25-Hydroxyvitamin D Status and Its Predictors in Greek and Cypriot Subsets of the UK Biobank Cohort
Abstract
1. Introduction
2. Methods
2.1. UK Biobank Cohort
2.2. Derivation of Variables
2.3. Statistical Analysis
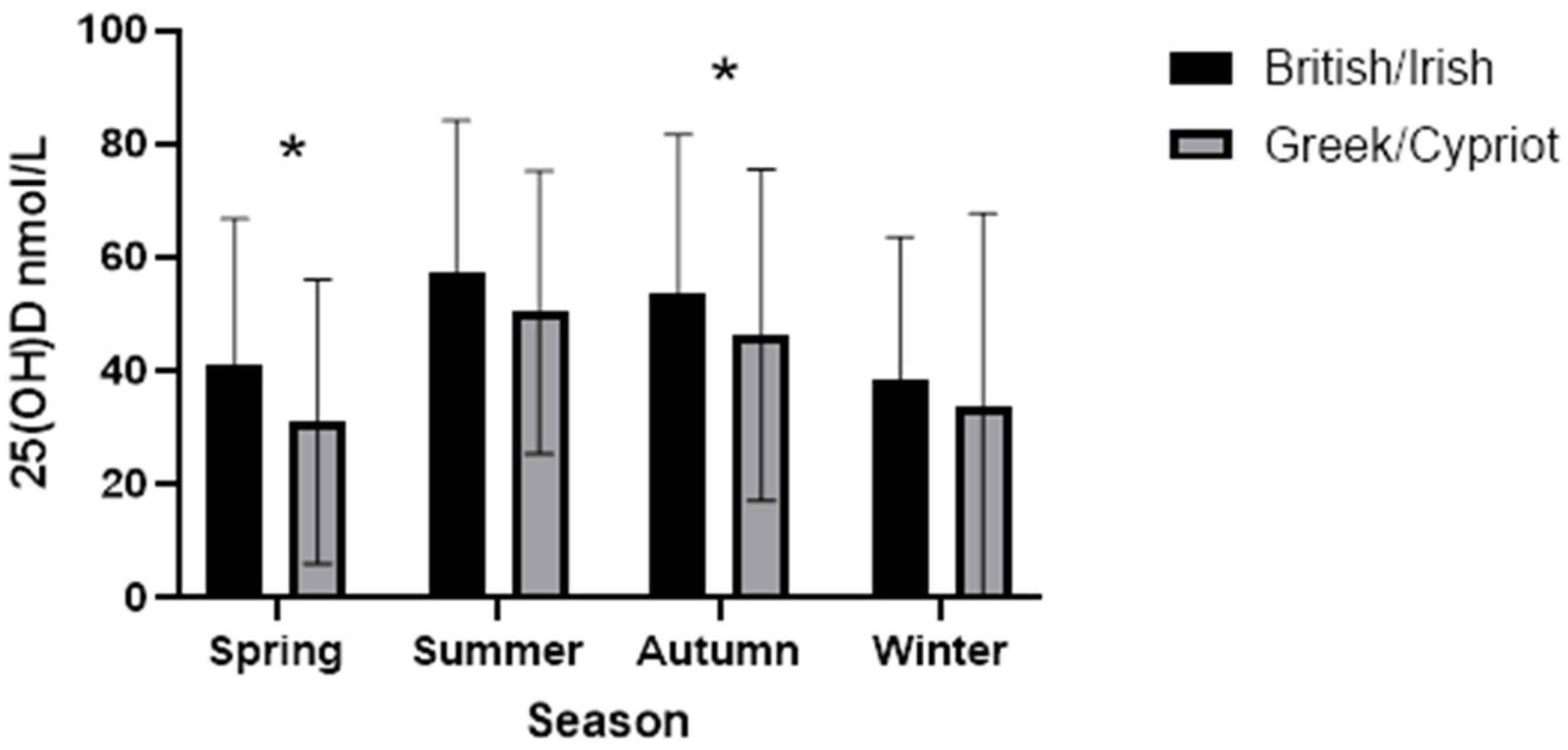
3. Results
3.1. Descriptive Statistics
3.1.1. Categorial Descriptives by Ethnicity: Sex, Socioeconomic Demographical Lifestyle and Dietary Factors
3.1.2. Ethnicity and Serum 25(OH)D Status
3.2. Logistic Regression Model for Prediction of Odds of 25(OH)D Levels > 50 nmol/L
3.2.1. All Participants
3.2.2. British/Irish Group Only
3.2.3. Greek/Cypriot Group Only
4. Discussion
4.1. Summary
4.1.1. Serum 25(OH)D Concentration
4.1.2. Vitamin D Intake
4.1.3. Lifestyle
4.1.4. Background Demographics
4.1.5. Geographical and Seasonal Factors
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef]
- Webb, A.R. Who, what, where and when-influences on cutaneous vitamin D synthesis. Prog. Biophys. Mol. Biol. 2006, 92, 17–25. [Google Scholar] [CrossRef]
- Schmid, A.; Walther, B. Natural vitamin D content in animal products. Adv. Nutr. 2013, 4, 453–462. [Google Scholar] [CrossRef]
- Snijder, M.B.; Lips, P.; Seidell, J.C.; Visser, M.; Deeg, D.J.; Dekker, J.M.; van Dam, R.M. Vitamin D status and parathyroid hormone levels in relation to blood pressure: A population-based study in older men and women. J. Intern. Med. 2007, 261, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Chapuy, M.C.; Preziosi, P.; Maamer, M.; Arnaud, S.; Galan, P.; Hercberg, S.; Meunier, P.J. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos. Int. 1997, 7, 439–443. [Google Scholar] [CrossRef]
- Lips, P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Isaia, G.; Giorgino, R.; Rini, G.B.; Bevilacqua, M.; Maugeri, D.; Adami, S. Prevalence of hypovitaminosis D in elderly women in Italy: Clinical consequences and risk factors. Osteoporos. Int. 2003, 14, 577–582. [Google Scholar] [CrossRef]
- Krieg, M.A.; Cornuz, J.; Jacquet, A.F.; Thiebaud, D.; Burckhardt, P. Influence of anthropometric parameters and biochemical markers of bone metabolism on quantitative ultrasound of bone in the institutionalized elderly. Osteoporos. Int. 1998, 8, 115–120. [Google Scholar] [CrossRef] [PubMed]
- van Dam, R.M.; Snijder, M.B.; Dekker, J.M.; Stehouwer, C.D.; Bouter, L.M.; Heine, R.J.; Lips, P. Potentially modifiable determinants of vitamin D status in an older population in the Netherlands: The Hoorn Study. Am. J. Clin. Nutr. 2007, 85, 755–761. [Google Scholar] [CrossRef]
- van der Wielen, R.P.; Lowik, M.R.; van den Berg, H.; de Groot, L.C.; Haller, J.; Moreiras, O.; van Staveren, W.A. Serum vitamin D concentrations among elderly people in Europe. Lancet 1995, 346, 207–210. [Google Scholar] [CrossRef]
- EFSA. Joint Explanatory Note by the European Food Safety Authority and the UK Scientific Advisory Committee on Nutrition Regarding Dietary Reference Values for Vitamin D. Available online: https://www.efsa.europa.eu/sites/default/files/documents/news/explanatory_note_EFSA_SACN_vitaminD.pdf (accessed on 13 August 2022).
- Kuchuk, N.O.; van Schoor, N.M.; Pluijm, S.M.; Chines, A.; Lips, P. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: Global perspective. J. Bone Miner. Res. 2009, 24, 693–701. [Google Scholar] [CrossRef]
- van Schoor, N.M.; Lips, P. Worldwide vitamin D status. Best. Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Worldwide status of vitamin D nutrition. J. Steroid Biochem. Mol. Biol. 2010, 121, 297–300. [Google Scholar] [CrossRef]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J.; et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef]
- Itkonen, S.T.; Andersen, R.; Bjork, A.K.; Brugard Konde, A.; Eneroth, H.; Erkkola, M.; Holvik, K.; Madar, A.A.; Meyer, H.E.; Tetens, I.; et al. Vitamin D status and current policies to achieve adequate vitamin D intake in the Nordic countries. Scand. J. Public Health 2021, 49, 616–627. [Google Scholar] [CrossRef]
- Challa, A.; Ntourntoufi, A.; Cholevas, V.; Bitsori, M.; Galanakis, E.; Andronikou, S. Breastfeeding and vitamin D status in Greece during the first 6 months of life. Eur. J. Pediatr. 2005, 164, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.; Bili, H.; Goulis, D.; Papadopoulou, F.; Harizopoulou, V.; Kintiraki, E. High prevalence of vitamin d deficiency among pregnant women at term and their neonates in Thessaloniki, Northern Greece. Bone 2012, 50, S104. [Google Scholar] [CrossRef]
- Manios, Y.; Moschonis, G.; Hulshof, T.; Bourhis, A.S.; Hull, G.L.J.; Dowling, K.G.; Kiely, M.E.; Cashman, K.D. Prevalence of vitamin D deficiency and insufficiency among schoolchildren in Greece: The role of sex, degree of urbanisation and seasonality. Br. J. Nutr. 2017, 118, 550–558. [Google Scholar] [CrossRef]
- Lapatsanis, P.; Deliyanni, V.; Doxiadis, S. Vitamin D deficiency rickets in Greece. J. Pediatr. 1968, 73, 195–202. [Google Scholar] [CrossRef]
- Anatoliotaki, M.; Tsilimigaki, A.; Tsekoura, T.; Schinaki, A.; Stefanaki, S.; Nicolaidou, P. Congenital rickets due to maternal vitamin D deficiency in a sunny island of Greece. Acta Paediatr. 2003, 92, 389–391. [Google Scholar] [CrossRef]
- Kolokotroni, O.; Papadopoulou, A.; Yiallouros, P.K.; Raftopoulos, V.; Kouta, C.; Lamnisos, D.; Nicolaidou, P.; Middleton, N. Association of vitamin D with adiposity measures and other determinants in a cross-sectional study of Cypriot adolescents. Public Health Nutr. 2015, 18, 112–121. [Google Scholar] [CrossRef]
- Kolokotroni, O.; Yiallouros, P.; Middleton, N. The association between vitamin D and cardio-metabolic indicators in adolescents in Cyprus. Eur. J. Public Health 2016, 26, ckw175.125. [Google Scholar] [CrossRef]
- Xyda, E.S.; Kosta, K.; Doumas, A.; Papanastasiou, M.; Samoutis, G.; Garyfallos, A.A. The prevalence of Vitamin D deficiency in a Greek and a Cypriot population sample. Endocr. Abstr. 2018, 56, P212. [Google Scholar] [CrossRef]
- Preece, M.A.; McIntosh, W.B.; Tomlinson, S.; Ford, J.A.; Dunnigan, M.G.; O’Riordan, J.L. Vitamin-D deficiency among Asian immigrants to Britain. Lancet 1973, 7809, 907–910. [Google Scholar] [CrossRef]
- Holvik, K.; Meyer, H.E.; Haug, E.; Brunvand, L. Prevalence and predictors of vitamin D deficiency in five immigrant groups living in Oslo, Norway: The Oslo Immigrant Health Study. Eur. J. Clin. Nutr. 2005, 59, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.E.; Falch, J.A.; Sogaard, A.J.; Haug, E. Vitamin D deficiency and secondary hyperparathyroidism and the association with bone mineral density in persons with Pakistani and Norwegian background living in Oslo, Norway, the Oslo Health Study. Bone 2004, 35, 412–417. [Google Scholar] [CrossRef]
- de Torrente de la Jara, G.; Pecoud, A.; Favrat, B. Musculoskeletal pain in female asylum seekers and hypovitaminosis D3. BMJ 2004, 329, 156–157. [Google Scholar] [CrossRef]
- Darling, A.L. Vitamin D deficiency in western dwelling South Asian populations: An unrecognised epidemic. Proc. Nutr. Soc. 2020, 79, 259–271. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Fry, D.; Almond, R.; Moffat, S.; Gordon, M.; Singh, P. UK Biobank Biomarker Project Companion Document to Accompany Serum Biomarker Data. 2019. Available online: https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/serum_biochemistry.pdf (accessed on 3 January 2024).
- DEQAS. DEQAS Review 2016–2017. 2017. Available online: http://www.deqas.org/downloads/DEQAS%20Review%20October%202017.pdf (accessed on 27 September 2023).
- UK Biobank. 24-Hour Dietary Recall Questionnaire. 2012. Available online: https://biobank.ndph.ox.ac.uk/showcase/ukb/docs/DietWebQ.pdf (accessed on 14 February 2024).
- Liu, B.; Young, H.; Crowe, F.L.; Benson, V.S.; Spencer, E.A.; Key, T.J.; Appleby, P.N.; Beral, V. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr. 2011, 14, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- UK Biobank. UK Biobank Touch-Screen Questionnaire: Final Version. Available online: https://biobank.ndph.ox.ac.uk/ukb/ukb/docs/TouchscreenQuestionsMainFinal.pdf (accessed on 14 February 2024).
- Townsend, P.; Phillimore, P.; Beattie, A. (Eds.) Health and Deprivation: Inequality and the North; Routledge: London, UK, 1988. [Google Scholar]
- EFSA. Scientific Opinion on Dietary Reference Values for Vitamin D. 2016. Available online: https://www.efsa.europa.eu/sites/default/files/consultation/160321.pdf (accessed on 20 August 2017).
- SACN. Vitamin D and Health. 2016. Available online: https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report (accessed on 1 June 2017).
- van Schoor, N.M.; Visser, M.; Pluijm, S.M.; Kuchuk, N.; Smit, J.H.; Lips, P. Vitamin D deficiency as a risk factor for osteoporotic fractures. Bone 2008, 42, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Lapatsanis, D.; Moulas, A.; Cholevas, V.; Soukakos, P.; Papadopoulou, Z.L.; Challa, A. Vitamin D: A necessity for children and adolescents in Greece. Calcif. Tissue Int. 2005, 77, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Magriplis, E.; Stratulat, A. Vitamin D deficiency in adult women in Greece. Clin. Nutr. ESPEN 2016, 13, E70. [Google Scholar] [CrossRef]
- Grigoriou, E.V.; Trovas, G.; Papaioannou, N.; Makras, P.; Kokkoris, P.; Dontas, I.; Makris, K.; Tournis, S.; Dedoussis, G.V. Serum 25-hydroxyvitamin D status, quantitative ultrasound parameters, and their determinants in Greek population. Arch. Osteoporos. 2018, 13, 111. [Google Scholar] [CrossRef]
- Papapetrou, P.D.; Triantaphyllopoulou, M.; Karga, H.; Zagarelos, P.; Aloumanis, K.; Kostakioti, E.; Vaiopoulos, G. Vitamin D deficiency in the elderly in Athens, Greece. J. Bone Miner. Metab. 2007, 25, 198–203. [Google Scholar] [CrossRef]
- Papapetrou, P.D.; Triantafyllopoulou, M.; Korakovouni, A. Severe vitamin D deficiency in the institutionalized elderly. J. Endocrinol. Investig. 2008, 31, 784–787. [Google Scholar] [CrossRef]
- Manios, Y.; Moschonis, G.; Lambrinou, C.P.; Mavrogianni, C.; Tsirigoti, L.; Hoeller, U.; Roos, F.F.; Bendik, I.; Eggersdorfer, M.; Celis-Morales, C.; et al. Associations of vitamin D status with dietary intakes and physical activity levels among adults from seven European countries: The Food4Me study. Eur. J. Nutr. 2018, 57, 1357–1368. [Google Scholar] [CrossRef]
- Public Health England; Food Standards Agency. National Diet and Nutrition Survey Rolling Programme Years 9 to 11 (2016/2017 to 2018/2019); Public Health England: London, UK, 2020.
- Dimakopoulos, I.; Magriplis, E.; Mitsopoulou, A.V.; Karageorgou, D.; Bakogianni, I.; Micha, R.; Michas, G.; Chourdakis, M.; Chrousos, G.P.; Roma, E.; et al. Intake and contribution of food groups to vitamin D intake in a representative sample of adult Greek population. Nutrition 2020, 72, 110641. [Google Scholar] [CrossRef]
- Hypponen, E.; Power, C. Hypovitaminosis D in British adults at age 45 y: Nationwide cohort study of dietary and lifestyle predictors. Am. J. Clin. Nutr. 2007, 85, 860–868. [Google Scholar] [CrossRef]
- Brustad, M.; Sandanger, T.; Aksnes, L.; Lund, E. Vitamin D status in a rural population of northern Norway with high fish liver consumption. Public Health Nutr. 2004, 7, 783–789. [Google Scholar] [CrossRef]
- Supervia, A.; Nogues, X.; Enjuanes, A.; Vila, J.; Mellibovsky, L.; Serrano, S.; Aubia, J.; Diez-Perez, A. Effect of smoking and smoking cessation on bone mass, bone remodeling, vitamin D, PTH and sex hormones. J. Musculoskelet. Neuronal Interact. 2006, 6, 234–241. [Google Scholar] [PubMed]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef]
- Wicherts, I.S.; van Schoor, N.M.; Boeke, A.J.; Visser, M.; Deeg, D.J.; Smit, J.; Knol, D.L.; Lips, P. Vitamin D status predicts physical performance and its decline in older persons. J. Clin. Endocrinol. Metab. 2007, 92, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, I.M.; Karamali, N.S.; Boeke, A.J.; Lips, P.; Middelkoop, B.J.; Verhoeven, I.; Wuister, J.D. High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am. J. Clin. Nutr. 2006, 84, 350–353; quiz 468–469. [Google Scholar] [CrossRef]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 138–145. [Google Scholar] [CrossRef]
- Grigoriou, E.; Trovas, G.; Papaioannou, N.; Dontas, I.; Makris, K.; Apostolou-Karampelis, K.; Dedoussis, G. Dietary Patterns of Greek Adults and Their Associations with Serum Vitamin D Levels and Heel Quantitative Ultrasound Parameters for Bone Health. Nutrients 2020, 12, 123. [Google Scholar] [CrossRef]
- Rhein, H.M. Vitamin D deficiency in Scotland. BMJ 2014, 348, g2821. [Google Scholar] [CrossRef]
- Fry, A.; Littlejohns, T.J.; Sudlow, C.; Doherty, N.; Adamska, L.; Sprosen, T.; Collins, R.; Allen, N.E. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants with Those of the General Population. Am. J. Epidemiol. 2017, 186, 1026–1034. [Google Scholar] [CrossRef]
- Pietilainen, K.H.; Korkeila, M.; Bogl, L.H.; Westerterp, K.R.; Yki-Jarvinen, H.; Kaprio, J.; Rissanen, A. Inaccuracies in food and physical activity diaries of obese subjects: Complementary evidence from doubly labeled water and co-twin assessments. Int. J. Obes. 2010, 34, 437–445. [Google Scholar] [CrossRef]
- Darling, A.L.; Blackbourn, D.J.; Ahmadi, K.R.; Lanham-New, S.A. Very high prevalence of 25-hydroxyvitamin D deficiency in 6433 UK South Asian adults: Analysis of the UK Biobank Cohort. Br. J. Nutr. 2021, 125, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.D.; Berry, J.; Durazo-Arvizu, R.; Gunter, E.; Jones, G.; Jones, J.; Makin, H.L.J.; Pattni, P.; Sempos, C.T.; Twomey, P.; et al. Hydroxyvitamin D assays: An historical perspective from DEQAS. J. Steroid Biochem. Mol. Biol. 2018, 177, 30–35. [Google Scholar] [CrossRef] [PubMed]
| Variables | All Participants (n 4483) | British/Irish (n 4158) | Greek/Cypriot (n 325) | * p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | n | Missing | Median | IQR | n | Missing | Median | IQR | n | Missing | ||
| 25(OH)D status baseline nmol/L | 47.10 | 30.9 | 3996 | 487 | 47.60 | 31.3 | 3707 | 451 | 40.30 | 21.5 | 289 | 36 | <0.001 |
| Vitamin D intake median over visits μg/day | 1.76 | 2.02 | 1911 | 2572 | 1.77 | 2.02 | 1779 | 2379 | 1.41 | 1.82 | 132 | 193 | 0.045 |
| Age at baseline assessment (years) | 58.00 | 12 | 4483 | 0 | 58.00 | 12 | 4158 | 0 | 57.00 | 12 | 325 | 0 | 0.004 |
| Townsend Deprivation Index | −2.24 | 4 | 4480 | 3 | −2.31 | 4 | 4155 | 3 | −0.66 | 4 | 325 | 0 | <0.001 |
| Age completed Full time education | 16.00 | 2 | 2964 | 1519 | 16.00 | 2 | 2820 | 1338 | 17.00 | 4 | 144 | 181 | <0.001 |
| British/Irish | Greek/Cypriot | Chi-Square | ||||
|---|---|---|---|---|---|---|
| % | n | % | n | p | ||
| Gender | ||||||
| Female | 55.7 | 2315 | 47.1 | 153 | 0.003 | |
| Male | 44.3 | 1843 | 52.9 | 172 | ||
| Household income | ||||||
| Less than 18,000 | 23.7 | 848 | 21.9 | 56 | <0.001 | |
| 18,000–30,999 | 25.8 | 924 | 19.1 | 49 | ||
| 31,000–51,999 | 25.9 | 925 | 21.5 | 55 | ||
| 52,000–100,000 | 19.4 | 695 | 27.3 | 70 | ||
| >100,000 | 5.2 | 186 | 10.2 | 26 | ||
| Oily Fish Intake | ||||||
| never | 10.8 | 448 | 4.4 | 14 | 0.02 | |
| Less than once a week | 32.2 | 1332 | 37.1 | 119 | ||
| Once per week | 39.8 | 1644 | 40.5 | 130 | ||
| 2–4 times per week | 16.3 | 672 | 16.5 | 53 | ||
| 5–6 times per week | 0.6 | 24 | 1.6 | 5 | ||
| Once or more daily | 0.3 | 11 | 0.0 | 0 | ||
| Alcohol Consumption | ||||||
| Almost daily or daily | 20.7 | 858 | 11.1 | 36 | <0.001 | |
| 3–4 times per week | 24.0 | 995 | 16.4 | 53 | ||
| 1–2 times per week | 27.1 | 1125 | 22.6 | 73 | ||
| 1–3 times per month | 11.3 | 468 | 11.8 | 38 | ||
| Special occasions only | 10.4 | 430 | 27.6 | 89 | ||
| never | 6.7 | 278 | 10.5 | 34 | ||
| Skin Colour | ||||||
| Very fair | 8.3 | 336 | 1.9 | 9 | <0.001 | |
| Fair | 72.1 | 2950 | 28.0 | 89 | ||
| Light Olive | 18.2 | 746 | 59.4 | 189 | ||
| Dark Olive | 1.0 | 41 | 5.7 | 18 | ||
| Brown | 0.3 | 13 | 5.0 | 16 | ||
| Smoking Status | ||||||
| Never | 54.6 | 2261 | 45.2 | 145 | <0.001 | |
| Previous | 34.7 | 1437 | 37.1 | 119 | ||
| Current | 10.7 | 443 | 17.8 | 57 | ||
| User of Single Vit D supplement or multivitamin/mineral | ||||||
| User | 23.6 | 970 | 28.4 | 91 | 0.051 | |
| Non user | 76.4 | 3141 | 71.6 | 229 | ||
| Region of assessment | ||||||
| Northern England | 47.3 | 1968 | 16.6 | 54 | <0.001 | |
| Southern England | 16.8 | 698 | 17.8 | 58 | ||
| Wales | 1.3 | 53 | 3.1 | 10 | ||
| Scotland | 8.1 | 335 | 2.2 | 7 | ||
| English Midlands | 16.0 | 667 | 12.9 | 42 | ||
| London | 10.5 | 437 | 47.4 | 154 | ||
| Summer sun exposure per day | ||||||
| Less than 30 min | 4.1 | 159 | 8.1 | 24 | 0.001 | |
| More than 30 min | 95.9 | 3730 | 91.9 | 273 | ||
| Season | ||||||
| Spring | 28.4 | 1180 | 36.0 | 117 | 0.019 | |
| Summer | 26.1 | 1084 | 20.6 | 67 | ||
| Winter | 24.2 | 1007 | 23.7 | 77 | ||
| Autumn | 21.3 | 887 | 19.7 | 64 | ||
| All Participants | British/Irish | Greeks/Cypriots | p | ||||
|---|---|---|---|---|---|---|---|
| N | % | n | % | n | % | ||
| ≥25 nmol/L | 3521 | 88.1 | 3298 | 89.0 | 223 | 77.2 | <0.001 |
| <25 nmol/L | 475 | 11.9 | 409 | 11.0 | 66 | 22.8 | |
| ≥50 nmol/L | 1848 | 46.2 | 1739 | 46.9 | 109 | 37.7 | <0.003 |
| <50 nmol/L | 2148 | 53.8 | 1968 | 53.1 | 180 | 62.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontea, F.E.; Lanham-New, S.A.; Darling, A.L. 25-Hydroxyvitamin D Status and Its Predictors in Greek and Cypriot Subsets of the UK Biobank Cohort. Nutrients 2025, 17, 3267. https://doi.org/10.3390/nu17203267
Kontea FE, Lanham-New SA, Darling AL. 25-Hydroxyvitamin D Status and Its Predictors in Greek and Cypriot Subsets of the UK Biobank Cohort. Nutrients. 2025; 17(20):3267. https://doi.org/10.3390/nu17203267
Chicago/Turabian StyleKontea, Francesca E., Susan A. Lanham-New, and Andrea L. Darling. 2025. "25-Hydroxyvitamin D Status and Its Predictors in Greek and Cypriot Subsets of the UK Biobank Cohort" Nutrients 17, no. 20: 3267. https://doi.org/10.3390/nu17203267
APA StyleKontea, F. E., Lanham-New, S. A., & Darling, A. L. (2025). 25-Hydroxyvitamin D Status and Its Predictors in Greek and Cypriot Subsets of the UK Biobank Cohort. Nutrients, 17(20), 3267. https://doi.org/10.3390/nu17203267







