1. Introduction
Generally, endothelial dysfunction initiates atherosclerosis, a major contributor to cardiovascular disease [
1]. Endothelial nitric oxide helps maintain vascular homeostasis through vasodilatory and antiatherosclerotic actions [
2]. Therefore, preserving vascular endothelial function (VEF) is critical for preventing cardiovascular disease. Flow-mediated dilatation (FMD) of the brachial artery is a widely accepted, noninvasive index of VEF [
3,
4], given that impaired FMD is associated with increased cardiovascular morbidity and mortality [
5,
6].
According to several epidemiological studies, habitual breakfast skipping is an independent risk factor for cardiovascular disease [
7,
8,
9,
10,
11]. An observational study also reported that individuals who habitually skip breakfast exhibit greater arterial stiffness than those who regularly eat breakfast, even after adjusting for potential confounders and cardiovascular risk factors [
12]. Despite this well-documented association, the underlying physiological mechanisms, particularly those related to vascular function, remain unclear. Given that VEF is an early marker of cardiovascular dysfunction, elucidating how breakfast habits influence VEF is crucial for both public health and clinical practice.
In healthy adults, skipping breakfast causes a higher glycemic response to lunch than eating breakfast [
13,
14,
15]. Postprandial hyperglycemia acutely suppresses VEF through oxidative stress [
16,
17,
18,
19]. One of the mechanisms underlying postprandial hyperglycemia after breakfast skipping may be the sustained increase in plasma free fatty acids (FFAs) by lunch [
13,
14]. Specifically, elevated FFAs inhibit insulin-dependent glucose uptake in skeletal muscle and impair insulin-dependent nitric oxide production in the endothelium [
20,
21]. Recently, we demonstrated that one-time breakfast skipping significantly suppressed postlunch VEF in healthy adults and that the degree of impairment negatively correlated with postprandial glucose levels [
22]. However, this finding is based primarily on single-day interventions. Thus, the cumulative vascular impact of repeated breakfast skipping remains unclear.
Currently, the metabolic consequences of skipping breakfast for several days in a controlled meal situation are insufficiently studied. In the study of Ogata et al. [
23], 24 h interstitial glucose levels elevated after 6 days of breakfast skipping when the participants remained in a metabolic chamber [
23]. Thus, given that postprandial hyperglycemia may impair VEF (16–18), repeated breakfast skipping might lead to VEF decline.
Interestingly, Ogata et al. [
23] also observed no difference in postlunch glucose response on Day 2 after breakfast skipping, suggesting a potential “metabolic adaptation.” However, their breakfast-skipping trial included a higher energy intake at lunch [
23], complicating the interpretation of whether this adaptation was physiological or nutritional. Furthermore, their study lacked detailed blood analyses (e.g., insulin and FFAs); thus, mechanistic links to postprandial glycemic control could not be elucidated.
Critically, no previous study has directly compared the vascular effects of regular (daily) and irregular (alternate-day) breakfast skipping, despite the fact that real-world eating patterns often vary. Therefore, this study aimed to investigate the effects of these two breakfast-skipping patterns for 8 days on VEF in the brachial artery, glucose, insulin, and lipid profiles in healthy young adults under controlled dietary trials.
4. Discussion
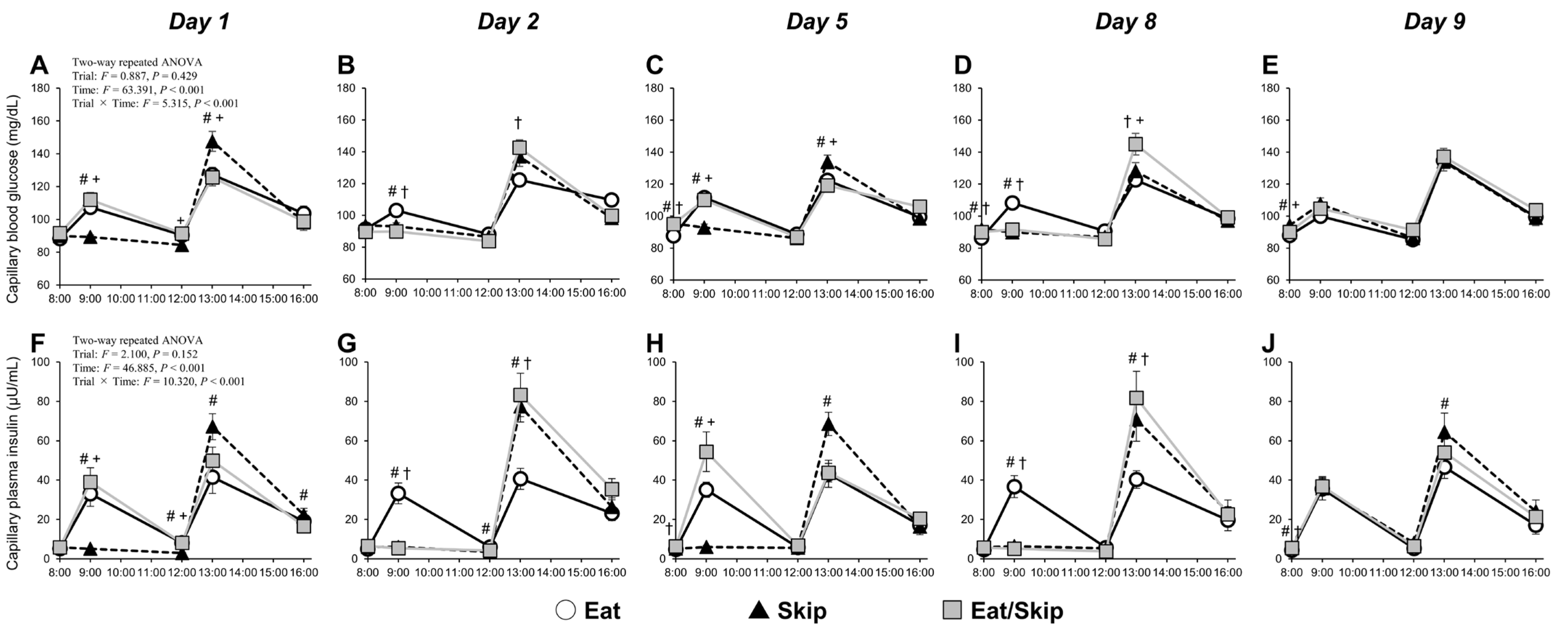
This study is the first to examine the effects of different breakfast consumption patterns (daily consumption [Eat], daily skipping [Skip], and alternate-day skipping [Eat/Skip]) over an eight-day period on VEF in the brachial artery. The VEF was significantly reduced by repeated breakfast skipping for eight consecutive days compared with that by regular breakfast consumption. Notably, even with only four instances of breakfast skipping, the alternate-day pattern (Eat/Skip) also resulted in decreased VEF; hence, not only the frequency but also the irregularity of breakfast intake may negatively affect vascular health. Furthermore, in the Skip and Eat/Skip trials, fasting blood glucose and plasma insulin showed a moderate negative correlation with FMD/SRAUC, suggesting a potential link between metabolic status and vascular function under conditions of breakfast skipping. Additionally, on breakfast-skipping days under the Eat/Skip trial, postprandial hyperglycemia consistently occurred at lunch. However, by Day 8, in the Skip trial, the glycemic response was attenuated despite the same meal pattern, suggesting that the body had adapted metabolically to repeated breakfast skipping. These findings suggest that both the consistency and regularity of breakfast intake are essential in maintaining vascular endothelial health.
In the Eat/Skip trial, postprandial glycemic responses after lunch were consistently higher on breakfast-skipping days than on breakfast-consumed days (Eat trial), consistent with previous reports [
13,
14,
15,
22]. Conversely, postlunch glycemic responses clearly elevated on days 1 and 5 in the Skip trial compared with those in the Eat trial; however, by Day 8, despite a greater total energy intake in the Skip trial, the postlunch glucose elevation was nearly equivalent between the two trials. This observation supports the findings of Ogata et al. [
23], who rigorously controlled meal quantity and timing. Interestingly, on Day 8, even under similar trials of breakfast skipping and high lunch volume, the glycemic response was lower in the Skip trial than in the Eat/Skip trial; thus, repeated exposure to skipping breakfast and/or excessive lunch intake may induce metabolic adaptations. Currently, the precise mechanisms underlying these adaptations remain unclear. Nonetheless, given the absence of a significant difference between the Skip and Eat/Skip trials in insulin levels at 30 min postlunch on Day 8, repeated breakfast skipping may improve insulin sensitivity in peripheral tissues. Supporting this notion, a cross-sectional study by Thomas et al. [
27] reported that postlunch following breakfast skipping glycemic responses were lower in individuals who habitually skip breakfast than in habitual breakfast eaters. Therefore, insulin sensitivity in peripheral tissues can potentially be remodeled according to habitual meal-skipping patterns and habitual meal size. Taken together, following a consistent pattern of daily breakfast skipping combined with a large lunch intake may initially impair glycemic control but could promote metabolic adaptation over time. Conversely, alternating between breakfast consumption and skipping (Eat/Skip) appears less likely to trigger such adaptive changes. Furthermore, the average nocturnal glucose levels (00:00–07:00) were consistently higher in the Skip trial than in the Eat trial from Day 3 onward, possibly reflecting increased macronutrient intake at dinner under the Skip trial and/or a reduction in nocturnal insulin secretion or sensitivity [
28,
29].
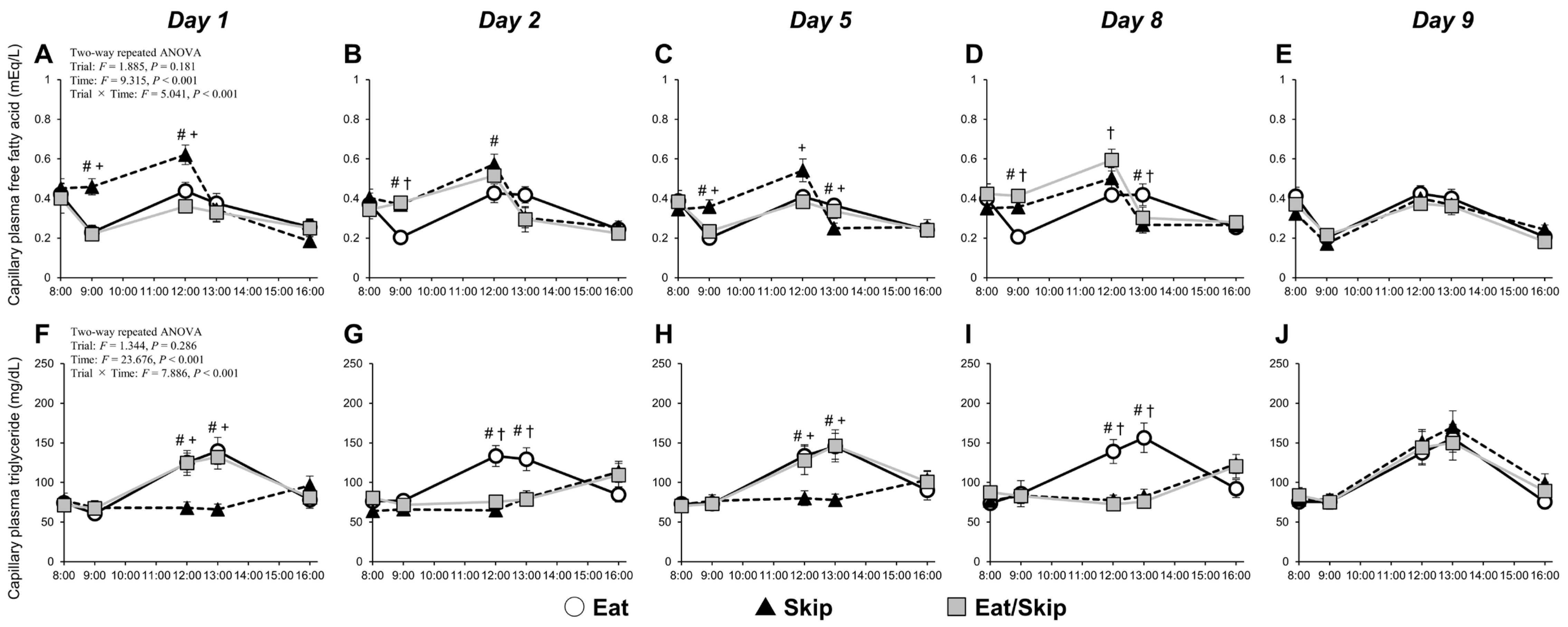
Breakfast consumption promotes insulin secretion, consequently reducing plasma FFA concentrations. When breakfast is skipped, the FFA levels tend to increase slightly before lunch and then rapidly decrease after lunch. Such postprandial FFA dynamics, which were observed in the present study, are generally consistent across studies [
13,
14,
22]. Notably, pre-lunch FFA elevation following breakfast skipping is reportedly one of the contributors to postprandial hyperglycemia via glucose uptake inhibition in peripheral tissues [
13]. This study revealed significantly higher postprandial glycemic and insulin responses after lunch in the Skip trial than in the Eat trial; hence, skipping breakfast may inhibit glucose uptake associated with reduced insulin sensitivity at lunch, consistent with previous study results [
14,
22]. Regarding glucose uptake inhibition in peripheral tissues, residual FFA levels after skipping breakfast are an important factor in inducing postprandial hyperglycemia. According to the study of Kim et al. [
20,
21], FFAs promote serine phosphorylation of the insulin receptor substrate (IRS) and downregulate insulin signaling related to tyrosine phosphorylation of the IRS, phosphatidylinositol 3-kinase (PI3-kinase), phosphoinositide-dependent kinase, and Akt, consequently reducing glucose transport into cells.
Interestingly, on Day 8 of the intervention, despite that pre-lunch FFA concentrations did not significantly differ between the Skip and Eat/Skip trials, only the Eat/Skip trial exhibited postprandial hyperglycemia. Therefore, repeated breakfast skipping may lead to a metabolic rhythm adaptation, thereby enhancing metabolic flexibility. Metabolic flexibility is the body’s ability to efficiently switch between lipid and carbohydrate metabolism in response to nutrient availability—a hallmark of metabolic health [
30]. In the Skip trial, the metabolic environment might have been reorganized temporarily following continuous breakfast skipping, potentially enhancing insulin sensitivity during the day’s first meal.
On Day 9, all participants from all trials received the same breakfast and lunch. While postprandial FFA or triglyceride concentrations showed no significant difference between trials, the peak interstitial glucose concentration after breakfast was significantly higher in the Skip trial than in the Eat and Eat/Skip trials. This result aligns with a previous finding [
27], which reported greater glycemic responses among breakfast skippers when reintroduced to breakfast. Although postlunch glucose responses did not differ significantly among trials, the Skip trial had significantly higher insulin secretion than the Eat trial, suggesting postprandial insulin resistance following breakfast skipping. This finding partially corresponds to those of Farshchi et al. [
31], who observed greater insulin responses in participants who resumed breakfast after 14 days of skipping. Thus, in the Skip trial, the metabolic adaptations established over 8 consecutive days of breakfast skipping may have been disrupted upon reintroducing breakfast on Day 9, leading to impaired glucose regulation. In the present study, skipped breakfast was reintroduced on Day 9 to standardize energy intake across trials. However, for weight-loss interventions aiming to maintain a hypocaloric diet, reintroduction of skipped meals should be avoided. Under such conditions, postprandial metabolic and vascular responses may differ from those observed in the current study.
In this study, both %FMD and %FMD/SR
AUC were significantly lower in the Skip trial than in the Eat trial and further decreased on Day 9 relative to baseline. Therefore, repeated postprandial hyperglycemia at lunch following breakfast skipping may contribute to sustained VEF suppression. Postprandial hyperglycemia has been reported to elevate reactive oxygen species production, acutely impairing the VEF of brachial artery [
16,
17,
18]. Moreover, FMD further decreases in cases wherein larger postlunch glucose responses occurred after breakfast skipping [
16]. Noteworthily, on Day 8 of the intervention, postprandial glucose responses in the Skip trial were comparable to those in the Eat trial, with no hyperglycemia observed, indicating metabolic adaptation resulting from repeated breakfast skipping. Therefore, further extending the intervention period could potentially lead to different effects on VEF. The higher fasting capillary blood glucose and plasma insulin in both breakfast skipping pattern trials during intervention may be partly associated with reductions in %FMD/SR
AUC observed in the Skip and Eat/Skip trials. These correlations do not include data from the Eat trial, as no significant FMD changes were observed there. Additionally, participants in the Skip and Eat/Skip (skip day) trials consumed consistently large evening meals despite timing of lower physical activity levels, possibly maintaining elevated glucose and insulin levels overnight; this effect may have contributed to impaired VEF.
Notably, FMD significantly decreased in the Eat/Skip trial. Although the number of skipped breakfasts was relatively low (4 days), the magnitude of FMD reduction was comparable to that in the continuous skipping trial; thus, irregular meal patterns, rather than the total frequency of breakfast skipping, may exert adverse effects on VEF. Furthermore, postlunch glucose levels on the skipping days (days 2 and 8) were consistently higher in the Eat/Skip trial than in the Eat trial, with no indication of metabolic adaptation seen in the continuous skipping group. Fasting blood glucose and plasma insulin also significantly elevated on certain days and tended to increase. Therefore, even with identical energy intake across trials, fluctuations in meal timing and quantity may exacerbate glucose metabolism. Indeed, epidemiological evidence indicates that insulin resistance indices are significantly higher in individuals with irregular eating patterns than in those who maintain regular meal schedules [
32].
Triglyceride levels measured between 12:00 and 13:00 were consistently higher in the Eat trial than in both the Skip trial and the skipping days of the Eat/Skip trial; hence, breakfast consumption may influence postprandial triglyceride responses. While triglyceride levels declined after 13:00 in the Eat trial, they continued to rise in the two other trials; this effect is expected, given the higher fat intake at lunch in the two latter conditions. Although postprandial triglyceride levels were not measured after 16:00, postdinner triglyceride levels might be also elevated on the Skip trial and the skipping days of the Eat/Skip trial compared with those in the Eat trial, likely attributable to the additional consumption of granola containing 13.1 g of fat at dinner in the Skip and Eat/Skip trials. If postprandial triglyceride levels increase, fatty acids are oxidized within the endothelium, inducing local oxidative stress and subsequently reducing nitric oxide bioavailability, thereby contributing to endothelial dysfunction [
33]. Additionally, postprandial lipid metabolism exhibits time-of-day dependency; when meals are consumed at night, triglyceride responses are heightened [
34]. However, triglyceride levels after dinner were not assessed in this study, thereby warranting further investigation.
Moreover, fasting triglycerides, total cholesterol, and HDL cholesterol showed no significant differences between the trials. Conversely, Farshchi et al. [
31] reported increases in total and LDL cholesterol after 14 days of breakfast omission, but only in the skipping condition. This discrepancy may be attributable to food intake differences during the intervention. In their study, food intake was greater in the skipping trial, whereas in our study, all meals were standardized in both quantity and composition across trials. These methodological differences may account for the divergent outcomes in fasting lipid levels.
This study has several limitations. First, postprandial blood samples were collected at only two time points (30 and 180 min after meals) based on our previous findings showing that most participants reached peak blood glucose concentrations at 30 min after a meal [
22]. However, blood sampling was impossible after 16:00. Consequently, we could not assess lipid profile changes after lunch and dinner, thereby limiting our understanding of postprandial lipid metabolism throughout the day. Second, the dietary fiber content of the test meals was not precisely quantified. Because the meals were composed of commercially available prepackaged products, and dietary fiber information was not provided for several items, potential effects of dietary fiber on postprandial metabolism and vascular responses could not be fully evaluated. However, as the same meals were used across all experimental conditions, daily dietary fiber intake was identical between conditions, and therefore unlikely to have affected the observed differences in vascular responses. Another limitation is that the menstrual cycle phases of the female participants were not standardized. VEF is influenced by hormonal fluctuations throughout the menstrual cycle [
35], and differences in cycle timing may have influenced the results. Our 10-day intervention period, including the day of dietary adjustment, made all tests within the early follicular phase (up to Day 8; when female hormone levels are relatively stable) difficult to complete, assuming that Day 0 corresponds to the menstruation onset. In contrast, the VEF in all three male participants decreased on Day 9 compared with that on Day 1 in both the Skip and Eat/Skip trials. Further investigation is needed to determine whether this trend is independent of sex differences. Moreover, our study has a small sample size; thus, we could not fully examine the sex-specific effects, which may be important in studies of vascular and metabolic function. All participants were also young and healthy, limiting the generalizability of these findings to older adults or those with metabolic or cardiovascular disease. Older adults or post-menopausal women often exhibit reduced metabolic adaptability, and breakfast skipping in these populations could potentially induce more pronounced metabolic and vascular impairments. Future studies are warranted to investigate age- and sex-specific responses to meal timing interventions. Furthermore, this study did not examine potential nocturnal effects or chronic adaptations, both of which may be important for understanding the long-term effects of meal timing and composition on vascular health. Finally, we did not assess endothelium-independent vasodilation; hence, we could not determine whether the observed changes in vascular function were exclusively endothelium-dependent. Therefore, further studies are warranted to address these limitations and elucidate the underlying mechanisms.