Randomized Controlled Trial. Acta Anaesthesiol. Scand. 2025, 69, e70118. [Google Scholar] [CrossRef]
- Fillenbaum, G.G.; Kuchibhatla, M.N.; Hanlon, J.T.; Artz, M.B.; Pieper, C.F.; Schmader, K.E.; Dysken, M.W.; Gray, S.L. Dementia and Alzheimer’s disease in community-dwelling elders taking vitamin C and/or vitamin E. Ann. Pharmacother. 2005, 39, 2009–2014. [Google Scholar] [CrossRef]
- Gokce, N.; Keaney, J.F.; Frei, B.; Holbrook, M.; Olesiak, M.; Zachariah, B.J.; Leeuwenburgh, C.; Heinecke, J.W.; Vita, J.A. Long-term ascorbic acid administration reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation 1999, 99, 3234–3240. [Google Scholar] [CrossRef]
- Chen, H.; Karne, R.J.; Hall, G.; Campia, U.; Panza, J.A.; Cannon, R.O.; Wang, Y.; Katz, A.; Levine, M.; Quon, M.J. High-dose oral vitamin C partially replenishes vitamin C levels in patients with Type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am. J. Physiol. Hear. Circ. Physiol. 2006, 290, H137–H145. [Google Scholar] [CrossRef]
- Carr, A.C. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit. Care 2020, 24, 133. [Google Scholar] [CrossRef]
- Van Gorkom, G.N.Y.; Lookermans, E.L.; Van Elssen, C.H.M.J.; Bos, G.M.J. The effect of vitamin C (Ascorbic acid) in the treatment of patients with cancer: A systematic review. Nutrients 2019, 11, 977. [Google Scholar] [CrossRef]
- Borst, P. Mega-dose vitamin C as therapy for human cancer? Proc. Natl. Acad. Sci. USA 2008, 105, 95. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Hoffer, L.J.; Levine, M. Intravenously administered vitamin C as cancer therapy: Three cases. Can. Med. Assoc. J. 2006, 174, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Assouline, S.; Miller, W.H. High-dose vitamin C therapy: Renewed hope or false promise? Can. Med. Assoc. J. 2006, 174, 956–957. [Google Scholar] [CrossRef] [PubMed]
- Mikirova, N.; Casciari, J.; Rogers, A.; Taylor, P. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J. Transl. Med. 2012, 10, 189. [Google Scholar] [CrossRef]
- Wang, L.; Sesso, H.D.; Glynn, R.J.; Christen, W.G.; Bubes, V.; Manson, J.A.E.; Buring, J.E.; Gaziano, J.M. Vitamin E and C supplementation and risk of cancer in men: Posttrial follow-up in the Physicians’ Health Study II randomized trial. Am. J. Clin. Nutr. 2014, 100, 915–923. [Google Scholar] [CrossRef]
- Vance, T.M.; Su, J.; Fontham, E.T.H.; Koo, S.I.; Chun, O.K. Dietary antioxidants and prostate cancer: A review. Nutr. Cancer 2013, 65, 793–801. [Google Scholar] [CrossRef]
- Ohno, S.; Ohno, Y.; Suzuki, N.; Soma, G.I.; Inoue, M. High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. Anticancer Res. 2009, 29, 809–815. [Google Scholar]
- Nielsen, T.K.; Højgaard, M.; Andersen, J.T.; Poulsen, H.E.; Lykkesfeldt, J.; Mikines, K.J. Elimination of ascorbic acid after high-dose infusion in prostate cancer patients: A pharmacokinetic evaluation. Basic. Clin. Pharmacol. Toxicol. 2015, 116, 343–348. [Google Scholar] [CrossRef]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef]
- Myriam, M.; Sabatier, M.; Steiling, H.; Williamson, G. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Br. J. Nutr. 2006, 96, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.R.; Cook, J.D. Interaction of vitamin C and iron. Ann. N. Y. Acad. Sci. 1980, 355, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Lőrincz, T.; Holczer, M.; Kapuy, O.; Szarka, A. The Interrelationship of Pharmacologic Ascorbate Induced Cell Death and Ferroptosis. Pathol. Oncol. Res. 2019, 25, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, S.; Zhang, L.; Cheng, X.; Yu, H.; Bao, J.; Lu, R. Vitamin C induces ferroptosis in anaplastic thyroid cancer cells by ferritinophagy activation. Biochem. Biophys. Res. Commun. 2021, 551, 46–53. [Google Scholar] [CrossRef]
- Zhong, B.; Zhao, L.; Yu, J.; Hou, Y.; Ai, N.; Lu, J.J.; Ge, W.; Chen, X. Exogenous iron impairs the anti-cancer effect of ascorbic acid both in vitro and in vivo. J. Adv. Res. 2023, 46, 149–158. [Google Scholar] [CrossRef]
- Vaishampayan, P.; Lee, Y. Redox-active vitamin C suppresses human osteosarcoma growth by triggering intracellular ROS-iron-calcium signaling crosstalk and mitochondrial dysfunction. Redox Biol. 2024, 75, 103288. [Google Scholar] [CrossRef]
- González-Montero, J.; Chichiarelli, S.; Eufemi, M.; Altieri, F.; Saso, L.; Rodrigo, R. Ascorbate as a Bioactive Compound in Cancer Therapy: The Old Classic Strikes Back. Molecules 2022, 27, 3818. [Google Scholar] [CrossRef]
- Patil, P.; Geevarghese, P.; Khaire, P.; Joshi, T.; Suryawanshi, A.; Mundada, S.; Pawar, S.; Farookh, A. Comparison of Therapeutic Efficacy of Ferrous Ascorbate and Iron Polymaltose Complex in Iron Deficiency Anemia in Children: A Randomized Controlled Trial. Indian J. Pediatr. 2019, 86, 1112–1117. [Google Scholar] [CrossRef]
- Chandra, J. Treating Iron Deficiency Anemia. Indian J. Pediatr. 2019, 86, 1085–1086. [Google Scholar] [CrossRef]
- Pachuta Węgier, L.; Kubiak, M.; Liebert, A.; Clavel, T.; Montagne, A.; Stennevin, A.; Roye, S.; Boudribila, A. Ferrous Sulfate Oral Solution in Young Children with Iron Deficiency Anemia. Pediatr. Int. 2020, 62, 820–827. [Google Scholar] [CrossRef]
- Pippard, M.J. Desferrioxamine-induced iron excretion in humans. Baillieres Clin. Haematol. 1989, 2, 323–343. [Google Scholar] [CrossRef]
- Hussain, M.A.M.; Green, N.; Flynn, D.M.; Hoffbrand, A.V. Effect of dose, time, and ascorbate on iron excretion after subcutaneous desferrioxamine. Lancet 1977, 1, 977–979. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Aldouri, M.A.; Hoffbrand, A.V.; Barr, J.; Wonke, B.; Kourouclaris, T.; Sheppard, L. Effective chelation of iron in β thalassaemia with the oral chelator 1,2-dimethyl-3-hydroxypyrid-4-one. Br. Med. J. (Clin. Res. Ed.) 1987, 295, 1509–1512. [Google Scholar] [CrossRef]
- Elalfy, M.S.; Saber, M.M.; Adly, A.A.M.; Ismail, E.A.; Tarif, M.; Ibrahim, F.; Elalfy, O.M. Role of vitamin C as an adjuvant therapy to different iron chelators in young β-thalassemia major patients: Efficacy and safety in relation to tissue iron overload. Eur. J. Haematol. 2016, 96, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Conte, D.; Brunelli, L.; Ferrario, L.; Mandelli, C.; Quatrini, M.; Velio, P.; Bianchi, P.A. Effect of ascorbic acid on desferrioxamine-induced urinary iron excretion in idiopathic hemochromatosis. Acta Haematol. 1984, 72, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Lamas, G.A.; Goertz, C.; Boineau, R.; Mark, D.B.; Rozema, T.; Nahin, R.L.; Lindblad, L.; Lewis, E.F.; Drisko, J.; Lee, K.L.; et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: The TACT randomized trial. JAMA 2013, 309, 1241–1250. [Google Scholar] [CrossRef]
- Lamas, G.A.; Boineau, R.; Goertz, C.; Mark, D.B.; Rosenberg, Y.; Stylianou, M.; Rozema, T.; Nahin, R.L.; Terry Chappell, L.; Lindblad, L.; et al. EDTA chelation therapy alone and in combination with oral high-dose multivitamins and minerals for coronary disease: The factorial group results of the Trial to Assess Chelation Therapy. Am. Hear. J. 2014, 168, 37–44.e5. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Chelators affecting iron absorption in mice. Arzneim.-Forsch. Drug Res. 1990, 40, 1332–1335. [Google Scholar]
- Hininger, I.; Waters, R.; Osman, M.; Garrel, C.; Fernholz, K.; Roussel, A.M.; Anderson, R.A. Acute prooxidant effects of vitamin C in EDTA chelation therapy and long-term antioxidant benefits of therapy. Free Radic. Biol. Med. 2005, 38, 1565–1570. [Google Scholar] [CrossRef]
- Mostert, L.J.; Van Dorst, J.A.L.M.; Koster, J.F.; van Eijk, H.G.; Kontoghiorghes, G.J. Free radical and cytotoxic effects of chelators and their iron complexes in the hepatocyte. Free Radic. Res. 1987, 3, 379–388. [Google Scholar] [CrossRef]
- Zhang, Y.; Balasooriya, H.; Sirisena, S.; Ng, K. The effectiveness of dietary polyphenols in obesity management: A systematic review and meta-analysis of human clinical trials. Food Chem. 2023, 404 Pt B, 134668. [Google Scholar] [CrossRef]
- Sweeney, M.; Burns, G.; Sturgeon, N.; Mears, K.; Stote, K.; Blanton, C. The Effects of Berry Polyphenols on the Gut Microbiota and Blood Pressure: A Systematic Review of Randomized Clinical Trials in Humans. Nutrients 2022, 14, 2263. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Walton, K.; Brodaty, H.; Chalton, K. Polyphenols and Diets as Current and Potential Nutrition Senotherapeutics in Alzheimer’s Disease: Findings from Clinical Trials. J. Alzheimer’s Dis. 2024, 101, S479–S501. [Google Scholar] [CrossRef] [PubMed]
- Tuong, W.; Walker, L.; Sivamani, R.K. Polyphenols as novel treatment options for dermatological diseases: A systematic review of clinical trials. J. Dermatol. Treat. 2015, 26, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, N.; Kakava, M.G.; Routsi, E.A.; Petsas, E.; Stavridis, N.; Freris, C.; Zoupanou, N.; Moschovou, K.; Kiriakidi, S.; Mavromoustakos, T. Quercetin: A Potential Polydynamic Drug. Molecules 2023, 28, 8141. [Google Scholar] [CrossRef]
- Kamal, R.; Paul, P.; Thakur, S.; Singh, S.K.; Awasthi, A. Quercetin in Oncology: A Phytochemical with Immense Therapeutic Potential. Curr. Drug Targets 2024, 25, 740–751. [Google Scholar] [CrossRef]
- Gugler, R.; Leschik, M.; Dengler, H.J. Disposition of quercetin in man after single oral and intravenous doses. Eur. J. Clin. Pharmacol. 1975, 9, 229–234. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Barnes, S.; Prasain, J.; D’Alessandro, T.; Arabshahi, A.; Botting, N.; Lila, M.A.; Jackson, G.; Janle, E.M.; Weaver, C.M. The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems. Food Funct. 2011, 2, 235–244. [Google Scholar] [CrossRef]
- Leite, N.B.; Martins, D.B.; Alvares, D.S.; Cabrera, M.P.D.S. Quercetin induces lipid domain-dependent permeability. Chem. Phys. Lipids 2022, 242, 105160. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, M. Studies on transition metal-quercetin complexes using electrospray ionization tandem mass spectrometry. Molecules 2015, 20, 8583–8594. [Google Scholar] [CrossRef] [PubMed]
- da Silva, W.M.B.; de Oliveira Pinheiro, S.; Alves, D.R.; de Menezes, J.E.S.A.; Magalhães, F.E.A.; Silva, F.C.O.; Silva, J.; Marinho, E.S.; de Morais, S.M. Synthesis of Quercetin-Metal Complexes, In Vitro and In Silico Anticholinesterase and Antioxidant Evaluation, and In Vivo Toxicological and Anxiolitic Activities. Neurotox. Res. 2020, 37, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.O.; Fatokun, D.I.; Ejidike, I.P.; Awolope, R.U.; Sanni, S.O. Quercetin Zinc and Iron Metal Complexes Protect against Sodium Arsenite Intoxication in the Hepato-Renal System of Wistar Rats via the Oxidative Stress Pathway. J. Toxicol. 2022, 2022, 6178261. [Google Scholar] [CrossRef] [PubMed]
- Bardestani, A.; Ebrahimpour, S.; Esmaeili, A.; Esmaeili, A. Quercetin attenuates neurotoxicity induced by iron oxide nanoparticles. J. Nanobiotechnology 2021, 19, 327. [Google Scholar] [CrossRef]
- Malacaria, L.; Corrente, G.A.; Beneduci, A.; Furia, E.; Marino, T.; Mazzone, G. A Review on Coordination Properties of Al(III) and Fe(III) toward Natural Antioxidant Molecules: Experimental and Theoretical Insights. Molecules 2021, 26, 2603. [Google Scholar] [CrossRef]
- Ravichandran, R.; Rajendran, M.; Devapiriam, D. Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem. 2014, 146, 472–478. [Google Scholar] [CrossRef]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2023, 80, 241–262. [Google Scholar] [CrossRef]
- El Hajji, H.; Nkhili, E.; Tomao, V.; Dangles, O. Interactions of quercetin with iron and copper ions: Complexation and autoxidation. Free. Radic. Res. 2006, 40, 303–320. [Google Scholar] [CrossRef]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin reduces blood pressure in hypertensive subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef]
- Kozhukhov, S.; Parkhomenko, A.; Lutay, Y.; Dovganych, N.; Study Investigators. Impact of quercetin in patients with myocardial infarction. A multicenter, randomized, and open-label pilot study. Hell. J. Cardiol. 2024, 76, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, F.; Vafa, M.; Ebrahimkhani, A.; Găman, M.A.; Sezavar Seyedi Jandaghi, S.H. Effects of quercetin supplementation on endothelial dysfunction biomarkers and depression in post-myocardial infarction patients: A double-blind, placebo-controlled, randomized clinical trial. Clin. Nutr. ESPEN 2023, 56, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Papakyriakopoulou, P.; Velidakis, N.; Khattab, E.; Valsami, G.; Korakianitis, I.; Kadoglou, N.P. Potential Pharmaceutical Applications of Quercetin in Cardiovascular Diseases. Pharmaceuticals 2022, 15, 1019. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Sharma, A.R.; Lee, Y.H.; Chatterjee, S.; Choi, Y.J.; Rajvansh, R.; Chakraborty, C.; Lee, S.S. Therapeutic Potential of Quercetin as an Antioxidant for Bone-Muscle-Tendon Regeneration and Aging. Aging Dis. 2024, 16, 1414–1437. [Google Scholar] [CrossRef]
- Farr, J.N.; Atkinson, E.J.; Achenbach, S.J.; Volkman, T.L.; Tweed, A.J.; Vos, S.J.; Ruan, M.; Sfeir, J.; Drake, M.T.; Saul, D.; et al. Effects of intermittent senolytic therapy on bone metabolism in postmenopausal women: A phase 2 randomized controlled trial. Nat. Med. 2024, 30, 2605–2612. [Google Scholar] [CrossRef]
- Bailly, A.R.; Hester, G.M.; Alesi, M.G.; Buresh, R.J.; Feito, Y.; Mermier, C.M.; Ducharme, J.B.; VanDusseldorp, T.A. Quercetins efficacy on bone and inflammatory markers, body composition, and physical function in postmenopausal women. J. Bone Miner. Metab. 2025, 43, 304–314. [Google Scholar] [CrossRef]
- Vaez, S.; Parivr, K.; Amidi, F.; Rudbari, N.H.; Moini, A.; Amini, N. Quercetin and polycystic ovary syndrome; inflammation, hormonal parameters and pregnancy outcome: A randomized clinical trial. Am. J. Reprod. Immunol. 2023, 89, e13644. [Google Scholar] [CrossRef]
- Wang, T.; Lu, S.Y.; Dan, L.; Sun, Y.; Fu, T.; Tian, L.; Chen, J. Higher Dietary Quercetin Intake Is Associated with Lower Risk of Adverse Outcomes among Individuals with Inflammatory Bowel Disease in a Prospective Cohort Study. J. Nutr. 2024, 154, 1861–1868. [Google Scholar] [CrossRef]
- Li, N.; Cui, C.; Xu, J.; Mi, M.; Wang, J.; Qin, Y. Quercetin intervention reduced hepatic fat deposition in patients with nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled crossover clinical trial. Am. J. Clin. Nutr. 2024, 120, 507–517. [Google Scholar] [CrossRef]
- Roy, D.; Kaur, P.; Ghosh, M.; Choudhary, D.; Rangra, N.K. The therapeutic potential of typical plant-derived compounds for the management of metabolic disorders. Phytother. Res. 2024, 38, 3986–4008. [Google Scholar] [CrossRef]
- Han, M.K.; Barreto, T.A.; Martinez, F.J.; Comstock, A.T.; Sajjan, U.S. Randomised clinical trial to determine the safety of supplementation in patients with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2020, 7, e000392. [Google Scholar] [CrossRef]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its Antioxidant Mechanism, Antibacterial Properties and Potential Application in Prevention and Control of Toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef]
- Nicolucci, C.; Rodrigues, F.C.; Marson, F.A.L.; Sciani, J.M. Potential use of quercetin in the reduction of craving in cocaine-dependent patients-A pilot clinical trial. Phytother. Res. 2024, 38, 1310–1312. [Google Scholar] [CrossRef]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456, Erratum in EBioMedicine 2020, 52, 102595. https://doi.org/10.1016/j.ebiom.2019.12.004. [Google Scholar] [CrossRef]
- Wissler Gerdes, E.O.; Misra, A.; Netto, J.M.E.; Tchkonia, T.; Kirkland, J.L. Strategies for late phase preclinical and early clinical trials of senolytics. Mech. Ageing Dev. 2021, 200, 111591. [Google Scholar] [CrossRef] [PubMed]
- Sajadi Hezaveh, Z.; Azarkeivan, A.; Janani, L.; Hosseini, S.; Shidfar, F. The effect of quercetin on iron overload and inflammation in β-thalassemia major patients: A double-blind randomized clinical trial. Complement. Ther. Med. 2019, 46, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yu, X.; Song, T.; Hou, X. Safety and efficacy of antioxidant therapy in children and adolescents with attention deficit hyperactivity disorder: A systematic review and network meta-analysis. PLoS ONE 2024, 19, e0296926. [Google Scholar] [CrossRef]
- Veith, C.; Drent, M.; Bast, A.; van Schooten, F.J.; Boots, A.W. The disturbed redox-balance in pulmonary fibrosis is modulated by the plant flavonoid quercetin. Toxicol. Appl. Pharmacol. 2017, 336, 40–48. [Google Scholar] [CrossRef]
- Vaiss, D.P.; Rodrigues, J.L.; Yurgel, V.C.; do Carmo Guedes, F., Jr.; da Matta, L.L.M.; Barros, P.A.B.; Vaz, G.R.; Dos Santos, R.N.; Matte, B.F.; Kupski, L.; et al. Curcumin and quercetin co-encapsulated in nanoemulsions for nasal administration: A promising therapeutic and prophylactic treatment for viral respiratory infections. Eur. J. Pharm. Sci. 2024, 197, 106766. [Google Scholar] [CrossRef]
- Boretti, A. Quercetin as a cancer chemopreventive or chemotherapeutic agent: Where we stand. Phytother. Res. 2023, 37, 1227–1231. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, H.; Li, X.; Zhou, S.; Song, X.; Ma, N.; Meng, M.; Chang, G.; Shen, X. Quercetin alleviates LPS/iE-DAP-induced liver injury by suppressing ferroptosis via regulating ferritinophagy and intracellular iron efflux. Redox. Biol. 2025, 81, 103557. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Wang, M.; Liu, X.; Luo, S.; Wang, X.; Yang, L.; Li, K.; Li, Y.; Wei, W.; Chen, H.; et al. Quercetin inhibits oligodendrocytes ferroptosis by blocking NCOA4-mediated ferritinophagy. Int. Immunopharmacol. 2025, 150, 114152. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gregorio, A.; Aranda-Rivera, A.K. Quercetin and Ferroptosis. Life 2023, 13, 1730. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2020, 28, 231–243. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, J.; Li, H.; Zhao, D.; Liu, Z.; Zhu, L.; Zhang, Z.; Peng, W. Quercetin: A promising therapy for diabetic encephalopathy through inhibition of hippocampal ferroptosis. Phytomedicine 2024, 126, 154887. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Hegde, M.; Parama, D.; Girisa, S.; Kumar, A.; Daimary, U.D.; Garodia, P.; Yenisetti, S.C.; Oommen, O.V.; Aggarwal, B.B. Role of Turmeric and Curcumin in Prevention and Treatment of Chronic Diseases: Lessons Learned from Clinical Trials. ACS Pharmacol. Transl. Sci. 2023, 6, 447–518. [Google Scholar] [CrossRef]
- Dehzad, M.J.; Ghalandari, H.; Nouri, M.; Askarpour, M. Antioxidant and anti-inflammatory effects of curcumin/turmeric supplementation in adults: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Cytokine 2023, 164, 156144. [Google Scholar] [CrossRef]
- Funk, J.L.; Schneider, C. Perspective on Improving the Relevance, Rigor, and Reproducibility of Botanical Clinical Trials: Lessons Learned From Turmeric Trials. Front. Nutr. 2021, 8, 782912. [Google Scholar] [CrossRef]
- Panknin, T.M.; Howe, C.L.; Hauer, M.; Bucchireddigari, B.; Rossi, A.M.; Funk, J.L. Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials. Int. J. Mol. Sci. 2023, 24, 4476. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Rapti, E.; Adamantidi, T.; Efthymiopoulos, P.; Kyzas, G.Z.; Tsoupras, A. Potential Applications of the Anti-Inflammatory, Antithrombotic and Antioxidant Health-Promoting Properties of Curcumin: A Critical Review. Nutraceuticals 2024, 4, 562–595. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Han, H.K. The effects of black pepper on the intestinal absorption and hepatic metabolism of drugs. Expert. Opin. Drug Metab. Toxicol. 2011, 7, 721–729. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, M.; Zong, J.; Zong, L.; Zhao, Z.; Wang, S.; Zhang, Z.; Han, M. Highly bioavailable curcumin preparation with a co-grinding and solvent-free process. Food Sci. Nutr. 2020, 8, 6415–6425. [Google Scholar] [CrossRef]
- Prasad, S.; DuBourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal-Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int. J. Mol. Sci. 2021, 22, 7094. [Google Scholar] [CrossRef]
- Mary, C.P.V.; Vijayakumar, S.; Shankar, R. Metal chelating ability and antioxidant properties of Curcumin-metal complexes—A DFT approach. J. Mol. Graph. Model. 2018, 79, 1–14. [Google Scholar] [CrossRef]
- Smirnova, E.; Moniruzzaman, M.; Chin, S.; Sureshbabu, A.; Karthikeyan, A.; Do, K.; Min, T. A Review of the Role of Curcumin in Metal Induced Toxicity. Antioxidants 2023, 12, 243. [Google Scholar] [CrossRef]
- Rainey, N.E.; Moustapha, A.; Saric, A.; Nicolas, G.; Sureau, F.; Petit, P.X. Iron chelation by curcumin suppresses both curcumin-induced autophagy and cell death together with iron overload neoplastic transformation. Cell Death Discov. 2019, 5, 150. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, M.; Wang, T.; Zhou, Y.; Sun, M.; Li, H.; Liu, Y.; Xu, A. The Differential Antagonistic Ability of Curcumin against Cytotoxicity and Genotoxicity Induced by Distinct Heavy Metals. Toxics 2023, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Ashar, B.H. Iron Deficiency Anemia Due to High-dose Turmeric. Cureus 2019, 11, e3858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chin, D.; Huebbe, P.; Frank, J.; Rimbach, G.; Pallauf, K. Curcumin may impair iron status when fed to mice for six months. Redox Biol. 2014, 2, 563–569. [Google Scholar] [CrossRef]
- Saeidnia, M.; Fazeli, P.; Erfani, M.; Nowrouzi-Sohrabi, P.; Tamaddon, G.; Karimi, M. The Effect of Curcumin on Iron Overload in Patients with Beta-Thalassemia Intermedia. Clin. Lab. 2022, 68, 545–550. [Google Scholar] [CrossRef]
- Eghbali, A.; Nourigheimasi, S.; Ghasemi, A.; Afzal, R.R.; Ashayeri, N.; Eghbali, A.; Khanzadeh, S.; Ghaffari, K. The effects of curcumin on hepatic T2*MRI and liver enzymes in patients with β-thalassemia major: A double-blind randomized controlled clinical trial. Front. Pharmacol. 2023, 14, 1284326. [Google Scholar] [CrossRef]
- Nasseri, E.; Mohammadi, E.; Tamaddoni, A.; Qujeq, D.; Zayeri, F.; Zand, H. Benefits of Curcumin Supplementation on Antioxidant Status in β-Thalassemia Major Patients: A Double-Blind Randomized Controlled Clinical Trial. Ann. Nutr. Metab. 2017, 71, 136–144. [Google Scholar] [CrossRef]
- Kose, T.; Sharp, P.A.; Latunde-Dada, G.O. Antioxidative Effects of Curcumin on Erastin-Induced Ferroptosis Through GPX4 Signalling. Gastrointest. Disord. 2025, 7, 4. [Google Scholar] [CrossRef]
- Yin, X.; Hou, X.; Feng, J. Role of Ferroptosis on Lung Epithelial Cells in Disease Progression and Treatment: A Review. Med. Sci. Monit. 2025, 31, e948226. [Google Scholar] [CrossRef]
- Tang, X.; Ding, H.; Liang, M.; Chen, X.; Yan, Y.; Wan, N.; Chen, Q.; Zhang, J.; Cao, J. Curcumin induces ferroptosis in non-small-cell lung cancer via activating autophagy. Thorac. Cancer 2021, 12, 1219–1230, Erratum in Thorac. Cancer 2024, 15, 1197. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, C.; Peng, C.; Peng, F. Potential Roles and Mechanisms of Curcumin and its Derivatives in the Regulation of Ferroptosis. Int. J. Biol. Sci. 2024, 20, 4838–4852. [Google Scholar] [CrossRef]
- Abah, M.O.; Ogenyi, D.O.; Zhilenkova, A.V.; Essogmo, F.E.; Uchendu, I.K.; Tchawe, Y.S.N.; Pascal, A.M.; Nikitina, N.M.; Oloche, O.S.; Pavliv, M.; et al. Comparative Transcriptomics Study of Curcumin and Conventional Therapies in Translocation, Clear Cell, and Papillary Renal Cell Carcinoma Subtypes. Int. J. Mol. Sci. 2025, 26, 6161. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Kolnagou, A.; Demetriou, T.; Neocleous, M.; Kontoghiorghe, C.N. New Era in the Treatment of Iron Deficiency Anaemia Using Trimaltol Iron and Other Lipophilic Iron Chelator Complexes: Historical Perspectives of Discovery and Future Applications. Int. J. Mol. Sci. 2021, 22, 5546. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Deferiprone and Iron-Maltol: Forty Years since Their Discovery and Insights into Their Drug Design, Development, Clinical Use and Future Prospects. Int. J. Mol. Sci. 2023, 24, 4970. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. The Design of Orally Active Iron Chelators for the Treatment of Thalassaemia. Ph.D. Thesis, University of Essex, Colchester, UK, 1982; pp. 1–243. Available online: https://www.pri.ac.cy/files/KGJ_thesis_1982.pdf (accessed on 7 September 2025).
- Shin, K.Y.; Lee, G.H.; Park, C.H.; Kim, H.J.; Park, S.H.; Kim, S.; Kim, H.S.; Lee, K.S.; Won, B.Y.; Lee, H.G.; et al. A novel compound, maltolyl p-coumarate, attenuates cognitive deficits and shows neuroprotective effects in vitro and in vivo dementia models. J. Neurosci. Res. 2007, 85, 2500–2511. [Google Scholar] [CrossRef] [PubMed]
- Kao, A.T.; Cabanlong, C.V.; Padilla, K.; Xue, X. Unveiling ferroptosis as a promising therapeutic avenue for colorectal cancer and colitis treatment. Acta Pharm. Sin. B 2024, 14, 3785–3801. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Wang, X.; Liang SLi, C.; Chen, X.; Piao, M.; Wang, Z.; Ge, P.; Luo, T. Maltol as a Novel Agent Protecting SH-SY5Y Cells Against Hemin-induced Ferroptosis. Chem. Res. Chin. Univ. 2022, 38, 1089–1096. [Google Scholar] [CrossRef]
- Maher, P. The flavonoid fisetin promotes nerve cell survival from trophic factor withdrawal by enhancement of proteasome activity. Arch. Biochem. Biophys. 2008, 476, 139–144. [Google Scholar] [CrossRef]
- Ozdemir, S.A.; Faizan, M.I.; Kaur, G.; Shaikh, S.B.; Ul Islam, K.; Rahman, I. Heterogeneity of Cellular Senescence, Senotyping, and Targeting by Senolytics and Senomorphics in Lung Diseases. Int. J. Mol. Sci. 2025, 26, 9687. [Google Scholar] [CrossRef]
- Mirza, M.A.; Padhi, S.; Mohapatra, S.; Mahmood, S.; Iqbal, Z. Fisetin from Dietary Supplement to a Drug Candidate: An Assessment of Potential. Curr. Pharm. Biotechnol. 2025, 26, 1143–1158. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Wang, L.; Cao, D.; Wu, H.; Jia, H.; Yang, C.; Zhang, L. Fisetin Prolongs Therapy Window of Brain Ischemic Stroke Using Tissue Plasminogen Activator: A Double-Blind Randomized Placebo-Controlled Clinical Trial. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619871359. [Google Scholar] [CrossRef] [PubMed]
- Siewe, N.; Friedman, A. Modeling treatment of osteoarthritis with standard therapy and senolytic drugs. PLoS ONE 2025, 20, e0332763. [Google Scholar] [CrossRef] [PubMed]
- Tavenier, J.; Nehlin, J.O.; Houlind, M.B.; Rasmussen, L.J.; Tchkonia, T.; Kirkland, J.L.; Andersen, O.; Rasmussen, L.J.H. Fisetin as a senotherapeutic agent: Evidence and perspectives for age-related diseases. Mech. Ageing Dev. 2024, 222, 111995. [Google Scholar] [CrossRef] [PubMed]
- Mullen, M.; Nelson, A.L.; Goff, A.; Billings, J.; Kloser, H.; Huard, C.; Mitchell, J.; Hambright, W.S.; Ravuri, S.; Huard, J. Fisetin Attenuates Cellular Senescence Accumulation During Culture Expansion of Human Adipose-Derived Stem Cells. Stem Cells 2023, 41, 698–710. [Google Scholar] [CrossRef]
- Maher, P. Modulation of the Neuroprotective and Anti-inflammatory Activities of the Flavonol Fisetin by the Transition Metals Iron and Copper. Antioxidants 2020, 9, 1113. [Google Scholar] [CrossRef]
- Dimitrić Marković, J.M.; Marković, Z.S.; Brdarić, T.P.; Filipović, N.D. Comparative spectroscopic and mechanistic study of chelation properties of fisetin with iron in aqueous buffered solutions. Implications on in vitro antioxidant activity. Dalton Trans. 2011, 40, 4560–4571. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Mylonis, I.; Simos, G.; Bonanou, S.; Tsakalof, A. Flavonoids induce HIF-1alpha but impair its nuclear accumulation and activity. Free. Radic. Biol. Med. 2008, 44, 657–670. [Google Scholar] [CrossRef]
- Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Jomova, K.; Kollar, V.; Rusko, M.; Valko, M. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch. Toxicol. 2019, 93, 2491–2513. [Google Scholar] [CrossRef]
- Sugihara, N.; Arakawa, T.; Ohnishi, M.; Furuno, K. Anti- and pro-oxidative effects of flavonoids on metal-induced lipid hydroperoxide-dependent lipid peroxidation in cultured hepatocytes loaded with alpha-linolenic acid. Free. Radic. Biol. Med. 1999, 27, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hong, Y.; Gong, M.; Cai, S.; Yuan, Z.; Feng, S.; Chen, Q.; Liu, X.; Mei, Z. Fisetin exerts neuroprotective effects in vivo and in vitro by inhibiting ferroptosis and oxidative stress after traumatic brain injury. Front. Pharmacol. 2024, 15, 1480345. [Google Scholar] [CrossRef]
- Li, D.; Liu, X.; Pi, W.; Zhang, Y.; Yu, L.; Xu, C.; Sun, Z.; Jiang, J. Fisetin Attenuates Doxorubicin-Induced Cardiomyopathy In Vivo and In Vitro by Inhibiting Ferroptosis Through SIRT1/Nrf2 Signaling Pathway Activation. Front. Pharmacol. 2022, 12, 808480. [Google Scholar] [CrossRef]
- Goujon, M.; Liang, Z.; Soriano-Castell, D.; Currais, A.; Maher, P. The Neuroprotective Flavonoids Sterubin and Fisetin Maintain Mitochondrial Health under Oxytotic/Ferroptotic Stress and Improve Bioenergetic Efficiency in HT22 Neuronal Cells. Antioxidants 2024, 13, 460. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Deng, Y.; Chen, Y.; Wu, C.; Zhao, X.; Chen, X.; Wang, X.; Zhou, Y.; Zhang, X.; et al. Fisetin suppresses ferroptosis through Nrf2 and attenuates intervertebral disc degeneration in rats. Eur. J. Pharmacol. 2024, 964, 176298. [Google Scholar] [CrossRef]
- Jiang, X.; Lei, Y.; Yin, Y.; Ma, F.; Zheng, M.; Liu, G. Fisetin Suppresses Atherosclerosis by Inhibiting Ferroptosis-Related Oxidative Stress in Apolipoprotein E Knockout Mice. Pharmacology 2024, 109, 169–179. [Google Scholar] [CrossRef]
- Szymczak, J.; Cielecka-Piontek, J. Fisetin-In Search of Better Bioavailability-From Macro to Nano Modifications: A Review. Int. J. Mol. Sci. 2023, 24, 14158. [Google Scholar] [CrossRef]
- Rezaei, H.; Ravankhah, M.; Ansari, M.; Alirezaee, A.; Keshavarzian, O.; Abdollahi, M.; Sabet, H.R. Effects of Alpha-Lipoic Acid Supplementation on Weight Loss, Inflammatory, Lipid, and Hematological Levels in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Ren. Nutr. 2024, 35, 289–299. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, J.; Guo, H. Alpha-lipoic acid on intermediate disease markers in overweight or obese adults: A systematic review and meta-analysis. BMJ Open 2025, 15, e088363. [Google Scholar] [CrossRef]
- Fasipe, B.; Faria, A.; Laher, I. Potential for Novel Therapeutic Uses of Alpha Lipoic Acid. Curr. Med. Chem. 2023, 30, 3942–3954. [Google Scholar] [CrossRef]
- Melli, G.; Taiana, M.; Camozzi, F.; Triolo, D.; Podini, P.; Quattrini, A.; Taroni, F.; Lauria, G. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp. Neurol. 2008, 214, 276–284. [Google Scholar] [CrossRef]
- Abdel Hamid, D.Z.; Nienaa, Y.A.; Mostafa, T.M. Alpha-lipoic acid improved anemia, erythropoietin resistance, maintained glycemic control, and reduced cardiovascular risk in diabetic patients on hemodialysis: A multi-center prospective randomized controlled study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2313–2329. [Google Scholar] [CrossRef]
- Pallardó, F.V.; Pagano, G.; Rodríguez, L.R.; Gonzalez-Cabo, P.; Lyakhovich, A.; Trifuoggi, M. Friedreich Ataxia: Current state-of-the-art, and future prospects for mitochondrial-focused therapies. Transl. Res. 2021, 229, 135–141. [Google Scholar] [CrossRef]
- Camiolo, G.; Tibullo, D.; Giallongo, C.; Romano, A.; Parrinello, N.L.; Musumeci, G.; Di Rosa, M.; Vicario, N.; Brundo, M.V.; Amenta, F.; et al. α-Lipoic Acid Reduces Iron-induced Toxicity and Oxidative Stress in a Model of Iron Overload. Int. J. Mol. Sci. 2019, 20, 609. [Google Scholar] [CrossRef]
- Sharifi-Zahabi, E.; Abdollahzad, H. Alpha Lipoic Acid Supplementation and Iron Homeostasis: A Comprehensive Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Int. J. Vitam. Nutr. Res. 2024, 95, 36623. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Moreau, R.; Heath, S.H.; Hagen, T.M. Dietary supplementation with (R)-α-lipoic acid reverses the age-related accumulation of iron and depletion of antioxidants in the rat cerebral cortex. Redox Rep. 2005, 10, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.; Zheng, Q.; Zhai, S.; Cai, T.; Xu, L.; Yang, L.; Jiao, L.; Zhang, C. Alpha-Lipoic Acid Mediates Clearance of Iron Accumulation by Regulating Iron Metabolism in a Parkinson’s Disease Model Induced by 6-OHDA. Front. Neurosci. 2020, 14, 612. [Google Scholar] [CrossRef] [PubMed]
- Mendes, P.R.; Félix Ddos, S.; Silva, P.C.; Pereira, G.H.; Simões, M.O. Effect of alpha lipoic acid on the blood cell count iron kinetics, in hypertensive patients. Nutr. Hosp. 2014, 31, 883–889. [Google Scholar] [CrossRef]
- Gorąca, A.; Huk-Kolega, H.; Piechota, A.; Kleniewska, P.; Ciejka, E.; Skibska, B. Lipoic acid—Biological activity and therapeutic potential. Pharmacol. Rep. 2011, 63, 849–858. [Google Scholar] [CrossRef]
- Cho, S.; Hong, S.J.; Kang, S.H.; Park, Y.; Kim, S.K. Alpha-Lipoic Acid Attenuates Apoptosis and Ferroptosis in Cisplatin-Induced Ototoxicity via the Reduction of Intracellular Lipid Droplets. Int. J. Mol. Sci. 2022, 23, 10981. [Google Scholar] [CrossRef]
- Zheng, Q.; Ma, P.; Yang, P.; Zhai, S.; He, M.; Zhang, X.; Tu, Q.; Jiao, L.; Ye, L.; Feng, Z.; et al. Alpha lipoic acid ameliorates motor deficits by inhibiting ferroptosis in Parkinson’s disease. Neurosci. Lett. 2023, 810, 137346. [Google Scholar] [CrossRef]
- Liu, L.; Yang, S.; Wang, H. α-Lipoic acid alleviates ferroptosis in the MPP+ -induced PC12 cells via activating the PI3K/Akt/Nrf2 pathway. Cell Biol. Int. 2021, 45, 422–431. [Google Scholar] [CrossRef]
- Born, T.; Kontoghiorghe, C.N.; Spyrou, A.; Kolnagou, A.; Kontoghiorghes, G.J. EDTA chelation reappraisal following new clinical trials and regular use in millions of patients: Review of preliminary findings and risk/benefit assessment. Toxicol. Mech. Methods 2013, 23, 11–17. [Google Scholar] [CrossRef]
- Woolf, S.H. The meaning of translational research and why it matters. JAMA 2008, 299, 211–213. [Google Scholar] [CrossRef]
- Woolf, S.H.; Purnell, J.Q.; Simon, S.M.; Zimmerman, E.B.; Camberos, G.J.; Haley, A.; Fields, R.P. Translating evidence into population health improvement: Strategies and barriers. Annu. Rev. Public Health 2015, 36, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Kleanthous, M.; Kontoghiorghe, C.N. The History of Deferiprone (L1) and the Paradigm of the Complete Treatment of Iron Overload in Thalassaemia. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020011. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzese, G.; Cossu, G.; Balocco, M.; Marchese, R.; Murgia, D.; Melis, M.; Galanello, R.; Barella, S.; Matta, G.; Ruffinengo, U.; et al. A pilot trial of deferiprone for neurodegeneration with brain iron accumulation. Haematologica 2011, 96, 1708–1711. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; Abbruzzese, G.; Matta, G.; Murgia, D.; Melis, M.; Ricchi, V.; Galanello, R.; Barella, S.; Origa, R.; Balocco, M.; et al. Efficacy and safety of deferiprone for the treatment of pantothenate kinase-associated neurodegeneration (PKAN) and neurodegeneration with brain iron accumulation (NBIA): Results from a four years follow-up. Park. Relat. Disord. 2014, 20, 651–654. [Google Scholar] [CrossRef]
- Zorzi, G.; Zibordi, F.; Chiapparini, L.; Bertini, E.; Russo, L.; Piga, A.; Longo, F.; Garavaglia, B.; Aquino, D.; Savoiardo, M.; et al. Iron-related MRI images in patients with pantothenate kinase-associated neurodegeneration (PKAN) treated with deferiprone: Results of a phase II pilot trial. Mov. Disord. 2011, 26, 1755–1759. [Google Scholar] [CrossRef]
- Forni, G.L.; Balocco, M.; Cremonesi, L.; Abbruzzese, G.; Parodi, R.C.; Marchese, R. Regression of symptoms after selective iron chelation therapy in a case of neurodegeneration with brain iron accumulation. Mov. Disord. 2008, 23, 904–907. [Google Scholar] [CrossRef]
- Rohani, M.; Razmeh, S.; Shahidi, G.A.; Alizadeh, E.; Orooji, M. A pilot trial of deferiprone in pantothenate kinase-associated neurodegeneration patients. Neurol. Int. 2018, 9, 7279. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. New Insights into Aspirin’s Anticancer Activity: The Predominant Role of Its Iron-Chelating Antioxidant Metabolites. Antioxidants 2024, 14, 29. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. The Puzzle of Aspirin and Iron Deficiency: The Vital Missing Link of the Iron-Chelating Metabolites. Int. J. Mol. Sci. 2024, 25, 5150. [Google Scholar] [CrossRef]
- He, Y.; Mao, S.; Zhao, Y.; Yang, J. Research Advances in the Synthesis, Metabolism, and Function of Chlorogenic Acid. Foods 2025, 14, 1914. [Google Scholar] [CrossRef]
- Lafay, S.; Morand, C.; Manach, C.; Besson, C.; Scalbert, A. Absorption and metabolism of caffeic acid and chlorogenic acid in the small intestine of rats. Br. J. Nutr. 2006, 96, 39–46. [Google Scholar] [CrossRef]
- Hambright, W.S.; Mu, X.; Gao, X.; Guo, P.; Kawakami, Y.; Mitchell, J.; Mullen, M.; Nelson, A.L.; Bahney, C.; Nishimura, H.; et al. The Senolytic Drug Fisetin Attenuates Bone Degeneration in the Zmpste24−/− Progeria Mouse Model. J. Osteoporos. 2023, 2023, 5572754. [Google Scholar] [CrossRef]


| Molecular Weight |
| Ascorbic acid: 176. Curcumin: 368. Fisetin: 286. Lipoic acid: 206. Maltol: 126. Quercetin: 302. |
| (Iron-chelating drugs—Deferasirox: 373. Deferiprone: 139. Deferoxamine: 561). |
Oil/water partition coefficient (LogP) |
| Ascorbic acid: 0.01 Curcumin: 4.16. Fisetin: 3.20. Lipoic acid: 2.11. Maltol: 1.23. Quercetin: 1.80. |
| (Iron-chelating drugs—Deferasirox: 6.30. Deferiprone: 0.05. Deferoxamine: 0.02). |
Comparative solubility and bioavailability |
| Ascorbic acid > Maltol > Quercetin, Lipoic acid, Fisetin, Curcumin. |
Main route of administration and posology used in clinical trials |
| Ascorbic acid: Oral and intravenous (0.20–10.0 g/day). Quercetin: Oral and intravenous (0.15–2.00 g/day). Curcumin: Oral and intravenous (0.50–12.0 g/day). Lipoic acid: Oral and intravenous (0.05–1.80 g/day). |
Clinical trials with iron-chelating/antioxidant nutraceuticals |
| Hundreds of clinical trials on different categories of patients have been carried out, showing improvements in antioxidant markers, but not robust evidence of therapeutic improvements or cures in most cases. |
Therapeutic targets related to iron and other metal ion metabolism |
| Iron binding and inhibition of the Fenton reaction and associated molecular, cellular, and tissue damage in diseases of free radical pathology. |
| Iron binding and modulation or inhibition of ferroptosis with potential therapeutic implications in associated diseases. |
| Metal-ion binding other than iron and metal intoxication, including reduction in associated oxidative stress. |
Toxicity implications in clinical trials |
| Low toxicity reported at the range of administered doses. |
Strategies for the development of nutraceuticals for clinical use |
| New formulations and posology protocols are needed for improving the bioavailability and efficacy of the lipophilic nutraceuticals. |
| There is a need for the identification of specific therapeutic targets in diseases of free radical pathology, which can lead to improvements or treatments of specific categories of patients. |
| The design of combination therapy protocols with other nutraceuticals, natural compounds, or drugs for the treatment of specific diseases associated with free radical pathology. |
| Efforts around the development of nutraceuticals for use in pharmaceuticals should adhere to existing legislation and fulfill all of the required regulatory route procedures. This process could be facilitated by involving academic groups that are experts on drug regulatory affairs, as well as government institutions involved in translational research. Similarly, this process could be facilitated by focusing on clinical investigations on orphan or emergency diseases. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontoghiorghes, G.J. Development of Iron-Chelating/Antioxidant Nutraceuticals and Natural Products as Pharmaceuticals for Clinical Use in Diseases with Free Radical Pathologies. Nutrients 2025, 17, 3270. https://doi.org/10.3390/nu17203270
Kontoghiorghes GJ. Development of Iron-Chelating/Antioxidant Nutraceuticals and Natural Products as Pharmaceuticals for Clinical Use in Diseases with Free Radical Pathologies. Nutrients. 2025; 17(20):3270. https://doi.org/10.3390/nu17203270
Chicago/Turabian StyleKontoghiorghes, George J. 2025. "Development of Iron-Chelating/Antioxidant Nutraceuticals and Natural Products as Pharmaceuticals for Clinical Use in Diseases with Free Radical Pathologies" Nutrients 17, no. 20: 3270. https://doi.org/10.3390/nu17203270
APA StyleKontoghiorghes, G. J. (2025). Development of Iron-Chelating/Antioxidant Nutraceuticals and Natural Products as Pharmaceuticals for Clinical Use in Diseases with Free Radical Pathologies. Nutrients, 17(20), 3270. https://doi.org/10.3390/nu17203270








