Gut Microbiota α- and β-Diversity, but Not Dietary Patterns, Differ Between Underweight and Normal-Weight Japanese Women Aged 20–39 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. DNA Extraction and Sequencing
2.4. PCR Amplification
2.5. Library Preparation and Sequencing
2.6. Data Processing and Analysis
2.7. Data Preprocessing
2.8. Statistical Analysis
2.8.1. Alpha Diversity Indices
2.8.2. Nonmetric Multidimensional Scaling (NMDS)
2.8.3. Redundancy Analysis (RDA)
2.8.4. ANOVA-like Differential Expression Tool, Version 2 (AldEx2)
3. Results
3.1. Participant Characteristics
3.2. Alpha Diversity of Dietary and Gut Microbiota Patterns
3.3. Correlations of Alpha Diversity with BMI and Nutrient Intake
3.4. The Beta Diversity of Gut Microbiota Patterns Rather Than Dietary Patterns Differed Between the Normal and Underweight Groups
3.5. The Difference in Bacterial Composition Between the Normal and Underweight Groups Reflects the Beta Diversity of the Gut Microbiota
3.6. Differential Abundance Analysis by ALDEx2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PERMANOVA | permutational multivariate analysis of variance |
| PERMDISP | permutational analysis of multivariate dispersions |
| ALDEx2 | ANOVA-Like Differential Expression (version 2) |
| NMDS | nonmetric multidimensional scaling |
| RDA | redundancy analysis |
References
- Tatsumi, Y.; Higashiyama, A.; Kubota, Y.; Sugiyama, D.; Nishida, Y.; Hirata, T.; Kadota, A.; Nishimura, K.; Imano, H.; Miyamatsu, N.; et al. Underweight Young Women without Later Weight Gain Are at High Risk for Osteopenia after Midlife: The KOBE Study. J. Epidemiol. 2016, 26, 572–578. [Google Scholar] [CrossRef]
- Boutari, C.; Pappas, P.D.; Mintziori, G.; Nigdelis, M.P.; Athanasiadis, L.; Goulis, D.G.; Mantzoros, C.S. The Effect of Underweight on Female and Male Reproduction. Metabolism 2020, 107, 154229. [Google Scholar] [CrossRef]
- Yamamoto, S.; Wada, Y. Awareness, Use and Information Sources of Folic Acid Supplementation to Prevent Neural Tube Defects in Pregnant Japanese Women. Public Health Nutr. 2018, 21, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Jaime, K.; Mank, V. Risks Associated with Excessive Weight Loss. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK603752/ (accessed on 29 February 2024).
- Ministry of Health, Labor and Welfare. National Nutrition Survey. 2013. Available online: http://www.mhlw.go.jp/file/04-Houdouhappyou-10904750-Kenkoukyoku-Gantaisakukenkouzoushinka/0000068070.pdf (accessed on 10 September 2015).
- Centers for Disease Control and Prevention (CDC). Prevalence of Underweight Among Adults Aged 20 and Over: United States, 1960–1962 Through 2015–2016; National Center for Health Statistics (NCHS): Hyattsville, MD, USA, 2018. [Google Scholar]
- Iizuka, K.; Sato, H.; Kobae, K.; Yanagi, K.; Yamada, Y.; Ushiroda, C.; Hirano, K.; Ichimaru, S.; Seino, Y.; Ito, A.; et al. Young Japanese Underweight Women with “Cinderella Weight” Are Prone to Malnutrition, Including Vitamin Deficiencies. Nutrients 2023, 15, 2216. [Google Scholar] [CrossRef] [PubMed]
- Hiraiwa, E.; Yamamoto-Wada, R.; Deguchi, K.; Ushiroda, C.; Naruse, H.; Iizuka, K. Skeletal Muscle Mass Index and Body Fat Percentage Reflect Different Nutritional Markers Independent of BMI in Underweight Women. Nutrients 2025, 17, 1766. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, K.; Yanagi, K.; Deguchi, K.; Ushiroda, C.; Yamamoto-Wada, R.; Ishihara, T.; Naruse, H. The Alpha and Beta Diversities of Dietary Patterns Differed by Age and Sex in Young and Middle-Aged Japanese Participants. Nutrients 2025, 17, 2205. [Google Scholar] [CrossRef]
- Matsumoto, M.; Tajima, R.; Fujiwara, A.; Yuan, X.; Okada, E.; Takimoto, H. Trends in Food Group Intake According to Body Size among Young Japanese Women: The 2001–2019 National Health and Nutrition Survey. Nutrients 2022, 14, 4078. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A Core Gut Microbiome in Obese and Lean Twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Zhang, H.; DiBaize, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human Gut Microbiota in Obesity and after Gastric Bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Kodancha, P.; Das, S. Gut Microbiome Changes in Anorexia Nervosa: A Comprehensive Review. Pathophysiology 2024, 31, 68–88. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Yuan, J.; Li, J.; Li, H.; Yin, K.; Wang, F.; Li, D. Overweight and Underweight Status Are Linked to Specific Gut Microbiota and Intestinal Tricarboxylic Acid Cycle Intermediates. Clin. Nutr. 2020, 39, 3189–3198. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yoshimura, Y.; Kaimoto, T.; Kunii, D.; Komatsu, T.; Yamamoto, S. Validation of a Food Frequency Questionnaire Based on Food Groups for Estimating Individual Nutrient Intake. Jpn. J. Nutr. Diet. 2001, 59, 221–232. (In Japanese) [Google Scholar] [CrossRef]
- Takahashi, K. Food Frequency Questionnaire Based on Food Groups for Estimating Individual Nutrient Intake. Jpn. J. Nutr. Diet. 2003, 61, 161–169. (In Japanese) [Google Scholar] [CrossRef]
- Kono, K.; Kozu, Y.; Yokota, S.; Hatayama, K.; Mizumura, K.; Maruoka, S.; Masuyama, H.; Gon, Y. Gut Microbiota Dysbiosis in Japanese Female Patients with Nontuberculous Mycobacteria-Associated Lung Disease: An Observational Study. Biomedicines 2025, 13, 1264. [Google Scholar] [CrossRef]
- Vargha, A.; Delaney, H.D. A Critique and Improvement of the CL Common Language Effect Size Statistics of McGraw and Wong. J. Educ. Behav. Stat. 2000, 25, 101–132. [Google Scholar] [CrossRef]
- Fernandes, A.D.; Reid, J.N.; Macklaim, J.M.; McMurrough, T.A.; Edgell, D.R.; Gloor, G.B. Unifying the Analysis of High-Throughput Sequencing Datasets: Characterizing RNA-Seq, 16S rRNA Gene Sequencing and Selective Growth Experiments by Compositional Data Analysis. Microbiome 2014, 2, 15. [Google Scholar] [CrossRef]
- Gloor, G.B.; Reid, G. Compositional Analysis: A Valid Approach to Analyze Microbiome High-Throughput Sequencing Data. Can. J. Microbiol. 2016, 62, 692–703. [Google Scholar] [CrossRef]
- Gloor, G.B.; Macklaim, J.M.; Pawlowsky-Glahn, V.; Egozcue, J.J. Microbiome Datasets Are Compositional: And This Is Not Optional. Front. Microbiol. 2017, 8, 2224. [Google Scholar] [CrossRef]
- Hata, T.; Seino, S.; Yokoyama, Y.; Narita, M.; Nishi, M.; Hida, A.; Shinkai, S.; Kitamura, A.; Fujiwara, Y. Interaction of Eating Status and Dietary Variety on Incident Functional Disability among Older Japanese Adults. J. Nutr. Health Aging 2022, 26, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, S.; Watanabe, S.; Shibata, H.; Amano, H.; Fujiwara, Y.; Shinkai, S.; Yoshida, H.; Suzuki, T.; Yukawa, H.; Yasumura, S.; et al. Effects of Dietary Variety on Declines in High-Level Functional Capacity in Elderly People Living in a Community. Nihon Koshu Eisei Zasshi 2003, 50, 1117–1124. [Google Scholar] [PubMed]
- Yokoyama, Y.; Nishi, M.; Murayama, H.; Amano, H.; Taniguchi, Y.; Nofuji, Y.; Narita, M.; Matsuo, E.; Seino, S.; Kawano, Y.; et al. Dietary Variety and Decline in Lean Mass and Physical Performance in Community-Dwelling Older Japanese: A 4-Year Follow-Up Study. J. Nutr. Health Aging 2017, 21, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Digitale, J.; Sié, A.; Coulibaly, B.; Ouermi, L.; Dah, C.; Tapsoba, C.; Bärnighausen, T.; Lebas, E.; Arzika, A.M.; Glymour, M.M.; et al. Gut Bacterial Diversity and Growth among Preschool Children in Burkina Faso. Am. J. Trop. Med. Hyg. 2020, 103, 2568–2574. [Google Scholar] [CrossRef]
- Camara, A.; Konate, S.; Alou, M.T.; Kodio, A.; Togo, A.H.; Cortaredona, S.; Henrissat, B.; Thera, M.A.; Doumbo, O.K.; Raoult, D.; et al. Clinical Evidence of the Role of Methanobrevibacter smithii in Severe Acute Malnutrition. Sci. Rep. 2021, 11, 5426. [Google Scholar] [CrossRef]
- Dinh, D.M.; Ramadass, B.; Kattula, D.; Sarkar, R.; Braunstein, P.; Tai, A.; A Wanke, C.; Hassoun, S.; Kane, A.V.; Naumova, E.N.; et al. Longitudinal Analysis of the Intestinal Microbiota in Persistently Stunted Young Children in South India. PLoS ONE 2016, 11, e0155405. [Google Scholar] [CrossRef]
- Surono, I.S.; Widiyanti, D.; Kusumo, P.D.; Venema, K. Gut Microbiota Profile of Indonesian Stunted Children and Children with Normal Nutritional Status. PLoS ONE 2021, 16, e0245399. [Google Scholar] [CrossRef]
- Kamil, R.Z.; Murdiati, A.; Juffrie, M.; Nakayama, J.; Rahayu, E.S. Gut Microbiota and Short-Chain Fatty Acid Profile between Normal and Moderate Malnutrition Children in Yogyakarta, Indonesia. Microorganisms 2021, 9, 127. [Google Scholar] [CrossRef]
- Shivakumar, N.; Sivadas, A.; Devi, S.; Jahoor, F.; McLaughlin, J.; Smith, C.P.; Kurpad, A.V.; Mukhopadhyay, A. Gut Microbiota Profiles of Young South Indian Children: Child Sex-Specific Relations with Growth. PLoS ONE 2021, 16, e0251803. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The Gut Microbiota and Host Health: A New Clinical Frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Candela, M.; Biagi, E.; Maccaferri, S.; Turroni, S.; Brigidi, P. Intestinal Microbiota Is a Plastic Factor Responding to Environmental Changes. Trends Microbiol. 2012, 20, 385–391. [Google Scholar] [CrossRef]
- Hills, R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Zoghi, S.; Sadeghpour Heravi, F.; Nikniaz, Z.; Shirmohamadi, M.; Moaddab, S.Y.; Ebrahimzadeh Leylabadlo, H. Gut Microbiota and Childhood Malnutrition: Understanding the Link and Exploring Therapeutic Interventions. Eng. Life Sci. 2023, 24, 2300070. [Google Scholar] [CrossRef]
- Chibuye, M.; Mende, D.R.; Spijker, R.; Simuyandi, M.; Luchen, C.C.; Bosomprah, S.; Chilengi, R.; Schultsz, C.; Harris, V.C. Systematic Review of Associations between Gut Microbiome Composition and Stunting in Under-Five Children. NPJ Biofilms Microbiomes 2024, 10, 46. [Google Scholar] [CrossRef]
- Al-Qadami, G.H.; Secombe, K.R.; Subramaniam, C.B.; Wardill, H.R.; Bowen, J.M. Gut Microbiota-Derived Short-Chain Fatty Acids: Impact on Cancer Treatment Response and Toxicities. Microorganisms 2022, 10, 2048. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Cai, J.; Rimal, B.; Rocha, E.R.; Coleman, J.P.; Zhang, C.; Nichols, R.G.; Luo, Y.; Kim, B.; et al. Bile Salt Hydrolase in Nonenterotoxigenic Bacteroides Potentiates Colorectal Cancer. Nat. Commun. 2023, 14, 755. [Google Scholar] [CrossRef]
- Kaakoush, N.O. Insights into the Role of Erysipelotrichaceae in the Human Host. Front. Cell Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef]
- Lee, Y.K. Effects of Diet on Gut Microbiota Profile and the Implications for Health and Disease. Biosci. Microbiota Food Health 2013, 32, 1–12. [Google Scholar] [CrossRef]
- Dahl, W.J.; Rivero Mendoza, D.; Lambert, J.M. Diet, Nutrients and the Microbiome. Prog. Mol. Biol. Transl. Sci. 2020, 171, 237–263. [Google Scholar] [CrossRef] [PubMed]
- Healey, G.; Murphy, R.; Butts, C.; Brough, L.; Whelan, K.; Coad, J. Habitual Dietary Fibre Intake Influences Gut Microbiota Response to an Inulin-Type Fructan Prebiotic: A Randomised, Double-Blind, Placebo-Controlled, Cross-Over, Human Intervention Study. Br. J. Nutr. 2018, 119, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Miyata, N.; Takakura, S.; Yoshihara, K.; Asano, Y.; Kimura-Todani, T.; Yamashita, M.; Zhang, X.T.; Watanabe, N.; Mikami, K.; et al. The Gut Microbiome Derived From Anorexia Nervosa Patients Impairs Weight Gain and Behavioral Performance in Female Mice. Endocrinology. 2019, 160, 2441–2452. [Google Scholar] [CrossRef] [PubMed]
- Ecklu-Mensah, G.; Choo-Kang, C.; Maseng, M.G.; Donato, S.; Bovet, P.; Viswanathan, B.; Bedu-Addo, K.; Plange-Rhule, J.; Oti Boateng, P.; Forrester, T.E.; et al. Gut microbiota and fecal short chain fatty acids differ with adiposity and country of origin: The METS-microbiome study. Nat. Commun. 2023, 14, 5160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakaji, S.; Sugawara, K.; Saito, D.; Yoshioka, Y.; MacAuley, D.; Bradley, T.; Kernohan, G.; Baxter, D. Trends in dietary fiber intake in Japan over the last century. Eur. J. Nutr. 2002, 41, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.nibn.go.jp/eiken/kenkounippon21/eiyouchousa/keinen_henka_eiyou.html (accessed on 11 October 2025). (In Japanese).
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.W.; Hirose, Y.; Morita, H.; Hattori, M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016, 23, 125–133. [Google Scholar] [CrossRef]
- Saviano, A.; Candelli, M.; Brigida, M.; Petruzziello, C.; Tilli, P.; Franceschi, F.; Ojetti, V. How Shift Work Affects Our Gut Microbiota: Impact on Gastrointestinal Diseases. Medicina 2025, 61, 995. [Google Scholar] [CrossRef]
- Yao, T.; Chao, Y.P.; Huang, C.M.; Lee, H.C.; Liu, C.Y.; Li, K.W.; Hsu, A.L.; Tung, Y.T.; Wu, C.W. Impacts of night shift on medical professionals: A pilot study of brain connectivity and gut microbiota. Front. Neurosci. 2025, 19, 1503176. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Mondragon, A.D.C.; Cardelle-Cobas, A.; Santos, E.M.; Porto-Arias, J.J.; Cepeda, A.; Miranda, J.M. Effects of Unconventional Work and Shift Work on the Human Gut Microbiota and the Potential of Probiotics to Restore Dysbiosis. Nutrients 2023, 15, 3070. [Google Scholar] [CrossRef]
- Iizuka, K.; Deguchi, K.; Ushiroda, C.; Yanagi, K.; Seino, Y.; Suzuki, A.; Yabe, D.; Sasaki, H.; Sasaki, S.; Saitoh, E.; et al. A Study on the Compatibility of a Food-Recording Application with Questionnaire-Based Methods in Healthy Japanese Individuals. Nutrients 2024, 16, 1742. [Google Scholar] [CrossRef]
- Murakami, K.; Sasaki, S.; Takahashi, Y.; Uenishi, K.; Yamasaki, M.; Hayabuchi, H.; Goda, T.; Oka, J.; Baba, K.; Ohki, K.; et al. Misreporting of dietary energy, protein, potassium and sodium in relation to body mass index in young Japanese women. Eur. J. Clin. Nutr. 2008, 62, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Burrows, T.L.; Ho, Y.Y.; Rollo, M.E.; Collins, C.E. Validity of Dietary Assessment Methods When Compared to the Method of Doubly Labeled Water: A Systematic Review in Adults. Front. Endocrinol. 2019, 10, 850. [Google Scholar] [CrossRef]
- de Freitas, M.; Steemburgo, T.; de Paula, T. Dietary Assessment Methods for Adults: A Systematic Review to Evaluate the Most Adequate Food Consumption Instrument. Curr. Dev. Nutr. 2022, 6 (Suppl. S1), 358. [Google Scholar] [CrossRef] [PubMed Central]




| Variable | Normal Weight Group (C) | Underweight Group (L) | p |
|---|---|---|---|
| (n = 40) | (n = 40) | ||
| Age, years | 27.88 (4.65) | 27.40 (4.49) | 0.65 |
| Weight, kg | 53.84 (5.78) | 43.36 (4.15) | <0.001 |
| Height, cm | 160.86 (6.05) | 159.17 (5.10) | 0.19 |
| BMI, kg/m2 | 20.77 (1.41) | 17.07 (0.97) | <0.001 |
| Energy intake, kcal/d | 1650.0 (666.6) | 1564.0 (341.3) | 0.48 |
| Carbohydrate, g/d | 212.73 (127.02) | 205.48 (44.24) | 0.74 |
| Protein intake, g/d | 55.21 (18.32) | 53.90 (14.70) | 0.73 |
| Lipid intake, g/d | 57.10 (15.52) | 55.15 (14.38) | 0.57 |
| Dietary fiber, g/d | 10.31 (4.54) | 10.16 (3.33) | 0.87 |
| Dietary Pattern | ||||
|---|---|---|---|---|
| Index | χ2 (df = 1) | p-Value | Cliff’s δ (95% CI) | Magnitude |
| Shannon index | 1.06 | 0.30 | −0.13 (−0.37, 0.12) | Negligible |
| Simpson’s diversity index | 0.84 | 0.36 | 0.12 (−0.13, 0.35) | Negligible |
| Pielou index | 0.37 | 0.54 | −0.079 (−0.32, 0.18) | Negligible |
| Gut Microbiota | ||||
| Index | χ2 (df = 1) | p-Value | Cliff’s δ (95% CI) | Magnitude |
| Shannon index | 20.37 | <0.001 | 0.59 (0.36, 0.75) | large |
| Simpson’s diversity index | 18.09 | <0.001 | 0.55 (0.32, 0.72) | large |
| Pielou index | 26.4 | <0.001 | 0.67 (0.46, 0.81) | large |
| Shannon Index (Diet) | Shannon Index (Gut Microbiota) | |||
|---|---|---|---|---|
| ρ (95% CI) | p (Two-Tailed) | ρ (95% CI) | p (Two-Tailed) | |
| BMI | −0.19 [−0.41, 0.029] | 0.077 | 0.49 [0.30, 0.65] | <0.0001 |
| Age | 0.026 [−0.20, 0.25] | 0.82 | 0.00042 [−0.22, 0.23] | 0.97 |
| Energy intake | 0.28 [0.062, 0.47] | 0.013 | −0.066 [−0.28, 0.16] | 0.56 |
| Carbohydrate intake | 0.20 [−0.017, 0.41] | 0.07 | −0.10 [−0.32, 0.12] | 0.37 |
| Protein intake | 0.49 [0.31, 0.64] | <0.0001 | −0.10 [−0.31, 0.2] | 0.36 |
| Lipid intake | 0.31 [0.99, 0.50] | 0.0048 | 0.027 [−0.19, 0.25] | 0.81 |
| Dietary fiber intake | 0.63 [0.47, 0.75] | <0.0001 | −0.13 [−0.35, 0.095] | 0.24 |
| Dietary Pattern | |||||||
|---|---|---|---|---|---|---|---|
| Section | Metric | C (n = 40) | L (n = 40) | Contrast (Pair) | R2 | F | p |
| PERMANOVA | Between-group composition | — | — | — | ≈0 | ≈0 | 0.99 |
| PERMDISP (overall) | Dispersion difference | — | — | — | — | 0.44 | 0.52 |
| PERMDISP (Tukey) | Pairwise | — | — | −0.013 (−0.050, 0.025) | — | — | 0.51 |
| Distance to centroid | Mean (BCa 95% CI) | 0.14 (0.13–0.17) | 0.13 (0.11–0.17) | — | — | — | — |
| Distance to centroid | Median (BCa 95% CI) | 0.14 (0.11–0.15) | 0.11 (0.087–0.13) | — | — | — | — |
| Dietary pattern | |||||||
| Gut Microbiome | |||||||
| Section | Metric | C (n = 40) | L (n = 40) | Contrast (Pair) | R2 | F | p |
| PERMANOVA | Between-group composition | — | — | — | 0.064 | 5.31 | 0.0001 |
| PERMDISP (overall) | Dispersion difference | — | — | — | — | 3.21 | 0.072 |
| PERMDISP (Tukey) | Pairwise | — | — | 0.025 (−0.0028, 0.053) | — | — | 0.079 |
| Distance to centroid | Mean (BCa 95% CI) | 0.28 (0.27, 0.30) | 0.31 (0.29, 0.33) | — | — | — | — |
| Distance to centroid | Median (BCa 95% CI) | 0.27 (0.25, 0.31) | 0.29 (0.27, 0.31) | — | — | — | — |
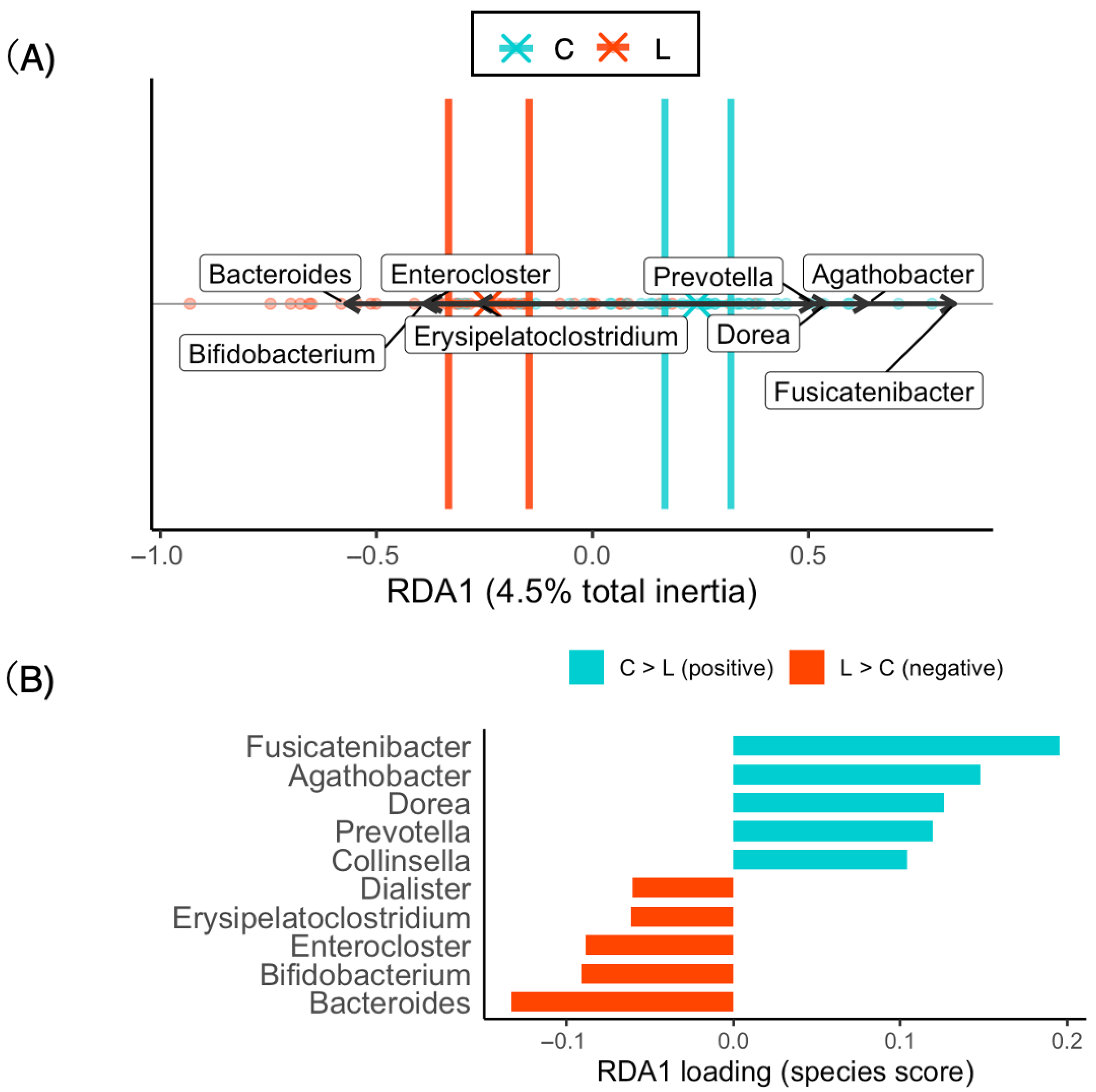
| Section | Metric | Value | Axis | Prop (Fraction) | Prop (%) | p | Term | F | df |
|---|---|---|---|---|---|---|---|---|---|
| Model summary | Total inertia | 0.28 | |||||||
| Model summary | R2 | 0.045 | |||||||
| Model summary | Adjusted R2 | 0.032 | |||||||
| Model summary | Permutation F | 3.65 | |||||||
| Model summary | Permutation p | 0.0005 | |||||||
| Model summary | Permutations (N) | ||||||||
| 9999 | |||||||||
| Model summary | Significant axes (p < 0.05) | 1 | |||||||
| Model summary | Significant terms (p < 0.05) | 1 | |||||||
| Model summary | List of significant terms | BMI group | |||||||
| Model summary | R2 | 0.045 | |||||||
| Per-axis | RDA1 | 1 | 100.00% | 0.0005 | |||||
| Per-term | 0.0005 | BMI group | 3.65 | 1 |
| Feature | diff.btw | Effect | wi.ep | wi.eBH | we.ep | we.eBH |
|---|---|---|---|---|---|---|
| Bacteroides | −0.94 | −0.54 | <0.001 | 0.011 | <0.001 | 0.013 |
| Enterocloster | −2.74 | −0.63 | <0.001 | <0.001 | <0.001 | 0.013 |
| Erysipelatoclostridium | −2.64 | −0.52 | <0.001 | 0.0085 | <0.001 | 0.015 |
| Dorea | 4.89 | 0.63 | <0.001 | 0.019 | <0.001 | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto-Wada, R.; Hiraiwa, E.; Okuma, K.; Yamada, M.; Ushiroda, C.; Deguchi, K.; Naruse, H.; Masuyama, H.; Iizuka, K. Gut Microbiota α- and β-Diversity, but Not Dietary Patterns, Differ Between Underweight and Normal-Weight Japanese Women Aged 20–39 Years. Nutrients 2025, 17, 3265. https://doi.org/10.3390/nu17203265
Yamamoto-Wada R, Hiraiwa E, Okuma K, Yamada M, Ushiroda C, Deguchi K, Naruse H, Masuyama H, Iizuka K. Gut Microbiota α- and β-Diversity, but Not Dietary Patterns, Differ Between Underweight and Normal-Weight Japanese Women Aged 20–39 Years. Nutrients. 2025; 17(20):3265. https://doi.org/10.3390/nu17203265
Chicago/Turabian StyleYamamoto-Wada, Risako, Eri Hiraiwa, Kana Okuma, Masako Yamada, Chihiro Ushiroda, Kanako Deguchi, Hiroyuki Naruse, Hiroaki Masuyama, and Katsumi Iizuka. 2025. "Gut Microbiota α- and β-Diversity, but Not Dietary Patterns, Differ Between Underweight and Normal-Weight Japanese Women Aged 20–39 Years" Nutrients 17, no. 20: 3265. https://doi.org/10.3390/nu17203265
APA StyleYamamoto-Wada, R., Hiraiwa, E., Okuma, K., Yamada, M., Ushiroda, C., Deguchi, K., Naruse, H., Masuyama, H., & Iizuka, K. (2025). Gut Microbiota α- and β-Diversity, but Not Dietary Patterns, Differ Between Underweight and Normal-Weight Japanese Women Aged 20–39 Years. Nutrients, 17(20), 3265. https://doi.org/10.3390/nu17203265






