Unveiling Dietary Complexity: A Scoping Review and Reporting Guidance for Network Analysis in Dietary Pattern Research
Abstract
1. Introduction
1.1. Dietary Patterns and Health
1.2. Traditional Dietary Pattern Analysis
1.3. Network Approaches
2. Methods
2.1. Study Design
2.2. Search Strategy and Selection Criteria
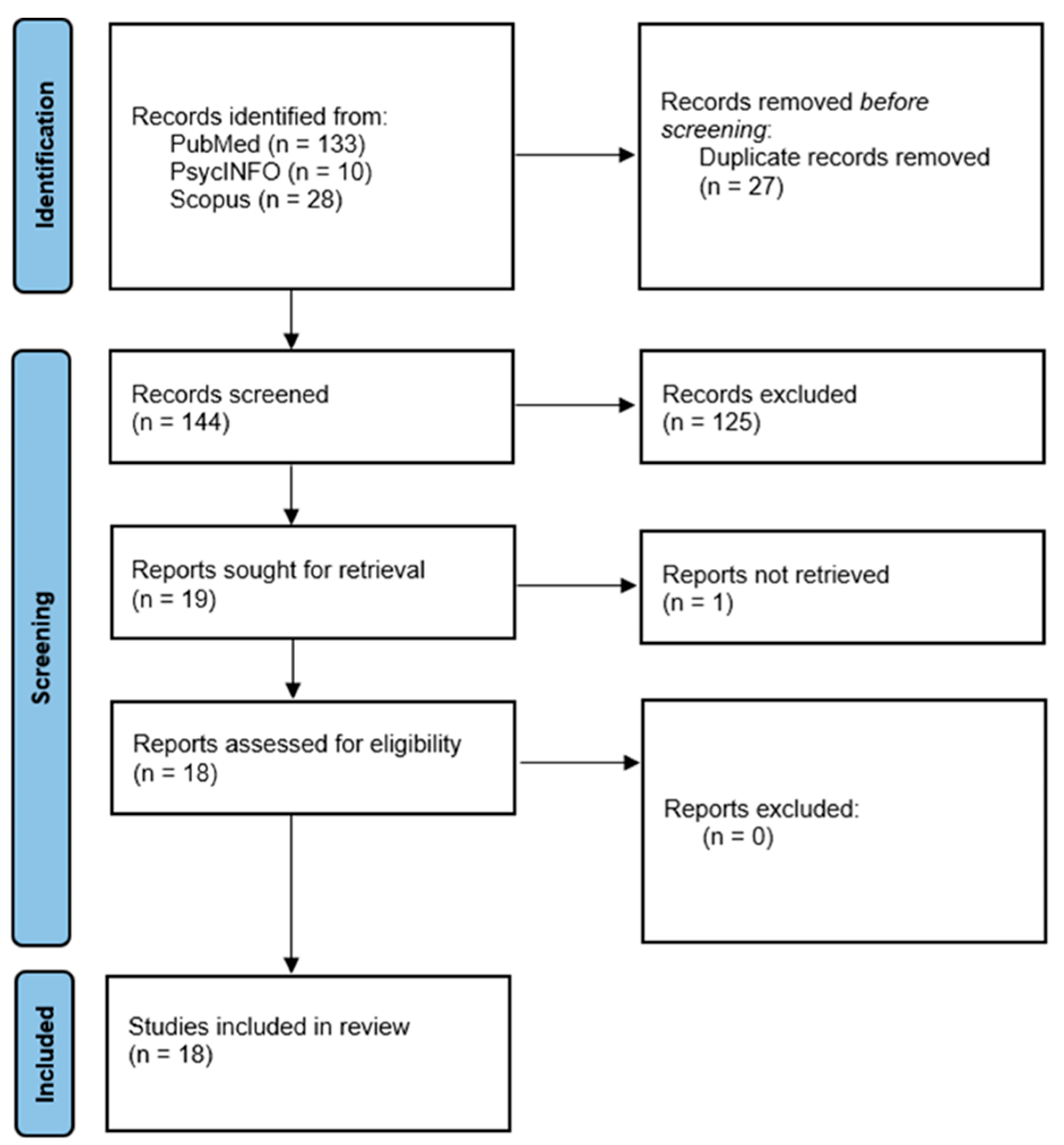
2.3. Screening and Article Selection
2.4. Data Extraction
3. Results
3.1. Search and Selection of Network Studies
3.2. Study Characteristics of Included Network Studies
3.3. Objectives of Included Network Studies
| Author (Year) | Population | Study Design | Dietary Assessment | Network Model | Aims | Findings |
|---|---|---|---|---|---|---|
| Slurink et al. (2023) [45] | 74,132 participants, Lifelines cohort study | Prospective Cohort | Flower-FFQ | MGM | To investigate associations of total dairy and dairy types with incident prediabetes. To assess how dairy intake is linked with metabolic risk factors, lifestyle behaviours, and foods, as potential explanations for these associations. | Low fat milk intake associated with higher prediabetes risk. High-fat yogurt intake had nonsignificant inverse association with prediabetes risk. Associations may be confounded by behaviours relating to dairy intake. |
| Schwedhelm et al. (2021) [47] | 365 women, 12 weeks gestation | Prospective Cohort | Three Automated Self-Administered 24 h dietary recalls | SGCGM | To investigate food networks across meals in pregnant women. To explore differences by overall diet quality classification. | Food combinations differed by meal and between dietary quality tertiles. High diet quality group: vegetables, whole-grain bread, cooked grains and nuts at breakfast. Low diet quality group: Sugar sweetened beverages, sandwiches, and fried potatoes at all main meals. |
| Felicetti et al. (2022) [48] | 424 participants with MS, 165 healthy controls | Cross-Sectional | MeDi adequacy questionnaire | MI | To investigate food networks across meals in people with multiple sclerosis (PwMS) and healthy controls (HC). To explore differences by overall diet quality classification. | PwMS Hubs: Fruit, vegetables, cereal, and fish. HC Hubs: Meat and alcohol. PwMS showed overall healthier dietary pattern than HC. |
| Samieri et al. (2020) [33] | 1522 participants (209 with dementia), 3C study | Nested Case–Control | FFQ | MI | To use network science to model complex diet relationships a decade before onset of dementia in a large French cohort. | Food networks substantially differed between cases and controls. Cases: Charcuterie was the main hub. Controls: Several disconnected subnetworks reflecting healthier choices. |
| Jayedi et al. (2021) [55] | 850 participants | Cross-Sectional | FFQ | GGM | To describe dietary networks identified by GGM, representing patterns of dietary intake in a sample of Iranian adults. To investigate the potential associations of these dietary patterns with general and abdominal adiposity. | Identified 3 dietary networks: healthy, unhealthy, saturated fats. Saturated fats network was associated with a higher likelihood of central obesity. No association with general obesity. |
| Iqbal et al. (2016) [49] | 27,120 participants, EPIC cohort | Cross-Sectional | FFQ | GGM (results confirmed through SGCGM) | To apply GGMs to derive sex-specific dietary intake networks representing consumption patterns in a German adult population. | Men: 1 major network including red meat, processed meat, and cooked vegetables. Women: similar network with addition of fried potatoes. |
| Schwedhelm et al. (2018) [46] | 814 participants, EPIC cohort | Cross-Sectional | Three 24 h recalls | SGCGM | To estimate and describe meal and habitual dietary networks derived through SGCGMs. To compare relations found in meal networks to the ones present in the habitual network. | Meal-specific networks (breakfast, lunch, dinner) had distinct food communities. Meal-specific dietary network only partly reflected in habitual network. |
| Gunathilake et al. (2022) [54] | 7477 participants (397 with cancer), Cancer Screening Cohort | Prospective Cohort | FFQ | GGM (also used PCA and RRR) | To investigate the association between dietary communities identified by a GGM and cancer risk. | A community composed of dairy products and bread was associated with a reduced cancer risk. In a matched population, poultry, seafood, bread, cakes and sweets, and meat byproducts showed significantly reduced risk of cancer. |
| Iqbal et al. (2019) [58] | 22,245 participants, EPIC cohort | Prospective Cohort | FFQ | GGM (also used PCA) | To investigate the association between previously identified GGMs food intake networks and risk of major chronic diseases as well as intermediate biomarkers in the EPIC-Potsdam cohort. | A Western-type pattern was associated with increased risk of type 2 diabetes in women. A high-fat dairy pattern associated with lower risk of type 2 diabetes in both sexes. |
| Hoang et al. (2021a) [53] | 10,777 participants (1049 with cancer), Cancer Screening Cohort | Cross-Sectional | FFQ | GGM | To identify major dietary patterns of Korean adults using a GGM. To examine the associations between dietary pattern (DP) scores and prevalence of self-reported cancer. | Identified 4 networks: principal, oil-sweet, meat, and fruit. Consumption of the oil-sweet pattern was lower in cancer patients, while meat and fruit pattern consumption was higher. |
| Jahanmiri et al. (2022) [56] | 850 participants | Cross-Sectional | FFQ | GGM | To derive dietary networks and assess their association with metabolic syndrome. | Identified 3 networks: healthy, unhealthy and saturated fats. The saturated fats network was associated with high odds of metabolic syndrome. |
| Aguirre-Quezada and Aranda-Ramírez (2024) [57] | 230 students | Cross-Sectional | FFQ | GGM | To apply GGMs to derived specific networks for groups of healthy and unhealthy obese individuals that represent the nutritional, psychological, and metabolic patterns in an Ecuadorian population. | Higher carbohydrate intake was associated with lower protein intake. For metabolically unhealthy obese individuals, intake of fibre, proteins, carbs, and fats was positively related to BMI. |
| Hoang et al. (2021b) [59] | 7423 participants, Cancer Screening Cohort | Cross-Sectional | FFQ | MGM | To elucidate the complex interrelatedness among dietary intake, demographics, and risk of comorbidities. | Normal and heavy eating significantly associated with increased risks of elevated BP, hypertension, and mild kidney impairment. |
| Landaeta-Díaz et al. (2023) [61] | 1242 participants | Cross-Sectional | FFQ | GGM | To explore food networks in the Chilean adult sample and in people with anhedonia symptoms. | Fruits, vegetables, and fast foods have central role in the main sample. In the anhedonia network, “pasta, rice and potatoes” and “bread” were more central. |
| Xia et al. (2020) [60] | 2043 matched controls for 2043 newly diagnosed non-alcoholic fatty liver disease (NAFLD) | Case–Control | FFQ | MI | To construct dietary networks from network science. To explore the associations between complex dietary networks and NAFLD. | Dietary structures differed between groups. The case group had two major networks while the control group had one. |
| Fereidani et al. (2021) [52] | 134 women with breast cancer, 266 hospital controls | Case–Control | FFQ | GGM | To compare food intake networks derived by GGMs for women with and without breast cancer to better understand how foods are consumed in relation to each other according to disease status. | Vegetables, fruits, sweets, and fried potatoes were central in both networks. The network of cases showed more conditional dependencies between foods than controls. |
| Gunathilake et al. (2020) [50] | 415 gastric cancer cases, 830 controls, Cancer Screening Cohort | Case–Control | FFQ | GGM | To apply GGMs to identify dietary patterns. To investigate the associations between dietary patterns and gastric cancer risk in a Korean population. | Vegetable/seafood and fruit networks were associated with a decreased risk of GC. Highest tertile of vegetable/seafood score had a reduced risk of GC. |
| Gunathilake et al. (2021) [51] | 268 patients with GC, 288 healthy controls | Case–Control | FFQ | GGM | To observe the combined effects of GGM-derived dietary patterns and the gastric microbiome on the risk of gastric cancer in a Korean population. | Vegetable/seafood pattern may interact with dysbiosis to attenuate the risk of GC in males. Dairy pattern may interact with dysbiosis to reduce GC risk in females. |
3.4. Adherence to Methodological Best Practices
3.4.1. Justifications for Using Network Models
3.4.2. Study Design and Causal Inference
3.4.3. Network Estimation and Regularisation
3.4.4. Use and Interpretation of Centrality Metrics
3.4.5. Handling of Non-Normal Data
| Author (Year) | Justification for Using Network Models | Study Design and Causal Inference | Network Estimation and Regularisation | Use of Centrality Metrics | Handling of Non-Normal Data |
|---|---|---|---|---|---|
| Slurink et al. (2023) [45] | Used to aid interpretation of regression models. Holistic approach to aid traditional reductionist methods. | Did not attempt to make inferences about causality. | Used LASSO regularisation. λ value of 0.5 reported. | Uses centrality metrics. Did not discuss limitation. | N/A (used MGM). |
| Schwedhelm et al. (2021) [47] | Addressed limitations of PCA. Better alternative for revealing meal-specific food combinations. | Did not attempt to make inferences about causality. | Used LASSO regularisation. Did not report λ. Made inferences despite being the first study to test these associations. | Did not use centrality metrics. | Addressed via SGCGM. Excluded episodically consumed foods. |
| Felicetti et al. (2022) [48] | Used to see complex relations hidden in eating behaviour. Complementary to other research. | Acknowledged cross-sectional design prevents causal claims. | Used permutation testing. | Did not use centrality metrics. | N/A (used MI). |
| Samieri et al. (2020) [33] | Provided complementary information to other approaches. Gained additional insights into food-disease associations. | Did not attempt to make inferences about causality. | Used permutation testing. Used thresholding (edge weight >40). | Used centrality metrics. Did not discuss limitations. | N/A (used MI). |
| Jayedi et al. (2021) [55] | Addressed limitations of PCA. | Acknowledged cross-sectional design as a limitation but did not specify why. | Used LASSO regularisation. Did not report λ. Made inferences despite being the first study to test these associations. | Used centrality metrics. Did not discuss limitations. | Acknowledged the normality assumption. Did not apply any correction. |
| Iqbal et al. (2016) [49] | Limitations of existing methods of dietary pattern analysis warrant investigation of complementary approaches. | Did not attempt to make inferences about causality. | Used LASSO regularisation. λ value of 0.25 reported. Performed network stability analysis (bootstrapping). | Did not use centrality metrics. | Addressed via log-transformation. Confirmed GGM results with SGCGM. |
| Schwedhelm et al. (2018) [46] | Addressed limitations of traditional methods in understanding how patterns arise. | Did not attempt to make inferences about causality. | Used LASSO regularisation with cross-validation. Did not report tuning parameter (λ). | Used centrality metrics to assist interpretation. Did not discuss limitations. | Addressed by using SGCGM instead of GGM. |
| Gunathilake et al. (2022) [54] | Used GGM to derive dietary communities. Compared with PCA and RRR. | Used a prospective cohort design, made causal inferences. | Used LASSO regularisation. Optimal λ values reported, 0.32 and 0.34. | Used centrality metrics. Did not discuss limitations. | Addressed via log-transformation. |
| Iqbal et al. (2019) [58] | Used GGM to investigate diet-disease relationships. Reconstructed PCA patterns for comparison. | Did not attempt to make inferences about causality. | Used LASSO regularisation. Referred to previous publication for regularisation details. | Used centrality metrics. Did not discuss limitations. | Addressed via log-transformation. |
| Hoang et al. (2021a) [53] | Addressed limitations of PCA and RRR. Used GGM to resolve issues between methods. | Acknowledged cross-sectional design was not strong enough for causal claims. | Used LASSO regularisation. Optimal λ values reported (0.48, 0.52, 0.46). | Used centrality metrics. Did not discuss limitations. | Addressed via log-transformation. |
| Jahanmiri et al. (2022) [56] | Framed GGM as a “commanding method” compared to reductionist traditional techniques. | Acknowledged cross-sectional design prevents cause-and-effect conclusions. | Used LASSO regularisation. Did not report λ. | Used centrality metrics. Did not discuss limitations. | Acknowledged the normality assumption as a limitation. Did not apply any correction. |
| Aguirre-Quezada and Aranda-Ramírez (2024) [57] | Addressed limitations in previous studies analyses. Praised GGM for providing a comprehensive overview. | Did not attempt to make inferences about causality. | Used LASSO regularisation. Explored a range of λ rather than selecting one. | Used centrality metrics. Did not discuss limitations. | Acknowledged the normality assumption as a limitation. Did not apply any correction. |
| Hoang et al. (2021b) [59] | Addressed limitations of conventional approaches. Used network analysis to explore complex interactions. | Acknowledged cross-sectional design may not allow for a full investigation of causality. | Used LASSO regularisation. λ value of 0.5 reported. Assessed network accuracy via bootstrapping. | Used centrality metrics. Did not discuss limitations. | N/A (used MGM). |
| Landaeta-Díaz et al. (2023) [61] | Addressed limitations of diet scores. Used GGM to represent the underlying structure of food groups. | Did not attempt to make inferences about causality. | Did not use any regularisation techniques. | Used centrality metrics. Did not discuss limitations. | Did not acknowledge the normality assumption. Did not apply any correction. |
| Xia et al. (2020) [60] | Addressed limitations of traditional statistical methods. Used network methods to provide new insight. | Did not attempt to make inferences about causality. | Used thresholding (edge weight ≥ 0.30) for interpretability. | Did not use centrality metrics. | N/A (used MI). |
| Fereidani et al. (2021) [52] | Addressed limitations of existing methods. Used GGM to show how foods are consumed in different combinations. | Did not attempt to make inferences about causality. | Used LASSO regularisation. λ value of 0.3 reported. | Used a nonstandard definition of “central food groups”. | Addressed via log-transformation. |
| Gunathilake et al. (2020) [50] | Used as a complementary strategy for investigating diet-disease relationships. | Did not attempt to make inferences about causality. | Used LASSO regularisation. Optimum λ value reported (0.38). | Used centrality metrics. Did not discuss limitations. | Addressed via log-transformation. |
| Gunathilake et al. (2021) [51] | Assessed diet as a pattern rather than a sum of single food items. | Did not attempt to make inferences about causality. | Used LASSO regularisation. Optimum λ value reported (0.37). | Used centrality metrics. Did not discuss limitations. | Addressed via log-transformation. |
3.5. Synthesis of Methodological Adherence
4. Discussion
4.1. Guiding Principles for Future Research
4.1.1. Principle 1: Selecting Appropriate Models
4.1.2. Principle 2: Aligning Study Designs with Research Questions
4.1.3. Principle 3: Best Practices for Reliable Network Estimation
4.1.4. Principle 4: Valid Interpretation of Network Metrics
4.1.5. Principle 5: Addressing Non-Normality in Dietary Data
4.2. Future Directions
4.3. Strengths and Limitations of the Current Work
4.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DASH | Dietary approach to stop hypertension |
| PCA | Principal component analysis |
| GGM | Gaussian graphical model |
| MGM | Mixed graphical model |
| MI | Mutual information |
| BN | Bayesian networks |
| EPIC | European Prospective Investigation into Cancer and Nutrition |
| SGCGM | Semiparametric Gaussian copula graphical model |
| RRR | Reduced rank regression |
| MeDi | Mediterranean diet |
| PwMS | People with multiple sclerosis |
| HC | Healthy controls |
References
- Becerra-Tomás, N.; Blanco Mejía, S.; Viguiliouk, E.; Khan, T.; Kendall, C.W.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1207–1227. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; García-López, M.; Bes-Rastrollo, M.; Toledo, E.; Martínez-Lapiscina, E.H.; Delgado-Rodriguez, M.; Vazquez, Z.; Benito, S.; Beunza, J.J. Mediterranean diet and the incidence of cardiovascular disease: A Spanish cohort. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Petersson, S.D.; Philippou, E. Mediterranean diet, cognitive function, and dementia: A systematic review of the evidence. Adv. Nutr. 2016, 7, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Sezaki, A.; Imai, T.; Miyamoto, K.; Kawase, F.; Shirai, Y.; Abe, C.; Sanada, M.; Inden, A.; Rato, T.; Sugihara, N. Association between the Mediterranean diet score and healthy life expectancy: A global comparative study. J. Nutr. Health Aging 2022, 26, 621–627. [Google Scholar] [CrossRef]
- Peng, W.; Liu, Y.; Liu, Y.; Zhao, H.; Chen, H. Major dietary patterns and their relationship to obesity among urbanized adult Tibetan pastoralists. Asia Pac. J. Clin. Nutr. 2019, 28, 507–519. [Google Scholar] [PubMed]
- Rakhra, V.; Galappaththy, S.L.; Bulchandani, S.; Cabandugama, P.K. Obesity and the western diet: How we got here. Mo. Med. 2020, 117, 536. [Google Scholar]
- Fabiani, R.; Minelli, L.; Bertarelli, G.; Bacci, S. A western dietary pattern increases prostate cancer risk: A systematic review and meta-analysis. Nutrients 2016, 8, 626. [Google Scholar] [CrossRef]
- Young, H.A.; Geurts, L.; Scarmeas, N.; Benton, D.; Brennan, L.; Farrimond, J.; Kiliaan, A.J.; Pooler, A.; Trovò, L.; Sijben, J.; et al. Multi-nutrient interventions and cognitive ageing: Are we barking up the right tree? Nutr. Res. Rev. 2023, 36, 471–483. [Google Scholar] [CrossRef]
- Bánáti, D.; Hellman-Regen, J.; Mack, I.; Young, H.A.; Benton, D.; Eggersdorfer, M.; Rohn, S.; Dulińska-Litewka, J.; Krężel, W.; Rühl, R. Defining a vitamin A5/X specific deficiency–vitamin A5/X as a critical dietary factor for mental health. Int. J. Vitam. Nutr. Res. 2024, 94, 443–475. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Gao, Q.; Zhao, H.; Chen, S.; Huang, L.; Wang, W.; Wang, T. A review of statistical methods for dietary pattern analysis. Nutr. J. 2021, 20, 37. [Google Scholar] [CrossRef]
- Naska, A.; Lagiou, A.; Lagiou, P. Dietary assessment methods in epidemiological research: Current state of the art and future prospects. F1000Research 2017, 6, 926. [Google Scholar] [CrossRef]
- Schulz, C.-A.; Oluwagbemigun, K.; Nöthlings, U. Advances in dietary pattern analysis in nutritional epidemiology. Eur. J. Nutr. 2021, 60, 4115–4130. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Wu, W.-K.; Panyod, S.; Wu, M.-S.; Sheen, L.-Y. The Protective Effect of Garlic Essential Oil in Carnitine-Induced Cardiovascular Disease apoE-/-Mice Model. Curr. Dev. Nutr. 2020, 4, nzaa062_029. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R.; Erlinger, T.P.; Appel, L.J. The effects of macronutrients on blood pressure and lipids: An overview of the DASH and OmniHeart trials. Curr. Cardiovasc. Risk Rep. 2007, 1, 46–51. [Google Scholar] [CrossRef]
- Morgenstern, J.D.; Rosella, L.C.; Costa, A.P.; de Souza, R.J.; Anderson, L.N. Perspective: Big Data and Machine Learning Could Help Advance Nutritional Epidemiology. Adv Nutr 2021, 12, 621–631. [Google Scholar] [CrossRef]
- O’Leary, D.; Smith, A.; Salehi, E.; Gross, J.J. Negative Affect, Affect Regulation, and Food Choice: A Value-Based Decision-Making Analysis. Soc. Psychol. Personal. Sci. 2023, 14, 295–304. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Newby, P.; Tucker, K.L. Empirically derived eating patterns using factor or cluster analysis: A review. Nutr. Rev. 2004, 62, 177–203. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Zhong, J.; Wang, Y.; Zhou, E.; Hong, F. Association between obesity phenotypes and dietary patterns: A two-step cluster analysis based on the China multi-ethnic cohort study. Prev. Med. 2024, 187, 108100. [Google Scholar] [CrossRef]
- Ma, T.; Tu, K.; Ou, Q.; Fang, Y.; Zhang, C. Comparing the associations of dietary patterns identified through principal component analysis and cluster analysis with colorectal cancer risk: A large case–control study in China. Nutrients 2023, 16, 147. [Google Scholar] [CrossRef]
- Jacques, P.F.; Tucker, K.L. Are dietary patterns useful for understanding the role of diet in chronic disease? 12. Am. J. Clin. Nutr. 2001, 73, 1–2. [Google Scholar] [CrossRef]
- Panagiotakos, D. α-Priori versus α-posterior methods in dietary pattern analysis: A review in nutrition epidemiology. Nutr. Bull. 2008, 33, 311–315. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Cartus, A.R.; Kirkpatrick, S.I.; Himes, K.P.; Kennedy, E.H.; Simhan, H.N.; Grobman, W.A.; Duffy, J.Y.; Silver, R.M.; Parry, S.; et al. Machine learning as a strategy to account for dietary synergy: An illustration based on dietary intake and adverse pregnancy outcomes. Am. J. Clin. Nutr. 2020, 111, 1235–1243. [Google Scholar] [CrossRef]
- Benton, D.; Young, H.A. Early exposure to sugar sweetened beverages or fruit juice differentially influences adult adiposity. Eur. J. Clin. Nutr. 2024, 78, 521–526. [Google Scholar] [CrossRef]
- Jacobs, D.R.; Tapsell, L.C.; Temple, N.J. Food synergy: The key to balancing the nutrition research effort. Public Health Rev. 2011, 33, 507–529. [Google Scholar] [CrossRef]
- Hevey, D. Network analysis: A brief overview and tutorial. Health Psychol. Behav. Med. 2018, 6, 301–328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Q.; Huang, J. Examining the network dynamics of daily movement and dietary behaviors among college students: A diary study. Appl. Psychol. Health Well-Being 2025, 17, e12631. [Google Scholar] [CrossRef]
- Brown, I.J.; Stamler, J.; Van Horn, L.; Robertson, C.E.; Chan, Q.; Dyer, A.R.; Huang, C.C.; Rodriguez, B.L.; Zhao, L.; Daviglus, M.L.; et al. Sugar-sweetened beverage, sugar intake of individuals, and their blood pressure: International study of macro/micronutrients and blood pressure. Hypertension 2011, 57, 695–701. [Google Scholar] [CrossRef]
- Altenbuchinger, M.; Weihs, A.; Quackenbush, J.; Grabe, H.J.; Zacharias, H.U. Gaussian and Mixed Graphical Models as (multi-)omics data analysis tools. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2020, 1863, 194418. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Baker, Y.; Ravikumar, P.; Allen, G.; Liu, Z. Mixed graphical models via exponential families. In Proceedings of the Artificial Intelligence and Statistics, Reykjavic, Iceland, 22–25 April 2014; pp. 1042–1050. [Google Scholar]
- Reshef, D.N.; Reshef, Y.A.; Finucane, H.K.; Grossman, S.R.; McVean, G.; Turnbaugh, P.J.; Lander, E.S.; Mitzenmacher, M.; Sabeti, P.C. Detecting novel associations in large data sets. Science 2011, 334, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Sonawane, A.R.; Lefèvre-Arbogast, S.; Helmer, C.; Grodstein, F.; Glass, K. Using network science tools to identify novel diet patterns in prodromal dementia. Neurology 2020, 94, e2014–e2025. [Google Scholar] [CrossRef] [PubMed]
- Conrady, S.; Jouffe, L. Introduction to Bayesian Networks & Bayesialab; Bayesia SAS: Change, France, 2013. [Google Scholar]
- Sadler, M.J.; McNulty, H.; Gibson, S. Sugar-fat seesaw: A systematic review of the evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 338–356. [Google Scholar] [CrossRef]
- Regazzoni, C.; Murino, V.; Vernazza, G. Distributed propagation of a-priori constraints in a Bayesian network of Markov random fields. IEE Proc. I (Commun. Speech Vis.) 1993, 140, 46–55. [Google Scholar] [CrossRef]
- Niedermayer, D. An introduction to Bayesian networks and their contemporary applications. In Innovations in Bayesian Networks: Theory and Applications; Springer: Berlin/Heidelberg, Germany, 2008; pp. 117–130. [Google Scholar]
- Casteigts, A.; Flocchini, P.; Quattrociocchi, W.; Santoro, N. Time-varying graphs and dynamic networks. Int. J. Parallel Emergent Distrib. Syst. 2012, 27, 387–408. [Google Scholar] [CrossRef]
- Holme, P.; Saramäki, J. Temporal networks. Phys. Rep. 2012, 519, 97–125. [Google Scholar] [CrossRef]
- Bretto, A. Hypergraph theory. In An introduction. Mathematical Engineering; Springer: Cham, Swiztherland, 2013; p. 1. [Google Scholar]
- Hayat, M.K.; Xue, S.; Wu, J.; Yang, J. Heterogeneous hypergraph embedding for node classification in dynamic networks. IEEE Trans. Artif. Intell. 2024, 5, 5465–5477. [Google Scholar] [CrossRef]
- Marinazzo, D.; Van Roozendaal, J.; Rosas, F.E.; Stella, M.; Comolatti, R.; Colenbier, N.; Stramaglia, S.; Rosseel, Y. An information-theoretic approach to hypergraph psychometrics. arXiv 2022, arXiv:2205.01035. [Google Scholar] [CrossRef]
- Kivelä, M.; Arenas, A.; Barthelemy, M.; Gleeson, J.P.; Moreno, Y.; Porter, M.A. Multilayer networks. J. Complex Netw. 2014, 2, 203–271. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, L.; Xiong, J.; Zhang, Q.; Cheng, G.; Ding, G. Children’s dietary patterns and dietary networks in five regions in China. Wei Sheng Yan Jiu 2024, 53, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Slurink, I.A.; Corpeleijn, E.; Bakker, S.J.; Jongerling, J.; Kupper, N.; Smeets, T.; Soedamah-Muthu, S.S. Dairy consumption and incident prediabetes: Prospective associations and network models in the large population-based Lifelines Study. Am. J. Clin. Nutr. 2023, 118, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, C.; Knüppel, S.; Schwingshackl, L.; Boeing, H.; Iqbal, K. Meal and habitual dietary networks identified through semiparametric Gaussian copula graphical models in a German adult population. PLoS ONE 2018, 13, e0202936. [Google Scholar] [CrossRef]
- Schwedhelm, C.; Lipsky, L.M.; Shearrer, G.E.; Betts, G.M.; Liu, A.; Iqbal, K.; Faith, M.S.; Nansel, T.R. Using food network analysis to understand meal patterns in pregnant women with high and low diet quality. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 101. [Google Scholar] [CrossRef]
- Felicetti, F.; Tommasin, S.; Petracca, M.; De Giglio, L.; Gurreri, F.; Ianniello, A.; Nistri, R.; Pozzilli, C.; Ruggieri, S. Eating hubs in multiple sclerosis: Exploring the relationship between Mediterranean diet and disability status in Italy. Front. Nutr. 2022, 9, 882426. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Buijsse, B.; Wirth, J.; Schulze, M.B.; Floegel, A.; Boeing, H. Gaussian graphical models identify networks of dietary intake in a German adult population. J. Nutr. 2016, 146, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, M.; Lee, J.; Choi, I.J.; Kim, Y.I.; Kim, J. Identification of Dietary Pattern Networks Associated with Gastric Cancer Using Gaussian Graphical Models: A Case-Control Study. Cancers 2020, 12, 1044. [Google Scholar] [CrossRef]
- Gunathilake, M.; Lee, J.H.; Choi, I.J.; Kim, Y.I.; Kim, J.S. Effect of the Interaction between Dietary Patterns and the Gastric Microbiome on the Risk of Gastric Cancer. Nutrients 2021, 13, 2692. [Google Scholar] [CrossRef]
- Fereidani, S.S.; Sedaghat, F.; Eini-Zinab, H.; Heidari, Z.; Jalali, S.; Mohammadi, E.; Naja, F.; Assadi, M.; Rashidkhani, B. Gaussian Graphical Models Identified Food Intake Networks among Iranian Women with and without Breast Cancer: A Case-Control Study. Nutr Cancer 2021, 73, 1890–1897. [Google Scholar] [CrossRef]
- Hoang, T.; Lee, J.; Kim, J. Differences in dietary patterns identified by the Gaussian graphical model in Korean adults with and without a self-reported cancer diagnosis. J. Acad. Nutr. Diet. 2021, 121, 1484–1496. [Google Scholar] [CrossRef]
- Gunathilake, M.; Hoang, T.; Lee, J.; Kim, J. Association between dietary intake networks identified through a Gaussian graphical model and the risk of cancer: A prospective cohort study. Eur. J. Nutr. 2022, 61, 3943–3960. [Google Scholar] [CrossRef]
- Jayedi, A.; Janbozorgi, N.; Djafarian, K.; Yekaninejad, M.S.; Shab-Bidar, S. Dietary networks identified by Gaussian graphical model and general and abdominal obesity in adults. Nutr. J. 2021, 20, 86. [Google Scholar] [CrossRef]
- Jahanmiri, R.; Djafarian, K.; Janbozorgi, N.; Dehghani-Firouzabadi, F.; Shab-Bidar, S. Saturated fats network identified using Gaussian graphical models is associated with metabolic syndrome in a sample of Iranian adults. Diabetol. Metab. Syndr. 2022, 14, 123. [Google Scholar] [CrossRef]
- Aguirre-Quezada, M.A.; Aranda-Ramírez, M.P. Irruption of Network Analysis to Explain Dietary, Psychological and Nutritional Patterns and Metabolic Health Status in Metabolically Healthy and Unhealthy Overweight and Obese University Students: Ecuadorian Case. Nutrients 2024, 16, 2924. [Google Scholar] [CrossRef]
- Iqbal, K.; Schwingshackl, L.; Floegel, A.; Schwedhelm, C.; Stelmach-Mardas, M.; Wittenbecher, C.; Galbete, C.; Knüppel, S.; Schulze, M.B.; Boeing, H. Gaussian graphical models identified food intake networks and risk of type 2 diabetes, CVD, and cancer in the EPIC-Potsdam study. Eur. J. Nutr. 2019, 58, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.; Lee, J.; Kim, J. Network Analysis of Demographics, Dietary Intake, and Comorbidity Interactions. Nutrients 2021, 13, 3563. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, Z.; Zhang, S.; Liu, Y.; Meng, G.; Zhang, Q.; Liu, L.; Wu, H.; Gu, Y.; Wang, Y.; et al. Complex Dietary Topologies in Non-alcoholic Fatty Liver Disease: A Network Science Analysis. Front Nutr 2020, 7, 579086. [Google Scholar] [CrossRef] [PubMed]
- Landaeta-Díaz, L.; Durán-Agüero, S.; González-Medina, G. Exploring food intake networks and anhedonia symptoms in a Chilean Adults sample. Appetite 2023, 190, 107042. [Google Scholar] [CrossRef] [PubMed]
- Neal, Z.P.; Forbes, M.K.; Neal, J.W.; Brusco, M.J.; Krueger, R.; Markon, K.; Steinley, D.; Wasserman, S.; Wright, A.G.C. Critiques of network analysis of multivariate data in psychological science. Nat. Rev. Methods Primers 2022, 2, 90. [Google Scholar] [CrossRef]
- Forbes, M.K.; Wright, A.G.; Markon, K.E.; Krueger, R.F. Quantifying the reliability and replicability of psychopathology network characteristics. Multivar. Behav. Res. 2021, 56, 224–242. [Google Scholar] [CrossRef]
- Epskamp, S.; Fried, E.I. A tutorial on regularized partial correlation networks. Psychol. Methods 2018, 23, 617. [Google Scholar] [CrossRef]
- Margolin, A.A.; Nemenman, I.; Basso, K.; Wiggins, C.; Stolovitzky, G.; Favera, R.D.; Califano, A. ARACNE: An algorithm for the reconstruction of gene regulatory networks in a mammalian cellular context. BMC Bioinform. 2006, 7, S7. [Google Scholar] [CrossRef]
- Mokhtari, E.B.; Ridenhour, B.J. Filtering asvs/otus via mutual information-based microbiome network analysis. BMC Bioinform. 2022, 23, 380. [Google Scholar] [CrossRef]
- Epskamp, S.; Borsboom, D.; Fried, E.I. Estimating psychological networks and their accuracy: A tutorial paper. Behav. Res. Methods 2018, 50, 195–212. [Google Scholar] [CrossRef]
- Hallquist, M.N.; Wright, A.G.; Molenaar, P.C. Problems with centrality measures in psychopathology symptom networks: Why network psychometrics cannot escape psychometric theory. Multivar. Behav. Res. 2021, 56, 199–223. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, S. Community detection in graphs. Phys. Rep. 2010, 486, 75–174. [Google Scholar] [CrossRef]
- Epskamp, S.; Waldorp, L.J.; Mõttus, R.; Borsboom, D. The Gaussian graphical model in cross-sectional and time-series data. Multivar. Behav. Res. 2018, 53, 453–480. [Google Scholar] [CrossRef] [PubMed]
- Yang, S. A Comparison of Different Methods of Zero-Inflated Data Analysis and Its Application in Health Surveys; University of Rhode Island: Kingston, RI, USA, 2014. [Google Scholar]
- Thelwall, M.; Wilson, P. Regression for citation data: An evaluation of different methods. J. Informetr. 2014, 8, 963–971. [Google Scholar] [CrossRef]
- Freedman, L.S.; Schatzkin, A.; Midthune, D.; Kipnis, V. Dealing With Dietary Measurement Error in Nutritional Cohort Studies. JNCI: J. Natl. Cancer Inst. 2011, 103, 1086–1092. [Google Scholar] [CrossRef]
- Arab, L.; Wesseling-Perry, K.; Jardack, P.; Henry, J.; Winter, A. Eight self-administered 24-hour dietary recalls using the Internet are feasible in African Americans and Whites: The energetics study. J. Am. Diet. Assoc. 2010, 110, 857–864. [Google Scholar] [CrossRef]

| Method | Algorithm | Linear/Nonlinear | Assumptions | Strengths/Limitations |
|---|---|---|---|---|
| Principal Component Analysis (PCA) | Eigenvalue decomposition | Linear | Assumes normally distributed data, linear relationships between variables, uncorrelated components. | Identifies what dietary patterns exist in a population. Can determine which foods are consumed together in a diet but does not reveal interactions between those foods. |
| Factor Analysis | Factor extraction | Linear | Assumes normally distributed data, linear relationships, data can be grouped into latent factors. | Can identify the underlying dietary factors that explain variations in food intake. However, does not provide information about how particular foods interact. |
| Cluster Analysis | k-means, hierarchical clustering | Nonlinear | Assumes defined clusters with similar characteristics and independent observations. | Groups individuals based on their dietary patterns. Useful for segmenting consumers based on dietary patterns. Can handle nonlinear associations between variables. Assumes pairwise similarity or proximity but does not explicitly capture direct or indirect interdependencies among multiple variables. |
| Dietary Index/Scores | Predefined scoring | Linear | Assumes each score represents healthfulness, often based on a reference diet. Each component is typically weighted (sometimes equally), ignoring potential interactions between components. Requires prior knowledge. | Can identify how closely an individual’s diet aligns with a healthy/reference dietary pattern. |
| Method | Algorithm | Linear/Nonlinear | Assumptions | Strengths/Limitations |
|---|---|---|---|---|
| Gaussian Graphical Models (GGMs) | Inverse covariance matrix estimation | Linear | Assumes normally distributed data, linear relationships, requires sparsity. | Measures the conditional dependencies between different foods. Reveals how certain foods are commonly consumed together, or how foods may displace each other in the diet. Can increase understanding how variables (e.g., foods, nutrients) directly interact, independent of others in the context of the whole diet. Relies on partial correlation matrix and is sensitive to non-normally distributed data. |
| Mixed Graphical Models (MGMs) | Combination of GGM and discrete modelling techniques | Both | Assumes mixed data types can be represented in a joint network, requires sparsity. | Can identify direct relationships while accommodating diverse variable types. Standard MGMs assume linear relationships but with extensions such as kernel methods nonlinear models can be developed. |
| Mutual Information Network | Information-theoretic methods | Nonlinear | No strict distributional assumptions, assumes mutual information represents dependence. | Uses entropy-based measures to quantify shared information. Reveals how certain foods are commonly consumed together, even in nonlinear relationships (e.g., nutrient thresholds or diminishing returns). Similar to GGM but without normality assumption. Does not differentiate direct and indirect associations. |
| Bayesian Networks (BNs) | Directed acyclic graphs | Both | Assumes probabilistic relationships between variables. | Provides insights into causality and allows the exploration of causal pathways. Can incorporate prior knowledge for enhanced interpretability. Computationally intensive when discovering unknown networks. |
| Dynamic Networks | Time-varying graph algorithms | Both | Requires longitudinal data with high temporal resolution. | Models time-varying dietary patterns and tracks changes in diet over time. Useful for predicting unintended consequences of interventions. Requires resource-intensive longitudinal data collection for accurate analysis. |
| Hypergraphs | Hyperedge-based graph algorithm | Both | Assumes interactions can involve more than two nodes. | Captures higher-order interactions. Useful for modelling the combined health impact of foods/nutrients which are unable to be explained by pairwise interactions. Computationally demanding and resource intensive. Complexity may affect interpretability. |
| Multilayered Graphs | Layered network construction | Both | Assume information is shared between all layers. | Enables analysis of intra- and inter-layer connections. Valuable for cross-domain analysis. Computationally demanding and complex. Challenging to interpret for large datasets. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, R.M.J.; Moore, J.A.; Griffiths, A.R.; Cousins, A.L.; Young, H.A. Unveiling Dietary Complexity: A Scoping Review and Reporting Guidance for Network Analysis in Dietary Pattern Research. Nutrients 2025, 17, 3261. https://doi.org/10.3390/nu17203261
Taylor RMJ, Moore JA, Griffiths AR, Cousins AL, Young HA. Unveiling Dietary Complexity: A Scoping Review and Reporting Guidance for Network Analysis in Dietary Pattern Research. Nutrients. 2025; 17(20):3261. https://doi.org/10.3390/nu17203261
Chicago/Turabian StyleTaylor, Rebecca M. J., Jack A. Moore, Amy R. Griffiths, Alecia L. Cousins, and Hayley A. Young. 2025. "Unveiling Dietary Complexity: A Scoping Review and Reporting Guidance for Network Analysis in Dietary Pattern Research" Nutrients 17, no. 20: 3261. https://doi.org/10.3390/nu17203261
APA StyleTaylor, R. M. J., Moore, J. A., Griffiths, A. R., Cousins, A. L., & Young, H. A. (2025). Unveiling Dietary Complexity: A Scoping Review and Reporting Guidance for Network Analysis in Dietary Pattern Research. Nutrients, 17(20), 3261. https://doi.org/10.3390/nu17203261






