Mitochondrial DNA DAMPs, Inflammation, and Insulin Sensitivity After Dietary Interventions in Adults with Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Diet Intervention Protocols
2.3. Measures
2.3.1. mtDNA DAMPs
2.3.2. Oral Glucose Tolerance Testing, Matsuda Index, and HOMA-IR
2.3.3. Cytokines, Cortisol, and CRP
2.4. Statistical Analysis
3. Results
3.1. Insulin Sensitivity/Resistance, Cytokines, and Diet Intervention
3.2. Cytokine-Derived Factors
3.3. Cytokine-Derived Factors and Insulin Sensitivity/Resistance Change
3.4. Cytokine-Derived Factors, Insulin Sensitivity/Resistance, and Diet Interventions
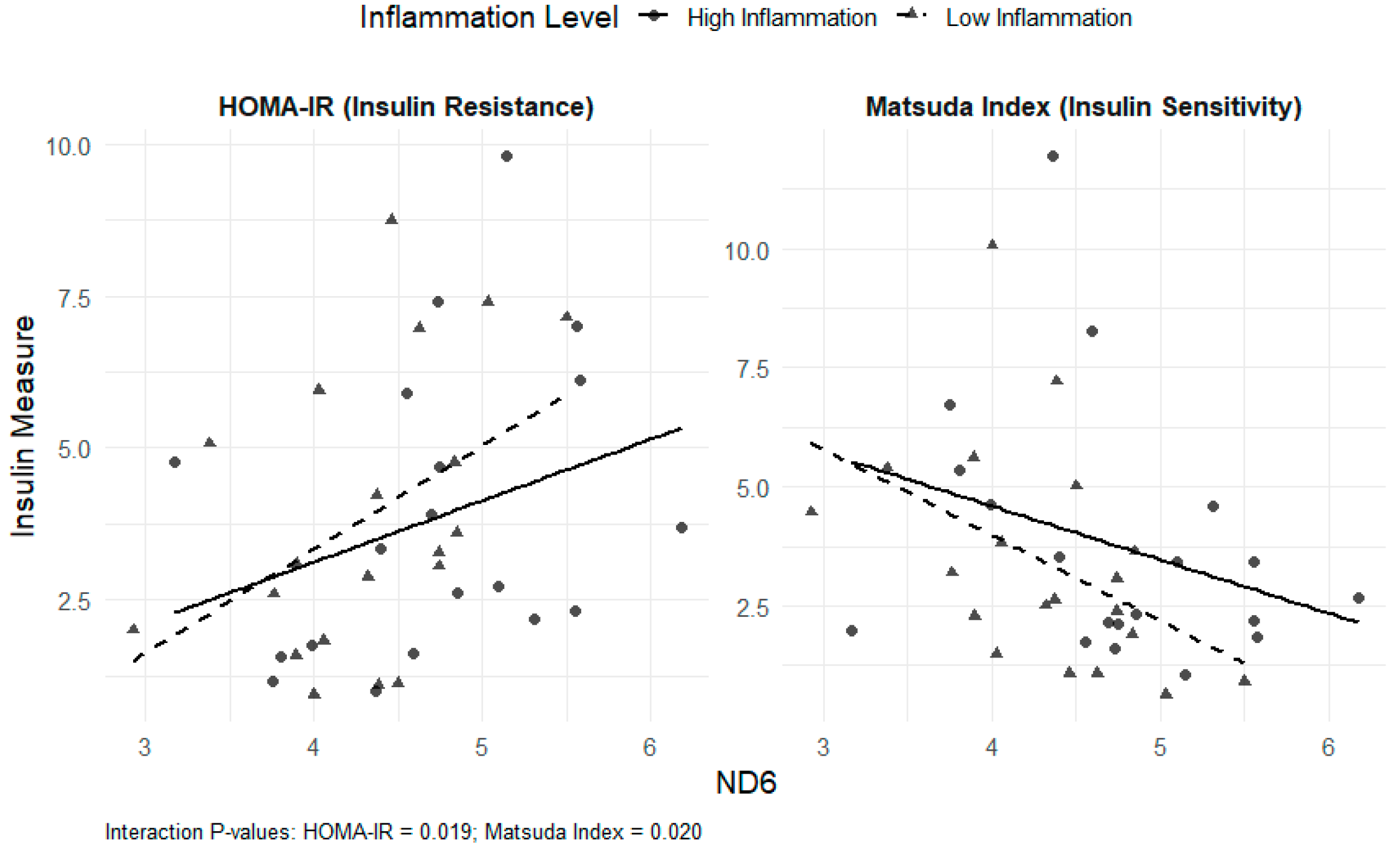
3.5. mtDNA DAMPs (ND1 and ND6), Cytokine-Derived Factors, Insulin Sensitivity/Resistance, and Dietary Intervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAMPs | Damage-associated molecular patterns |
| mtDNA | Mitochondrial DNA |
| ROS | Reactive oxygen species |
| BMI | Body mass index |
| OGTT | Oral glucose tolerance test |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| CRP | C-reactive protein |
| NADH | Nicotinamide adenine dinucleotide + hydrogen |
References
- Diabetes Mortality by State: National Center for Health Statistics. 2022 [Updated 1 March 2022]. Available online: https://www.cdc.gov/nchs/state-stats/deaths/diabetes.html (accessed on 23 June 2025).
- National Diabetes Statistics Report: Center for Disease Control and Prevention 2020 [Updated 15 May 2024]. Available online: https://www.cdc.gov/diabetes/php/data-research/methods.html?CDC_AAref_Val=https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 23 June 2025).
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef]
- Lee, Y.S.; Olefsky, J. Chronic tissue inflammation and metabolic disease. Genes Dev. 2021, 35, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Wlodarczyk, M.; Nowicka, G. Obesity, DNA Damage, and Development of Obesity-Related Diseases. Int. J. Mol. Sci. 2019, 20, 1146. [Google Scholar] [CrossRef]
- de Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef]
- Garcia-Martinez, I.; Shaker, M.E.; Mehal, W.Z. Therapeutic Opportunities in Damage-Associated Molecular Pattern-Driven Metabolic Diseases. Antioxid. Redox Signal. 2015, 23, 1305–1315. [Google Scholar] [CrossRef]
- Shin, J.J.; Lee, E.K.; Park, T.J.; Kim, W. Damage-associated molecular patterns and their pathological relevance in diabetes mellitus. Ageing Res. Rev. 2015, 24 Pt A, 66–76. [Google Scholar] [CrossRef]
- Xu, L.; Yan, X.; Zhao, Y.; Wang, J.; Liu, B.; Yu, S.; Fu, J.; Liu, Y.; Su, J. Macrophage Polarization Mediated by Mitochondrial Dysfunction Induces Adipose Tissue Inflammation in Obesity. Int. J. Mol. Sci. 2022, 23, 9252. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef] [PubMed]
- Yuzefovych, L.; Pastukh, V.M.; Rachek, L. Mitochondrial DNA DAMPs Induce Inflammation and Insulin Resistance. Diabetes 2018, 67, 1783. [Google Scholar] [CrossRef]
- Jialal, I.; Kaur, H.; Devaraj, S. Toll-like receptor status in obesity and metabolic syndrome: A translational perspective. J. Clin. Endocrinol. Metab. 2014, 99, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Land, W.G. The Role of Damage-Associated Molecular Patterns (DAMPs) in Human Diseases: Part II: DAMPs as diagnostics, prognostics and therapeutics in clinical medicine. Sultan Qaboos Univ. Med. J. 2015, 15, e157–e170. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Hisata, S.; Choi, A.M. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxid. Redox Signal. 2015, 23, 1329–1350. [Google Scholar] [CrossRef]
- Skuratovskaia, D.; Komar, A.; Vulf, M.; Litvinova, L. Mitochondrial destiny in type 2 diabetes: The effects of oxidative stress on the dynamics and biogenesis of mitochondria. PeerJ 2020, 8, e9741. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Tao, S.-Y.; Liang, Z.; Xie, R.; Liu, N.-N.; Deng, R.; Zhang, Y.; Deng, D.; Jiang, G. Mitochondrial damage-associated molecular patterns: A new insight into metabolic inflammation in type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2024, 40, e3733. [Google Scholar] [CrossRef]
- Gao, C.-L.; Zhu, C.; Zhao, Y.-P.; Chen, X.-H.; Ji, C.-B.; Zhang, C.-M.; Zhu, J.-G.; Xia, Z.-K.; Tong, M.-L.; Guo, X.-R. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol. Cell. Endocrinol. 2010, 320, 25–33. [Google Scholar] [CrossRef]
- de Souza Freire, T.; de Souza-Pinto, N.C. Chapter 26—Dietary modulation and mitochondrial DNA damage. In Molecular Nutrition and Mitochondria; Ostojic, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 651–665. [Google Scholar]
- Kaliszewska, A.; Allison, J.; Martini, M.; Arias, N. Improving Age-Related Cognitive Decline through Dietary Interventions Targeting Mitochondrial Dysfunction. Int. J. Mol. Sci. 2021, 22, 3574. [Google Scholar] [CrossRef]
- Pollicino, F.; Veronese, N.; Dominguez, L.J.; Barbagallo, M. Mediterranean diet and mitochondria: New findings. Exp. Gerontol. 2023, 176, 112165. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E. The role of the Mediterranean diet on weight loss and obesity-related diseases. Rev. Endocr. Metab. Disord. 2020, 21, 315–327. [Google Scholar] [CrossRef]
- Del Bo’, C.; Marino, M.; Martini, D.; Tucci, M.; Ciappellano, S.; Riso, P.; Porrini, M. Overview of Human Intervention Studies Evaluating the Impact of the Mediterranean Diet on Markers of DNA Damage. Nutrients 2019, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y. Optimal Diet Strategies for Weight Loss and Weight Loss Maintenance. J. Obes. Metab. Syndr. 2021, 30, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Martin-Pelaez, S.; Fito, M.; Castaner, O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef] [PubMed]
- Gower, B.A.; Goss, A.M.; Yurchishin, M.L.; Deemer, S.E.; Sunil, B.; Garvey, W.T. Effects of a Carbohydrate-Restricted Diet on beta-Cell Response in Adults with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2025, 110, 1811–1817. [Google Scholar] [CrossRef]
- Hanick, C.J.; Berg, K.J.; Garvey, W.T.; Goss, A.M.; Steger, F.L.; Richman, J.S.; Peterson, C.M. Study protocol, menu design, and rationale for a study testing the effects of a whole fruit-rich diet on glycemic control, liver fat, pancreatic fat, and cardiovascular health in adults with type 2 diabetes. Nutr. Res. 2025, 135, 82–100. [Google Scholar] [CrossRef]
- Butts, B.; Brown, J.A.; Denney, T.S., Jr.; Ballinger, S.; Lloyd, S.G.; Oparil, S.; Sanders, P.; Merriman, T.R.; Gaffo, A.; Singh, J.; et al. Racial Differences in XO (Xanthine Oxidase) and Mitochondrial DNA Damage-Associated Molecular Patterns in Resistant Hypertension. Hypertension 2022, 79, 775–784. [Google Scholar] [CrossRef]
- Gilstrap, S.R.; Hobson, J.M.; Owens, M.A.; White, D.M.; Sammy, M.J.; Ballinger, S.; Sorge, R.E.; Goodin, B.R. Mitochondrial reactivity following acute exposure to experimental pain testing in people with HIV and chronic pain. Mol. Pain 2023, 19, 17448069231195975. [Google Scholar] [CrossRef]
- Simmons, J.D.; Lee, Y.-L.; Mulekar, S.B.; Kuck, J.L.B.; Brevard, S.B.; Gonzalez, R.P.; Gillespie, M.N.; Richards, W.O. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann. Surg. 2013, 258, 591–596, discussion 6–8. [Google Scholar] [CrossRef]
- Yuzefovych, L.V.; Pastukh, V.M.; Ruchko, M.V.; Simmons, J.D.; Richards, W.O.; Rachek, L.I. Plasma mitochondrial DNA is elevated in obese type 2 diabetes mellitus patients and correlates positively with insulin resistance. PLoS ONE 2019, 14, e0222278. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Wei, Y.; Sowers, J.R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R.S. The Impacts of Animal-Based Diets in Cardiovascular Disease Development: A Cellular and Physiological Overview. J. Cardiovasc. Dev. Dis. 2023, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Jaagura, M.; Viiard, E.; Karu-Lavits, K.; Adamberg, K. Low-carbohydrate high-fat weight reduction diet induces changes in human gut microbiota. Microbiologyopen 2021, 10, e1194. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Ebbeling, C.B.; Rimm, E.B. Carbohydrates, Insulin Secretion, and “Precision Nutrition”. Diabetes Care 2022, 45, 1303–1305. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Diet Intervention (n, %) | |||
|---|---|---|---|---|
| Total | Low-Carbohydrate Diet (n = 9) | Low-Fat Diet (n = 20) | Fruit-Rich Mediterranean Diet (n = 10) | |
| Race | ||||
| African American | 25(64) | 6(67) | 14(70) | 5(50) |
| Caucasian American | 14(36) | 3(33) | 6(30) | 5(50) |
| Sex | ||||
| Female | 29(75) | 6(67) | 15(75) | 8(80) |
| Male | 10(25) | 3(33) | 5(25) | 2(20) |
| Age | 55.6 ± 7.7 | 54.1 ± 6.3 | 56.3 ± 8 | 55.7 ± 8.2 |
| BMI (kg/m2) baseline | 33.5 ± 6.1 | 33.9 ± 5.8 | 34.7 ± 6.2 | 30.6 ± 5.9 |
| BMI (kg/m2) post-intervention | 32.4 ± 6.1 | 32.7 ± 5.5 | 33.3 ± 6.4 | 30.4 ± 6.2 |
| Fasting and glucose measures | ||||
| Fasting glucose (mg/dL) baseline | 131.3 ± 20.3 | 136.9 ± 19.4 | 132.6 ± 20.4 | 123.5 ± 20.6 |
| Fasting glucose (mg/dL) post-intervention | 123.6 ± 22.9 | 121.0 ± 15.2 | 120.9 ± 19.8 | 131.5 ± 33.1 |
| Fasting insulin (μIU/mL) baseline | 16.6 ± 13.0 | 14.6 ± 8.6 | 18.4 ± 15.7 | 14.7 ± 10.8 |
| Fasting insulin (μIU/mL) post-intervention | 13.3 ± 8.5 | 12.1 ± 8.3 | 13.7 ± 9.3 | 13.5 ± 7.8 |
| Matsuda index baseline | 3.1 ± 3.3 | 2.7 ± 1.2 | 2.6 ± 1.6 | 4.5 ± 5.9 |
| Matsuda index post-intervention | 3.6 ± 2.5 | 4.7 ± 2.6 | 3.0 ± 1.9 | 3.7 ± 3.4 |
| HOMA-IR baseline | 4.9 ± 2.9 | 4.7 ± 1.8 | 5.3 ± 2.8 | 4.7 ± 4.2 |
| HOMA-IR post-intervention | 3.9 ± 2.4 | 2.7 ± 1.7 | 4.2 ± 2.3 | 4.3 ± 2.9 |
| Markers | Low-Carbohydrate Diet (n = 9) | p-Value | Low-Fat Diet (n = 20) | p-Value | Fruit-Rich Mediterranean Diet (n = 10) | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-Intervention | Baseline | Post-Intervention | Baseline | Post-Intervention | ||||
| IFN-γ (pg/mL) | 7.3 ± 7.1 | 5.2 ± 2.6 | 0.91 | 6.3 ± 5.8 | 6.6 ± 6.6 | 0.96 | 6.3 ± 5.7 | 3.8 ± 2.1 | 0.048 * |
| IL-10 (pg/mL) | 0.3 ± 0.09 | 0.2 ± 0.08 | 0.30 | 0.3 ± 0.2 | 0.3 ± 0.3 | 0.62 | 0.2 ± 0.1 | 0.8 ± 1.5 | 0.048 * |
| IL-6 (pg/mL) | 1.2 ± 0.3 | 1.3 ± 0.5 | 0.65 | 1.4 ± 0.8 | 1.4 ± 1.5 | 0.33 | 1.1 ± 0.7 | 1.0 ± 0.4 | 0.49 |
| IL-8 (pg/mL) | 9.6 ± 2.9 | 9.6 ± 3.1 | 0.82 | 14.3 ± 12.9 | 14.7 ± 13.4 | 0.25 | 11.9 ± 4.0 | 12.9 ± 5.7 | 0.43 |
| TNF-α (pg/mL) | 1.7 ± 0.7 | 1.5 ± 0.6 | 0.004 * | 2.2 ± 0.8 | 2.0 ± 0.7 | 0.35 | 2.3 ± 0.5 | 2.4 ± 0.5 | 0.43 |
| CRP (mg/L) | 3.1 ± 2.5 | 3.6 ± 2.9 | 0.36 | 3.9 ± 3.7 | 3.34 ± 3.2 | 0.21 | 3.5 ± 2.8 | 3.5 ± 2.9 | 0.57 |
| Cortisol (ug/mL) | 11.5 ± 3.5 | 9.7 ± 2.2 | 0.05 | 11.1 ± 4.0 | 11.5 ± 3.1 | 0.78 | 12.1 ± 4.2 | 12.7 ± 3.5 | 0.56 |
| Time Point | Total mtDNA DAMPs Mean (SD) | ND1 Mean (SD) | ND6 Mean (SD) | Change in ND1 Copies | Change in ND6 Copies | |
|---|---|---|---|---|---|---|
| All participants | Baseline | 218.2 (133.7) | 125.9 (79.6) | 92.3 (60.4) | – | – |
| Post-intervention | 277.3 (202.6) | 160.8 (120.0) | 116.4 (89.2) | 34.9 | 24.1 | |
| Dietary Intervention | ||||||
| Low-Carbohydrate | Baseline | 189.9 (81.3) | 110.9 (66.7) | 79.0 (35.0) | – | – |
| Post-intervention | 206.1 (86.1) | 117.9 (43.7) | 88.2 (53.5) | 7.0 | 9.2 | |
| Low-Fat | Baseline | 205.6 (118.1) | 123.0 (69.9) | 82.6 (51.5) | – | – |
| Post-intervention | 278.0 (197.8) | 170.7 (127.3) | 107.3 (74.4) | 47.7 | 24.7 | |
| Fruit-rich Mediterranean | Baseline | 268.9 (189.9) | 145.1 (108.7) | 123.9 (85.1) | – | – |
| Post-intervention | 339.8 (273.3) | 179.7 (149.8) | 160.1 (127.4) | 34.6 | 36.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cedillo, Y.E.; Sammy, M.J.; Taylor, M.G.; Hanick, C.J.; Peterson, C.M.; Gower, B.A. Mitochondrial DNA DAMPs, Inflammation, and Insulin Sensitivity After Dietary Interventions in Adults with Type 2 Diabetes. Nutrients 2025, 17, 3248. https://doi.org/10.3390/nu17203248
Cedillo YE, Sammy MJ, Taylor MG, Hanick CJ, Peterson CM, Gower BA. Mitochondrial DNA DAMPs, Inflammation, and Insulin Sensitivity After Dietary Interventions in Adults with Type 2 Diabetes. Nutrients. 2025; 17(20):3248. https://doi.org/10.3390/nu17203248
Chicago/Turabian StyleCedillo, Yenni E., Melissa J. Sammy, Meghan G. Taylor, Cody J. Hanick, Courtney M. Peterson, and Barbara A. Gower. 2025. "Mitochondrial DNA DAMPs, Inflammation, and Insulin Sensitivity After Dietary Interventions in Adults with Type 2 Diabetes" Nutrients 17, no. 20: 3248. https://doi.org/10.3390/nu17203248
APA StyleCedillo, Y. E., Sammy, M. J., Taylor, M. G., Hanick, C. J., Peterson, C. M., & Gower, B. A. (2025). Mitochondrial DNA DAMPs, Inflammation, and Insulin Sensitivity After Dietary Interventions in Adults with Type 2 Diabetes. Nutrients, 17(20), 3248. https://doi.org/10.3390/nu17203248







