Risk of Sarcopenic Obesity Across Menopausal Transition Stages in Middle-Aged Korean Women
Abstract
1. Introduction
2. Materials and Methods
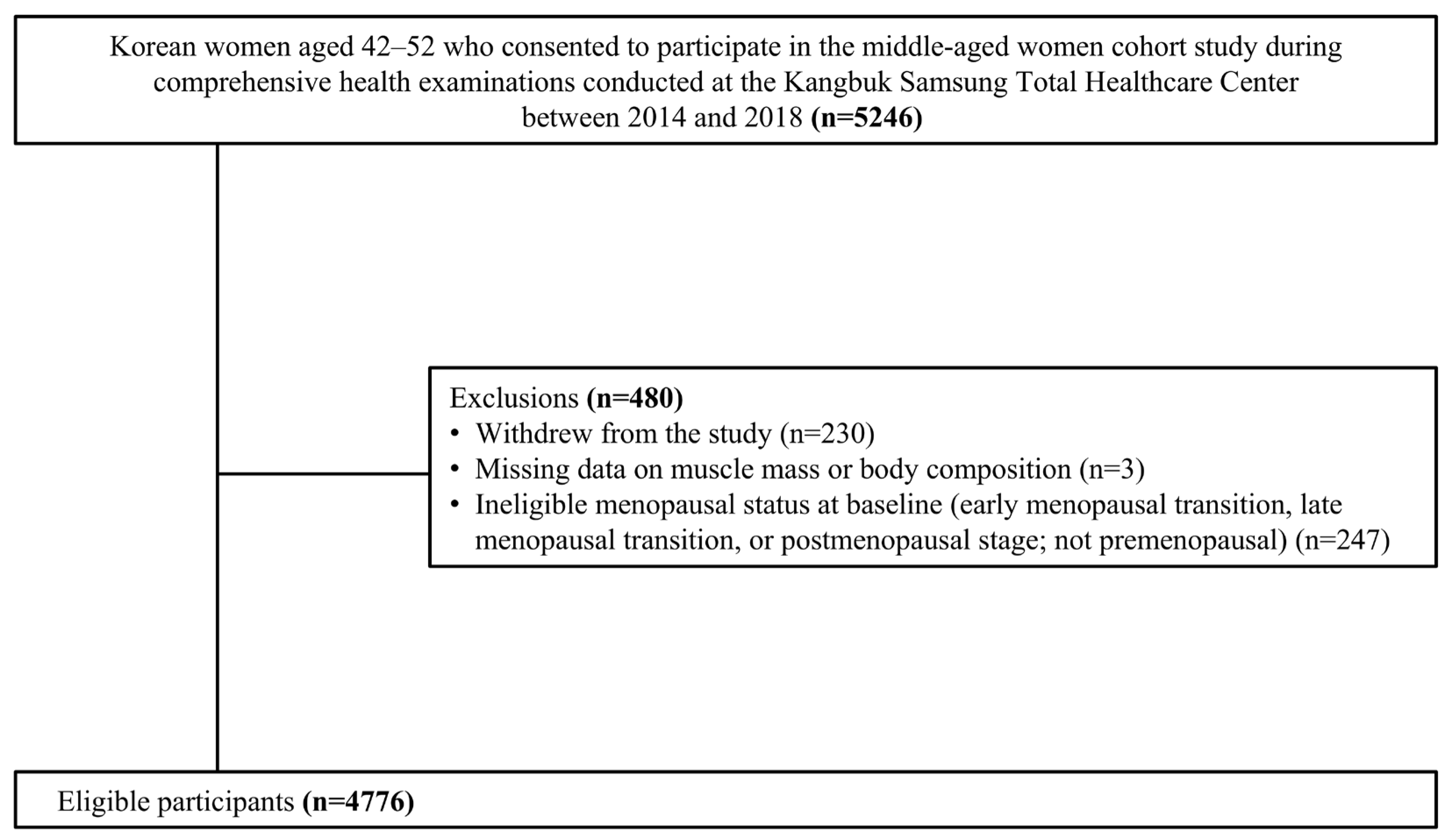
2.1. Sample Description
2.2. Measurement
2.3. Definition of Menopausal Stages
2.4. Definition of Sarcopenic Obesity
2.5. Statistical Analysis
3. Results
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASM | Appendicular skeletal muscle mass |
| BIA | Bioelectrical impedance analysis |
| BMI | Body mass index |
| CI | Confidence interval |
| DXA | Dual-energy X-ray absorptiometry |
| FMP | Final menstrual period |
| FSH | Follicle-stimulating hormone |
| GEE | Generalized estimating equation |
| IQR | Interquartile range |
| MI | Multiple imputation |
| MT | Menopausal transition |
| OR | Odds ratio |
| PBF | Percent body fat |
| SD | Standard deviation |
| STRAW + 10 | Stages of Reproductive Aging Workshop + 10 criteria |
| WC | Waist circumference |
References
- Hurtado, M.D.; Saadedine, M.; Kapoor, E.; Shufelt, C.L.; Faubion, S.S. Weight Gain in Midlife Women. Curr. Obes. Rep. 2024, 13, 352–363. [Google Scholar] [CrossRef]
- Greendale, G.A.; Sternfeld, B.; Huang, M.; Han, W.; Karvonen-Gutierrez, C.; Ruppert, K.; Cauley, J.A.; Finkelstein, J.S.; Jiang, S.-F.; Karlamangla, A.S. Changes in body composition and weight during the menopause transition. JCI Insight 2019, 4, e124865. [Google Scholar] [CrossRef]
- Maltais, M.L.; Desroches, J.; Dionne, I.J. Changes in muscle mass and strength after menopause. J. Musculoskelet. Neuronal Interact. 2009, 9, 186–197. [Google Scholar] [PubMed]
- Juppi, H.-K.; Sipilä, S.; Cronin, N.J.; Karvinen, S.; Karppinen, J.E.; Tammelin, T.H.; Aukee, P.; Kovanen, V.; Kujala, U.M.; Laakkonen, E.K. Role of Menopausal Transition and Physical Activity in Loss of Lean and Muscle Mass: A Follow-Up Study in Middle-Aged Finnish Women. J. Clin. Med. 2020, 9, 1588. [Google Scholar] [CrossRef] [PubMed]
- Boonpor, J.; Pell, J.P.; Ho, F.K.; Celis-Morales, C.; Gray, S.R. In people with type 2 diabetes, sarcopenia is associated with the incidence of cardiovascular disease: A prospective cohort study from the UK Biobank. Diabetes Obes. Metab. 2024, 26, 524–531. [Google Scholar] [CrossRef]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.-Y.; Bruyère, O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef]
- Batsis, J.A.; Haudenschild, C.; Crow, R.S.; Gilliam, M.; Mackenzie, T.A. Sarcopenia Definition Outcome Consortium—Defined Weakness and Risk of Falls: The National Health and Aging Trends Survey. Geriatr. Gerontol. Int. 2023, 23, 213–220. [Google Scholar] [CrossRef]
- Geraci, A.; Calvani, R.; Ferri, E.; Marzetti, E.; Arosio, B.; Cesari, M. Sarcopenia and Menopause: The Role of Estradiol. Front. Endocrinol. 2021, 12, 682012. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Writing Group European Working Group. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Chen, T.; Kao, H.-H.; Ogawa, W.; Arai, H.; Tahapary, D.L.; Assantachai, P.; Tham, K.-W.; Chan, D.-C.; Yuen, M.M.-A.; Appannah, G.; et al. The Asia-Oceania consensus: Definitions and diagnostic criteria for sarcopenic obesity. Obes. Res. Clin. Pract. 2025, 19, 185–192. [Google Scholar] [CrossRef]
- Dionne, I.J.; Kinaman, K.A.; Poehlman, E.T. Sarcopenia and muscle function during menopause and hormone-replacement therapy. J. Nutr. Health Aging 2000, 4, 156–161. [Google Scholar]
- Moccia, P.; Belda-Montesinos, R.; Monllor-Tormos, A.; Chedraui, P.; Cano, A. Body weight and fat mass across the menopausal transition: Hormonal modulators. Gynecol. Endocrinol. 2022, 38, 99–104. [Google Scholar] [CrossRef]
- Collins, B.C.; Arpke, R.W.; Larson, A.A.; Baumann, C.W.; Xie, N.; Cabelka, C.A.; Nash, N.L.; Juppi, H.-K.; Laakkonen, E.K.; Sipilä, S.; et al. Estrogen Regulates the Satellite Cell Compartment in Females. Cell Rep. 2019, 28, 368–381.e6. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.L.; Whincup, P.H.; Morris, R.W.; Lennon, L.T.; Papacosta, O.; Wannamethee, S.G. Sarcopenic obesity and risk of cardiovascular disease and mortality: A population-based cohort study of older men. J. Am. Geriatr. Soc. 2014, 62, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.H.; Lee, H.J.; Kim, H.J.; Kim, I.H.; Kil Joo, J.; Na, Y.J. Correlation of Sarcopenic Obesity on Various Cardiometabolic Risk Factors and Fracture Risk in Mid-Aged Korean Women. J. Menopausal Med. 2023, 29, 58–65. [Google Scholar] [CrossRef]
- Bouchard, D.R.; Dionne, I.J.; Brochu, M. Sarcopenic/obesity and physical capacity in older men and women: Data from the Nutrition as a Determinant of Successful Aging (NuAge)-the Quebec longitudinal Study. Obesity 2009, 17, 2082–2088. [Google Scholar] [CrossRef]
- Benz, E.; Pinel, A.; Guillet, C.; Capel, F.; Pereira, B.; De Antonio, M.; Pouget, M.; Cruz-Jentoft, A.J.; Eglseer, D.; Topinkova, E.; et al. Sarcopenia and Sarcopenic Obesity and Mortality Among Older People. JAMA Netw. Open 2024, 7, e243604. [Google Scholar] [CrossRef]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Wong, B.W.X.; Tan, D.Y.Z.; Li, L.; Yong, E. Individual and combined effects of muscle strength and visceral adiposity on incident prediabetes and type 2 diabetes in a longitudinal cohort of midlife Asian women. Diabetes Obes. Metab. 2025, 27, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Sowers, M.; Zheng, H.; Tomey, K.; Karvonen-Gutierrez, C.; Jannausch, M.; Li, X.; Yosef, M.; Symons, J. Changes in body composition in women over six years at midlife: Ovarian and chronological aging. J. Clin. Endocrinol. Metab. 2007, 92, 895–901. [Google Scholar] [CrossRef]
- Razmjou, S.; Abdulnour, J.; Bastard, J.-P.; Fellahi, S.; Doucet, É.; Brochu, M.; Lavoie, J.-M.; Rabasa-Lhoret, R.; Prud’Homme, D. Body composition, cardiometabolic risk factors, physical activity, and inflammatory markers in premenopausal women after a 10-year follow-up: A MONET study. Menopause 2018, 25, 89–97. [Google Scholar] [CrossRef]
- Jang, Y.; Chang, Y.; Park, J.; Kim, C.; Jeon, S.W.; Kang, J.; Kwon, R.; Lim, G.-Y.; Kim, K.-H.; Kim, H.; et al. Menopausal stage transitions and their associations with overall and individual sleep quality in middle-aged Korean women. J. Affect. Disord. 2024, 368, 82–89. [Google Scholar] [CrossRef]
- Park, J.; Chang, Y.; Choi, H.R.; Kim, J.H.; Seo, S.W.; Ryu, H.J.; Cho, Y.; Kim, C.; Kwon, R.; Lim, G.-Y.; et al. Overactive bladder and cognitive impairment in middle-aged women: A cross-sectional study. Maturitas 2024, 187, 108042. [Google Scholar] [CrossRef]
- Tran, T.X.M.; Chang, Y.; Choi, H.R.; Kwon, R.; Lim, G.-Y.; Kim, E.Y.; Ryu, S.; Park, B. Adiposity, Body Composition Measures, and Breast Cancer Risk in Korean Premenopausal Women. JAMA Netw. Open 2024, 7, e245423. [Google Scholar] [CrossRef]
- He, M.; Tan, K.; Li, E.; Kung, A. Body fat determination by dual energy X-ray absorptiometry and its relation to body mass index and waist circumference in Hong Kong Chinese. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 748–752. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Thomas, R.J.; Collazo-Clavell, M.L.; Korinek, J.; Allison, T.G.; Batsis, J.A.; Sert-Kuniyoshi, F.H.; Lopez-Jimenez, F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int. J. Obes. 2008, 32, 959–966. [Google Scholar] [CrossRef] [PubMed]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Vikram, N.K.; Gupta, R.; Pandey, R.M.; Wasir, J.S.; Gupta, V.P. Waist circumference cutoff points and action levels for Asian Indians for identification of abdominal obesity. Int. J. Obes. 2006, 30, 106–111. [Google Scholar] [CrossRef]
- Agaku, I.T.; King, B.A.; Dube, S.R. Current cigarette smoking among adults—United States, 2005–2012. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 29–34. [Google Scholar]
- Babor, T.F.; Robaina, K. The Alcohol Use Disorders Identification Test (AUDIT): A review of graded severity algorithms and national adaptations. Int. J. Alcohol Drug Res. 2016, 5, 17–24. [Google Scholar] [CrossRef]
- Oh, J.Y.; Yang, Y.J.; Kim, B.S.; Kang, J.H. Validity and reliability of Korean version of International Physical Activity Questionnaire (IPAQ) short form. Korean J. Fam. Med. 2007, 28, 532–541. [Google Scholar]
- Park, S.E.; Ko, S.-H.; Kim, J.Y.; Kim, K.; Moon, J.H.; Kim, N.H.; Han, K.D.; Choi, S.H.; Cha, B.S. Diabetes Fact Sheets in Korea 2024. Diabetes Metab. J. 2025, 49, 24–33. [Google Scholar] [CrossRef]
- Kim, H.C.; Lee, H.; Lee, H.-H.; Ahn, S.V.; Lee, J.-M.; Cheon, D.Y.; Jhee, J.H.; Yoon, M.; Shin, M.-H.; Heo, J.; et al. Korea Hypertension Fact Sheet 2024: Nationwide population-based analysis with a focus on young adults. Clin. Hypertens. 2025, 31, e11. [Google Scholar] [CrossRef]
- Jin, E.-S.; Shim, J.-S.; Kim, S.E.; Bae, J.H.; Kang, S.; Won, J.C.; Shin, M.-J.; Jin, H.Y.; Moon, J.; Lee, H.; et al. Dyslipidemia Fact Sheet in South Korea, 2022. Diabetes Metab. J. 2023, 47, 632–642. [Google Scholar] [CrossRef]
- World Health Organization. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Ho-Pham, L.T.; Campbell, L.V.; Nguyen, T.V. More on body fat cutoff points. Mayo Clin. Proc. 2011, 86, 584. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Wang, K.; Wang, W.; Dong, F.; Qian, Y.; Gong, H.; Xu, G.; Li, G.; Pan, L.; et al. Optimal body fat percentage cut-off values for identifying cardiovascular risk factors in Mongolian and Han adults: A population-based cross-sectional study in Inner Mongolia, China. BMJ Open 2017, 7, e014675. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, C.; Bao, Y.; Peng, L.; Gu, H.; Jia, W. Optimal body fat percentage cut-offs for obesity in Chinese adults. Clin. Exp. Pharmacol. Physiol. 2012, 39, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Lear, S.A.; James, P.T.; Ko, G.T.; Kumanyika, S. Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. Eur. J. Clin. Nutr. 2010, 64, 42–61. [Google Scholar] [CrossRef] [PubMed]
- Cui, J. QIC program and model selection in GEE analyses. Stata J. 2007, 7, 209–220. [Google Scholar] [CrossRef]
- Celentano, D.D.; Szklo, M.; Gordis, L. Gordis Epidemiology, 6th ed.; ClinicalKey; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Pan, W. Sample Size and Power Calculations with Correlated Binary Data. Control. Clin. Trials 2001, 22, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Furushima, T.; Miyachi, M.; Iemitsu, M.; Murakami, H.; Kawano, H.; Gando, Y.; Kawakami, R.; Sanada, K. Comparison between clinical significance of height-adjusted and weight-adjusted appendicular skeletal muscle mass. J. Physiol. Anthropol. 2017, 36, 15. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef]
- Van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Davies, K.; Heaney, R.; Recker, R.; Barger-Lux, M.; Lappe, J. weight change and menopause. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 874–879. [Google Scholar] [CrossRef]
- Kammerlander, A.A.; Lyass, A.; Mahoney, T.F.; Massaro, J.M.; Long, M.T.; Vasan, R.S.; Hoffmann, U. Sex Differences in the Associations of Visceral Adipose Tissue and Cardiometabolic and Cardiovascular Disease Risk: The Framingham Heart Study. J. Am. Heart Assoc. 2021, 10, e019968. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, S.H.; Jung, K.J.; Goo, J.M.; Yoon, S.H. High visceral fat attenuation and long-term mortality in a health check-up population. J. Cachexia Sarcopenia Muscle 2023, 14, 1495–1507. [Google Scholar] [CrossRef]
- Müller, M.J.; Lagerpusch, M.; Enderle, J.; Schautz, B.; Heller, M.; Bosy-Westphal, A. Beyond the body mass index: Tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obes. Rev. 2012, 13 (Suppl. 2), 6–13. [Google Scholar] [CrossRef] [PubMed]
- La Colla, A.; Pronsato, L.; Milanesi, L.; Vasconsuelo, A. 17beta-Estradiol and testosterone in sarcopenia: Role of satellite cells. Ageing Res. Rev. 2015, 24 Pt B, 166–177. [Google Scholar] [CrossRef]
- Kodoth, V.; Scaccia, S.; Aggarwal, B. Adverse Changes in Body Composition During the Menopausal Transition and Relation to Cardiovascular Risk: A Contemporary Review. Womens Health Rep. 2022, 3, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gomez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- McLester, C.N.; Nickerson, B.S.; Kliszczewicz, B.M.; McLester, J.R. Reliability and agreement of various InBody body composition analyzers as compared to dual-energy X-ray absorptiometry in healthy men and women. J. Clin. Densitom. 2020, 23, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.H.; de Craen, A.J.; Slagboom, P.E.; Gunn, D.A.; Stokkel, M.P.; Westendorp, R.G.; Maier, A.B. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin. Nutr. 2011, 30, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Park, K.S.; Ahn, S.; Ku, E.J.; Jung, K.Y.; Kim, Y.J.; Kim, K.M.; Moon, J.H.; Choi, S.H.; Park, K.S.; et al. Comparison of Abdominal Visceral Adipose Tissue Area Measured by Computed Tomography with That Estimated by Bioelectrical Impedance Analysis Method in Korean Subjects. Nutrients 2015, 7, 10513–10524. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.; Genton, L.; Hans, D.; Pichard, C. Validation of a bioelectrical impedance analysis equation to predict appendicular skeletal muscle mass (ASMM). Clin. Nutr. 2003, 22, 537–543. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Sipilä, S.; Törmäkangas, T.; Sillanpää, E.; Aukee, P.; Kujala, U.M.; Kovanen, V.; Laakkonen, E.K. Muscle and bone mass in middle-aged women: Role of menopausal status and physical activity. J. Cachexia Sarcopenia Muscle 2020, 11, 698–709. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, N.; Luo, J.; Liu, Y.; Ossowski, Z. Exercise and Nutrition for Sarcopenia: A Systematic Review and Meta-Analysis with Subgroup Analysis by Population Characteristics. Nutrients 2025, 17, 2342. [Google Scholar] [CrossRef]
| Variable | Frequency (%) |
|---|---|
| Age at baseline † | 42.2 ± 3.0 |
| Age at menarche | |
| <12 years old | 169 (3.6) |
| 12–13 years old | 1665 (34.9) |
| 14–16 years old | 2666 (55.9) |
| ≥17 years old | 184 (3.9) |
| Unknown | 82 (1.7) |
| Smoking | |
| Non-smoker | 4072 (85.4) |
| Ever-smoker | 533 (11.2) |
| Unknown | 161 (3.4) |
| Alcohol consumption | |
| <10 g/day | 3918 (82.2) |
| ≥10 g/day | 403 (8.5) |
| Unknown | 445 (9.3) |
| Parity | |
| Nulliparous | 332 (7.0) |
| Parous | 4282 (89.8) |
| Unknown | 152 (3.2) |
| Marital status | |
| Unmarried | 207 (4.3) |
| Married/cohabitating | 4378 (91.9) |
| Divorced/separated/widowed | 78 (1.6) |
| Unknown | 103 (2.2) |
| Education | |
| ≤High school | 960 (20.2) |
| ≥College | 3629 (76.1) |
| Unknown | 177 (3.7) |
| Physical activity ‡ | |
| Active § | 543 (11.4) |
| Minimally active | 1795 (37.7) |
| Inactive | 2343 (49.1) |
| Unknown | 85 (1.8) |
| Hypertension ¶ | 144 (3.0) |
| Diabetes mellitus †† | 79 (1.7) |
| Hypercholesterolemia ‡‡ | 389 (8.2) |
| Low muscle mass §§ | 742 (15.6) |
| Obesity | |
| PBF ≥ 35% | 767 (16.1) |
| WC ≥ 80 cm | 1348 (28.3) |
| BMI ≥ 23 kg/m2 | 1487 (31.2) |
| Sarcopenic obesity | |
| ASM < 5.7 kg/m2 + PBF ≥ 35% | 65 (1.4) |
| ASM < 5.7 kg/m2 + WC ≥ 80 cm | 24 (0.5) |
| ASM < 5.7 kg/m2 + BMI ≥ 23 kg/m2 | 13 (0.3) |
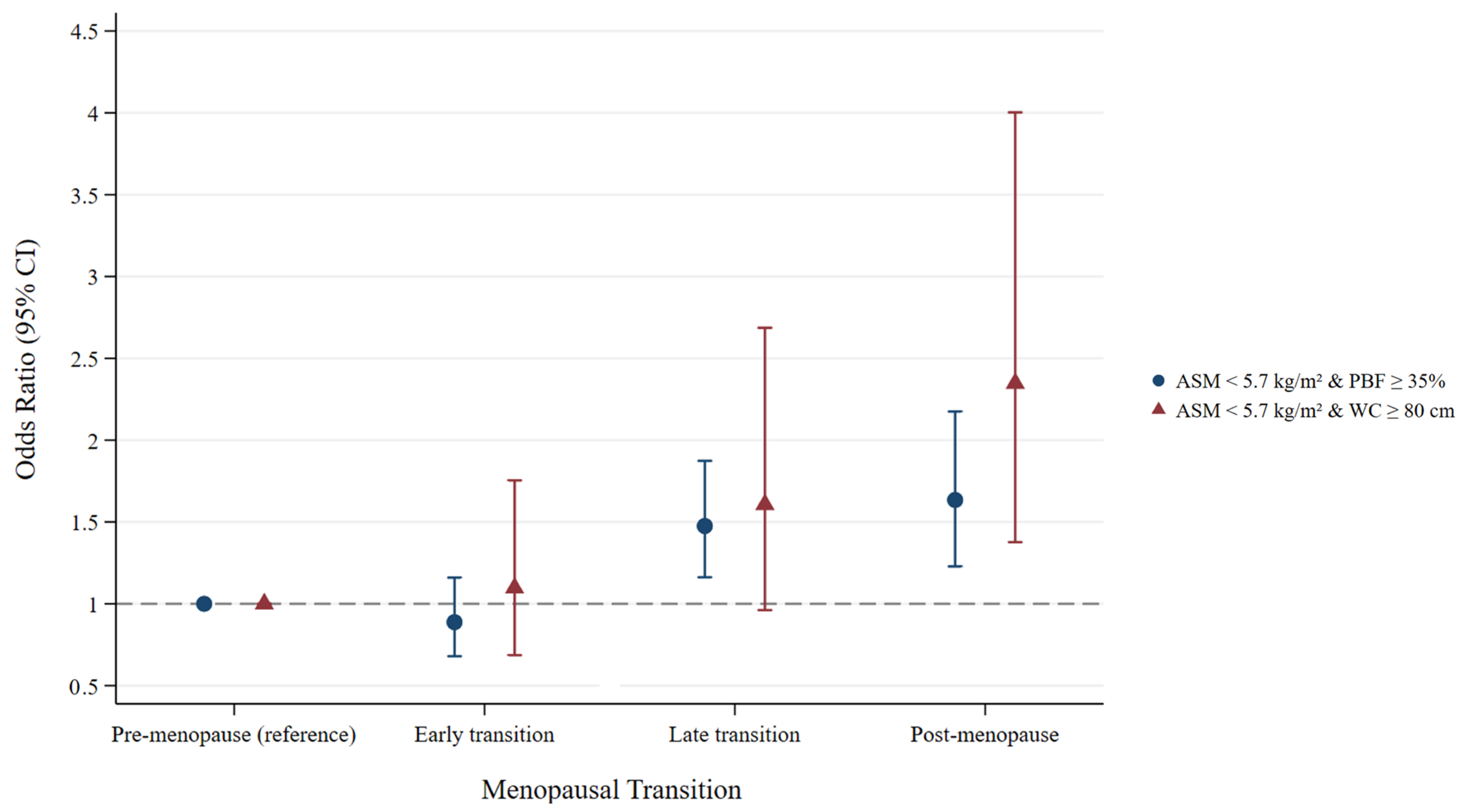
| ASM < 5.7 kg/m2 and PBF ≥ 35% | Model 1 † | Model 2 ‡ | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| Time-varying age (year) | 1.07 (1.04–1.10) | <0.001 | 1.07 (1.04–1.10) | <0.001 |
| Menopausal transition | ||||
| Premenopause | Ref (1) | – | Ref (1) | – |
| Early transition | 0.89 (0.68–1.16) | 0.375 | 0.89 (0.68–1.16) | 0.384 |
| Late transition | 1.47 (1.16–1.87) | <0.001 | 1.48 (1.16–1.87) | <0.001 |
| Postmenopause | 1.63 (1.22–2.16) | <0.001 | 1.63 (1.23–2.17) | <0.001 |
| ASM < 5.7 kg/m2 and WC ≥ 80 cm | Model 1 † | Model 2 ‡ | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| Time-varying age (year) | 1.00 (0.95–1.05) | 0.944 | 1.00 (0.95–1.05) | 0.944 |
| Menopausal transition | ||||
| Premenopause | Ref (1) | – | Ref (1) | – |
| Early transition | 1.09 (0.69–1.72) | 0.717 | 1.10 (0.69–1.75) | 0.699 |
| Late transition | 1.57 (0.94–2.61) | 0.082 | 1.61 (0.96–2.69) | 0.070 |
| Postmenopause | 2.27 (1.33–3.88) | 0.003 | 2.35 (1.38–4.00) | 0.002 |
| ASM < 5.7 kg/m2 and BMI ≥ 23 kg/m2 | Model 1 † | Model 2 ‡ | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| Time-varying age (year) | 1.06 (1.00–1.12) | 0.038 | 1.06 (1.01–1.13) | 0.025 |
| Menopausal transition | ||||
| Premenopause | Ref (1) | – | Ref (1) | – |
| Early transition | 0.96 (0.61–1.50) | 0.861 | 0.94 (0.59–1.48) | 0.781 |
| Late transition | 1.45 (0.86–2.44) | 0.165 | 1.45 (0.85–2.47) | 0.167 |
| Postmenopause | 1.40 (0.82–2.40) | 0.214 | 1.44 (0.84–2.47) | 0.180 |
| ASM < 5.7 kg/m2 and PBF ≥ 38% | Model 1 † | Model 2 ‡ | ||
| Adjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| Time-varying age (year) | 1.06 (1.01–1.12) | 0.014 | 1.07 (1.02–1.12) | 0.008 |
| Menopausal transition | ||||
| Premenopause | Ref (1) | – | Ref (1) | - |
| Early transition | 0.99 (0.64–1.52) | 0.946 | 0.97 (0.63–1.49) | 0.898 |
| Late transition | 1.98 (1.17–3.36) | 0.011 | 1.97 (1.16–3.33) | 0.012 |
| Postmenopause | 2.15 (1.25–3.71) | 0.006 | 2.16 (1.26–3.70) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.; Jang, Y.; Park, J.H.; Chang, Y.; Ryu, S. Risk of Sarcopenic Obesity Across Menopausal Transition Stages in Middle-Aged Korean Women. Nutrients 2025, 17, 3238. https://doi.org/10.3390/nu17203238
Cho Y, Jang Y, Park JH, Chang Y, Ryu S. Risk of Sarcopenic Obesity Across Menopausal Transition Stages in Middle-Aged Korean Women. Nutrients. 2025; 17(20):3238. https://doi.org/10.3390/nu17203238
Chicago/Turabian StyleCho, Yoosun, Yoonyoung Jang, Jae Ho Park, Yoosoo Chang, and Seungho Ryu. 2025. "Risk of Sarcopenic Obesity Across Menopausal Transition Stages in Middle-Aged Korean Women" Nutrients 17, no. 20: 3238. https://doi.org/10.3390/nu17203238
APA StyleCho, Y., Jang, Y., Park, J. H., Chang, Y., & Ryu, S. (2025). Risk of Sarcopenic Obesity Across Menopausal Transition Stages in Middle-Aged Korean Women. Nutrients, 17(20), 3238. https://doi.org/10.3390/nu17203238







