Immunomodulatory Effect of Raspberry (Rubus idaeus L.) Fruit Extracts on Activated Macrophages and Dysfunctional Vascular Endothelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Raspberry Fruit Extract and Anthocyanin and Non-Anthocyanin Polyphenol Fractions
2.3. Polyphenol Identification and Quantification
2.4. Cell Cultures and Anti-Inflammatory Experiment Procedure
2.5. Cell Viability Assay
2.6. RNA Extraction and Real-Time PCR Analysis
2.7. Determination of IL-6 and MCP-1 Production
2.8. Statistical Analysis
3. Results
3.1. Composition of Polyphenols in Raspberry Fruit Extract and Its Anthocyanin and Non-Anthocyanin Fractions
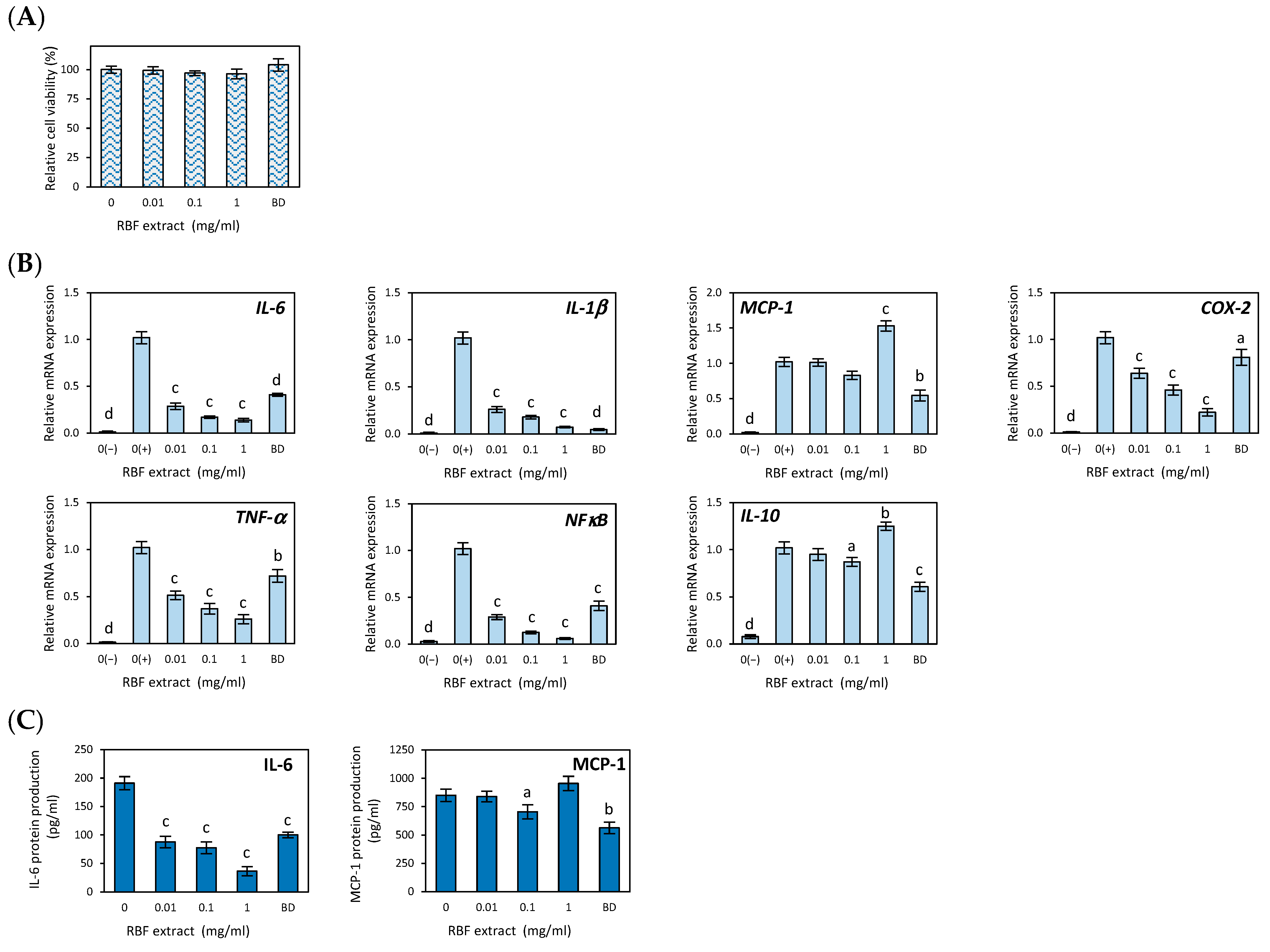
3.2. Anti-Inflammatory Effects of Raspberry Fruit Extract
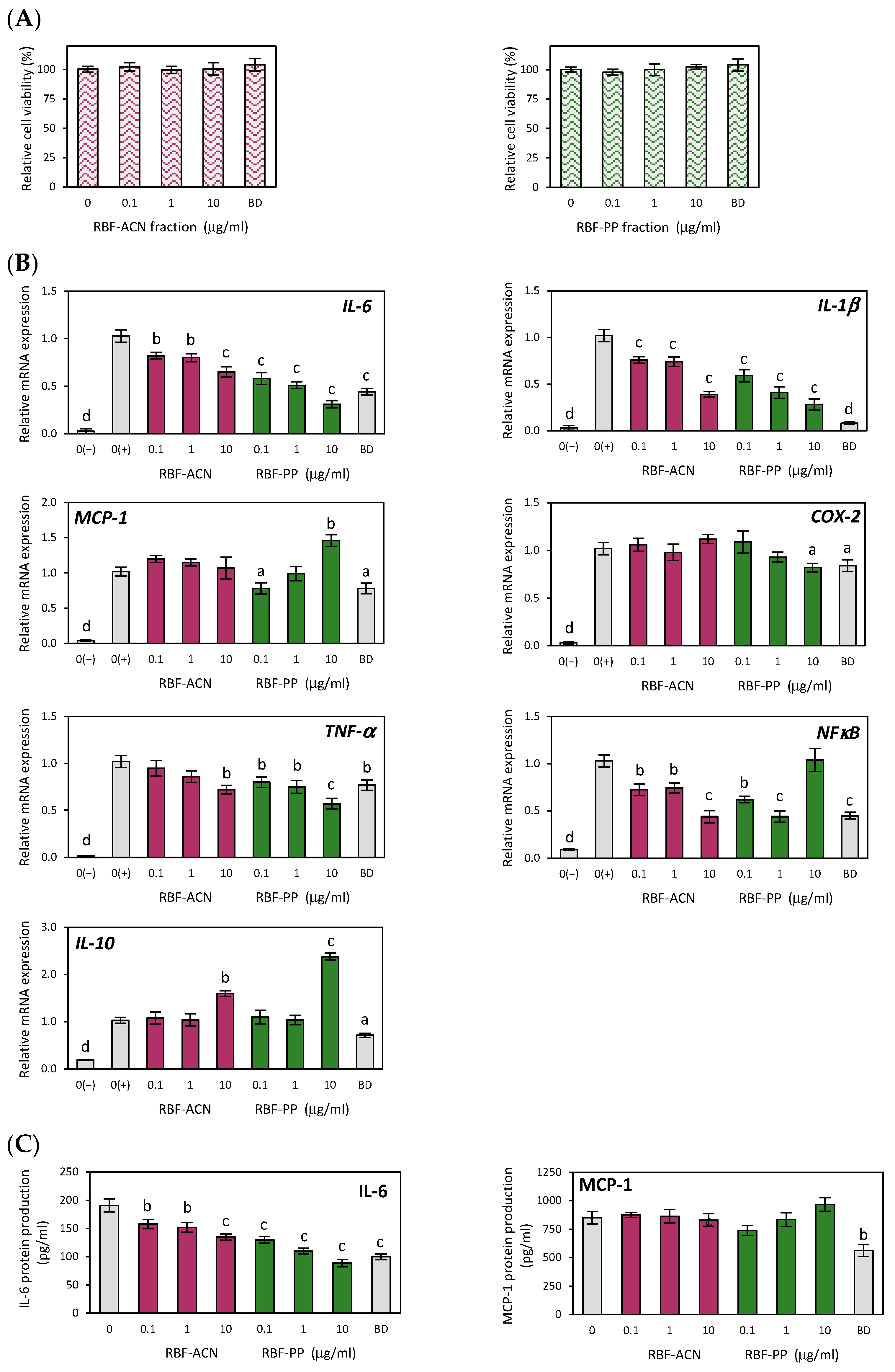
3.3. Anti-Inflammatory Effects of Anthocyanin and Non-Anthocyanin Polyphenol Fractions of Raspberry Fruit Extract
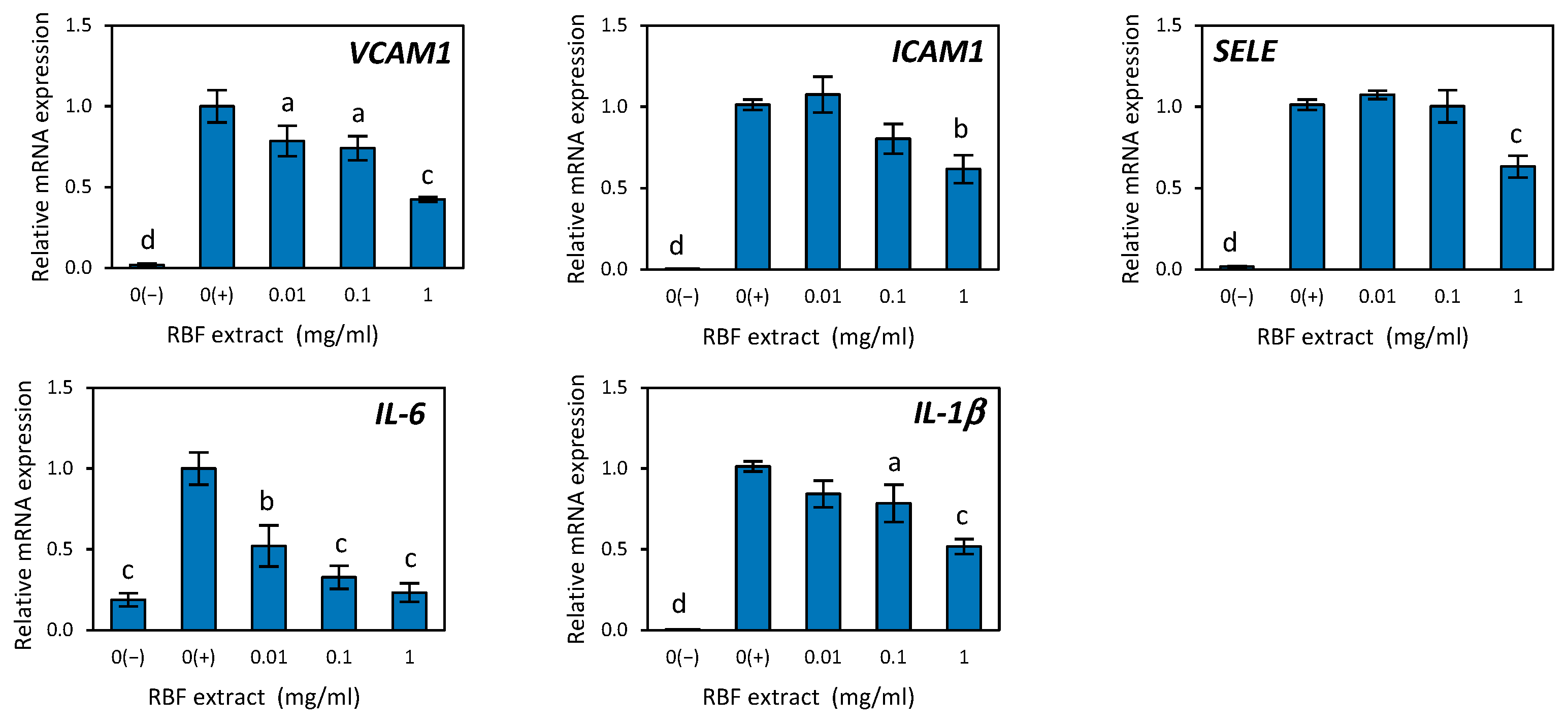
3.4. Potential of Raspberry Fruit Extract, Anthocyanin Fraction, and Non-Anthocyanin Polyphenol Fraction in Counteracting Vascular Endothelial Dysfunction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Susser, L.I.; Rayner, K.J. Through the layers: How macrophages drive atherosclerosis across the vessel wall. J. Clin. Investig. 2022, 132, e157011. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef]
- Auffray, C.; Fogg, D.; Garfa, M.; Elain, G.; Join-Lambert, O.; Kayal, S.; Sarnacki, S.; Cumano, A.; Lauvau, G.; Geissmann, F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007, 317, 666–670. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef]
- Rahman, K.; Vengrenyuk, Y.; Ramsey, S.A.; Vila, N.R.; Girgis, M.; Liu, J.; Gusarova, V.; Gromada, J.; Weinstock, A.; Moore, K.J.; et al. Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression. J. Clin. Investig. 2017, 127, 2904–2915. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Everett, B.M. Novel antiatherosclerotic therapies. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 538–545. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.J.; Celermajer, D.S.; Patel, S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis 2017, 269, 262–271. [Google Scholar] [CrossRef]
- Ezzati, M.; Riboli, E. Behavioral and dietary risk factors for noncommunicable diseases. N. Engl. J. Med. 2013, 369, 954–964. [Google Scholar]
- Cassidy, A. Berry anthocyanin intake and cardiovascular health. Mol. Aspects. Med. 2018, 61, 76–82. [Google Scholar] [CrossRef]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Red Raspberries and Their Bioactive Polyphenols: Cardiometabolic and Neuronal Health Links. Adv. Nutr. 2016, 7, 44–65. [Google Scholar] [CrossRef]
- Suh, J.H.; Romain, C.; González-Barrio, R.; Cristol, J.P.; Teissèdre, P.L.; Crozier, A.; Rouanet, J.M. Raspberry juice consumption, oxidative stress and reduction of atherosclerosis risk factors in hypercholesterolemic golden Syrian hamsters. Food Funct. 2011, 2, 400–405. [Google Scholar] [CrossRef]
- Jia, H.; Liu, J.W.; Ufur, H.; He, G.S.; Liqian, H.; Chen, P. The antihypertensive effect of ethyl acetate extract from red raspberry fruit in hypertensive rats. Pharmacogn. Mag. 2011, 7, 19–24. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A.; Zielińska-Wasielica, J.; Olkowicz, M. Raspberry (Rubus idaeus L.) fruit extract decreases oxidation markers, improves lipid metabolism and reduces adipose tissue inflammation in hypertrophied 3T3-L1 adipocytes. J. Funct. Foods 2019, 62, 103568. [Google Scholar] [CrossRef]
- Jean-Gilles, D.; Li, L.; Ma, H.; Yuan, T.; Chichester, C.O., III; Seeram, N.P. Anti-inflammatory effects of polyphenolic-enriched red raspberry extract in an antigen-induced arthritis rat model. J. Agric. Food. Chem. 2012, 60, 5755–5762. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A.; Rychlik, J.; Grajek, W. Cranberries (Oxycoccus quadripetalus) inhibit adipogenesis and lipogenesis in 3T3-L1 cells. Food Chem. 2014, 148, 246–252. [Google Scholar] [CrossRef]
- Kowalska, K.; Dembczyński, R.; Gołąbek, A.; Olkowicz, M.; Olejnik, A. ROS Modulating Effects of Lingonberry (Vaccinium vitis-idaea L.) Polyphenols on Obese Adipocyte Hypertrophy and Vascular Endothelial Dysfunction. Nutrients 2021, 13, 885. [Google Scholar] [CrossRef]
- Zhang, H.; Park, Y.; Wu, J.; Chen, X.; Lee, S.; Yang, J.; Dellsperger, K.C.; Zhang, C. Role of TNF-alpha in vascular dysfunction. Clin. Sci. 2009, 116, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Speer, H.; D’Cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and Human Health-A Focus on Oxidative Stress, Inflammation and Disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef]
- Stevens, T.W.; Khalaf, F.K.; Soehnlen, S.; Hegde, P.; Storm, K.; Meenakshisundaram, C.; Dworkin, L.D.; Malhotra, D.; Haller, S.T.; Kennedy, D.J.; et al. Dirty Jobs: Macrophages at the Heart of Cardiovascular Disease. Biomedicines 2022, 10, 1579. [Google Scholar] [CrossRef]
- Vendrame, S.; Klimis-Zacas, D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-κB and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015, 73, 348–358. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Wu, Z.; Yao, L.; Wu, Y.; Huang, L.; Liu, K.; Zhou, X.; Gou, D. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci. Rep. 2014, 4, 6234. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, B.; Yang, Y.; Pham, T.X.; Park, Y.K.; Manatou, J.; Koo, S.I.; Chun, O.K.; Lee, J.Y. Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-κB independent of NRF2-mediated mechanism. J. Nutr. Biochem. 2014, 25, 404–411. [Google Scholar] [CrossRef]
- Zhang, Y.; Lian, F.; Zhu, Y.; Xia, M.; Wang, Q.; Ling, W.; Wang, X.D. Cyanidin-3-O-beta-glucoside inhibits LPS-induced expression of inflammatory mediators through decreasing IkappaBalpha phosphorylation in THP-1 cells. Inflamm. Res. 2010, 59, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.D.; Chew, B.P.; Atienza, L.M. Red raspberry (Rubus idaeus L.) intake decreases oxidative stress in obese diabetic (db/db) mice. Food Chem. 2017, 227, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Mykkanen, O.T.; Huotari, A.; Herzig, K.H.; Dunlop, T.W.; Mykkanen, H.; Kirjavainen, P.V. Wild blueberries (Vaccinium myrtillus) alleviate inflammation and hypertension associated with developing obesity in mice fed with a high-fat diet. PLoS ONE 2014, 9, e114790. [Google Scholar] [CrossRef]
- Schell, J.; Betts, N.M.; Lyons, T.J.; Basu, A. Raspberries Improve Postprandial Glucose and Acute and Chronic Inflammation in Adults with Type 2 Diabetes. Ann. Nutr. Metab. 2019, 74, 165–174. [Google Scholar] [CrossRef]
- Aboonabi, A.; Aboonabi, A. Anthocyanins reduce inflammation and improve glucose and lipid metabolism associated with inhibiting nuclear factor-kappaB activation and increasing PPAR-γ gene expression in metabolic syndrome subjects. Free Radic. Biol. Med. 2020, 150, 30–39. [Google Scholar] [CrossRef]
- Moens, U.; Kostenko, S.; Sveinbjørnsson, B. The Role of Mitogen-Activated Protein Kinase-Activated Protein Kinases (MAPKAPKs) in Inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef] [PubMed]
- Land Lail, H.; Feresin, R.G.; Hicks, D.; Stone, B.; Price, E.; Wanders, D. Berries as a Treatment for Obesity-Induced Inflammation: Evidence from Preclinical Models. Nutrients 2021, 13, 334. [Google Scholar] [CrossRef]
- Farahi, L.; Sinha, S.K.; Lusis, A.J. Roles of Macrophages in Atherogenesis. Front. Pharmacol. 2021, 12, 785220. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Speciale, A.; Canali, R.; Chirafisi, J.; Saija, A.; Virgili, F.; Cimino, F. Cyanidin-3-O-glucoside protection against TNF-a-induced endothelial dysfunction: Involvement of nuclear factor-ĸB signaling. J. Agric. Food Chem. 2010, 58, 12048–12054. [Google Scholar] [CrossRef]
- Yang, F.; Suo, Y.; Chen, D.; Tong, L. Protection against vascular endothelial dysfunction by polyphenols in sea buckthorn berries in rats with hyperlipidemia. Biosci. Trends 2016, 10, 188–196. [Google Scholar] [CrossRef]
- Basu, A.; Fu, D.X.; Wilkinson, M.; Simmons, B.; Wu, M.; Betts, N.M.; Du, M.; Lyons, T.J. Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutr. Res. 2010, 30, 462–469. [Google Scholar] [CrossRef]
- Jeong, H.S.; Hong, S.J.; Lee, T.B.; Kwon, J.W.; Jeong, J.T.; Joo, H.J.; Park, J.H.; Ahn, C.M.; Yu, C.W.; Lim, D.S. Effects of black raspberry on lipid profiles and vascular endothelial function in patients with metabolic syndrome. Phytother. Res. 2014, 28, 1492–1498. [Google Scholar] [CrossRef]
- Calvano, A.; Izuora, K.; Oh, E.C.; Ebersole, J.L.; Lyons, T.J.; Basu, A. Dietary berries, insulin resistance and type 2 diabetes: An overview of human feeding trials. Food Funct. 2019, 10, 6227–6243. [Google Scholar] [CrossRef] [PubMed]

| Peak No. | RT (min) | UV λmax (nm) | [M − H]−/ /[M + H]+ (m/z) | MS/MS (m/z) | Tentative Identification | Concentration (mg/g) * | ||
|---|---|---|---|---|---|---|---|---|
| RBF | RBF-ACN | RBF-PP | ||||||
| RBF anthocyanins | ||||||||
| 1 | 15.77 | 280, 520 | 611.1642 (+) | 449.1078 | Cyanidin-3-O-sophoroside | 3.07 ± 0.18 | 520.02 ± 12.54 | 28.29 ± 1.95 |
| 287.0615 | ||||||||
| 2 | 16.44 | 280, 520 | 757.2220 (+) | 595.1663 | Cyanidin-3-O-glucosyl-rutinoside | 0.71 ± 0.05 | 127.25 ± 6.23 | Trace amounts |
| 287.0572 | ||||||||
| 3 | 17.18 | 280, 520 | 449.1123 (+) | 287.0589 | Cyanidin-3-O-glucoside | 1.41 ± 0.12 | 235.26 ± 5.22 | Trace amounts |
| 4 | 18.20 | 280, 520 | 595.1734 (+) | 287.0578 | Cyanidin-3-O-rutinoside | 0.51 ± 0.04 | 97.25 ± 3.41 | Trace amounts |
| RBF flavanols | ||||||||
| 5 | 9.84 | 280 | 577.1355 (−) | 289.0712 | Procyanidin B | 0.30 ± 0.03 | - | 42.43 ± 2.05 |
| 6 | 10.74 | 280 | 289.1139 (−) | 245.1203 | Catechin | 0.12 ± 0.02 | - | 26.06 ± 1.13 |
| 7 | 12.61 | 280 | 289.1142 (−) | 245.1210 | Epicatechin | 0.58 ± 0.04 | - | 140.39 ± 5.97 |
| RBF hydroxycinnamic acid derivatives | ||||||||
| 8 | 9.61 | 278, 320 | 341.0880 (−) | 161.0238 | Caffeoyl hexoside | 0.10 ± 0.01 | - | 19.98 ± 0.96 |
| 9 | 12.60 | 234, 316 | 325.0933 (−) | 145.0294 | p-Coumaryl hexoside isomer 1 | 0.08 ± 0.01 | - | 27.72 ± 1.08 |
| 10 | 14.30 | 234, 316 | 325.0933 (−) | 145.0293 | p-Coumaryl hexoside isomer 2 | 0.07 ± 0.01 | Trace amounts | Trace amounts |
| 11 | 14.93 | 235, 325 | 163.0402 (−) | 119.0496 | p-Coumaric acid | 0.09 ± 0.02 | Trace amounts | Trace amounts |
| RBF flavonols | ||||||||
| 12 | 22.19 | 254, 360 | 433.0417 (−) | 300.9982 | Ellagic acid-O-pentoside | Trace amounts | - | Trace amounts |
| 13 | 23.35 | 262, 363 | 300.9992 (−) | 229.0131 | Ellagic acid | 0.21 ± 0.03 | - | Trace amounts |
| 14 | 23.69 | 266, 354 | 463.0874 (−) | 301.0334 | Quercetin-3-O-galactoside | 0.14 ± 0.01 | - | 19.45 ± 1.37 |
| 15 | 24.41 | 268, 354 | 463.0882 (−) | 301.0354 | Quercetin-3-O-glucoside | Trace amounts | - | Trace amounts |
| 16 | 24.62 | 272, 355 | 477.0679 (−) | 301.0345 | Quercetin 3-O-glucuronide | Trace amounts | - | Trace amounts |
| 17 | 25.88 | 266, 348 | 461.0733 (−) | 285.0391 | Kaempferol 3-O-glucuronide | Trace amounts | - | Trace amounts |
| 18 | 28.45 | 256, 360 | 475.0525 (−) | 300.9978 | Ellagic acid acetyl pentoside isomer 1 | Trace amounts | - | Trace amounts |
| 19 | 29.52 | 256, 360 | 475.0525 (−) | 299.9904 | Ellagic acid acetyl pentoside isomer 2 | Trace amounts | - | Trace amounts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, K.; Dembczyński, R.; Olejnik, A. Immunomodulatory Effect of Raspberry (Rubus idaeus L.) Fruit Extracts on Activated Macrophages and Dysfunctional Vascular Endothelial Cells. Nutrients 2025, 17, 3257. https://doi.org/10.3390/nu17203257
Kowalska K, Dembczyński R, Olejnik A. Immunomodulatory Effect of Raspberry (Rubus idaeus L.) Fruit Extracts on Activated Macrophages and Dysfunctional Vascular Endothelial Cells. Nutrients. 2025; 17(20):3257. https://doi.org/10.3390/nu17203257
Chicago/Turabian StyleKowalska, Katarzyna, Radosław Dembczyński, and Anna Olejnik. 2025. "Immunomodulatory Effect of Raspberry (Rubus idaeus L.) Fruit Extracts on Activated Macrophages and Dysfunctional Vascular Endothelial Cells" Nutrients 17, no. 20: 3257. https://doi.org/10.3390/nu17203257
APA StyleKowalska, K., Dembczyński, R., & Olejnik, A. (2025). Immunomodulatory Effect of Raspberry (Rubus idaeus L.) Fruit Extracts on Activated Macrophages and Dysfunctional Vascular Endothelial Cells. Nutrients, 17(20), 3257. https://doi.org/10.3390/nu17203257







