Synergistic Association of Glycemic Variability and Severe Vitamin D Deficiency with Proliferative Diabetic Retinopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants and Group Allocation
- High-GV group (n = 29)—patients who met the clinic’s variability criterion (see below).
- Low-GV group (n = 29)—patients whose glucose profiles showed no clinically relevant variability.
2.3. Inclusion Criteria
- Age ≥ 18 years.
- Diagnosis of type 1 or type 2 diabetes ≥ 1 year.
- Availability of at least three months of glucose records and a recent ophthalmic examination.
2.4. Exclusion Criteria
- Active ocular infection or other retinal disease (e.g., vein occlusion, age-related macular degeneration).
- Current vitamin D supplementation > 2000 IU Day−1 or parenteral vitamin D within six months.
- Chronic kidney disease stage ≥ 4 or hepatic failure (conditions altering vitamin D metabolism).
2.5. Biochemical Measurements
2.6. Ophthalmic Evaluation
2.7. Optical Coherence Tomography
2.8. Covariates
2.9. Statistical Analysis
2.10. Ethical Considerations
3. Results
3.1. Cohort Profile and Baseline Characteristics
3.2. Diabetes Subtype and Treatment Profile
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teo, Z.L.; Tham, Y.-C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Tan, T.-E. The Diabetic Retinopathy “Pandemic” and Evolving Global Strategies: The 2023 Friedenwald Lecture. Investig. Opthalmol. Vis. Sci. 2023, 64, 47. [Google Scholar] [CrossRef] [PubMed]
- Vision Loss Expert Group of the Global Burden of Disease Study; GBD 2019 Blindness and Vision Impairment Collaborators. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: A meta-analysis from 2000 to 2020. Eye 2024, 38, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- Ajjan, R.A. The clinical importance of measuring glycaemic variability: Utilising new metrics to optimise glycaemic control. Diabetes Obes. Metab. 2024, 26 (Suppl. 7), 3–16. [Google Scholar] [CrossRef] [PubMed]
- Urakami, T.; Terada, H.; Tanabe, S.; Mine, Y.; Aoki, M.; Aoki, R.; Suzuki, J.; Morioka, I. Clinical significance of coefficient of variation in continuous glucose monitoring for glycemic management in children and adolescents with type 1 diabetes. J. Diabetes Investig. 2024, 15, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, F.D.; Poopak, A.; Samimi, S.; Deravi, N.; Nakhaei, P.; Sheikhy, A.; Moosaie, F.; Rabizadeh, S.; Meysamie, A.; Nakhjavani, M.; et al. Glycemic profile variability as an independent predictor of diabetic retinopathy in patients with type 2 diabetes: A prospective cohort study. Front. Endocrinol. 2024, 15, 1383345. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef] [PubMed]
- Mangal, D.K.; Shaikh, N.; Tolani, H.; Gautam, D.; Pandey, A.K.; Sonnathi, Y.; Gupta, S.D.; Kalra, S.; Sharma, K.C.; Prasad, J.; et al. Fahmina Anwar—Burden of micronutrient deficiency among patients with type 2 diabetes: Systematic review and meta-analysis. BMJ Nutr. Prev. Health 2025, 8, e000950. [Google Scholar] [CrossRef]
- Petrea, C.E.; Ghenciu, L.A.; Iacob, R.; Stoicescu, E.R.; Săndesc, D. Vitamin D Deficiency as a Risk Factor for Diabetic Retinopathy: A Systematic Review and Meta-Analysis. Biomedicines 2024, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Lazzara, F.; Longo, A.M.; Giurdanella, G.; Lupo, G.; Platania, C.B.M.; Rossi, S.; Drago, F.; Anfuso, C.D.; Bucolo, C. Vitamin D3 preserves blood retinal barrier integrity in an in vitro model of diabetic retinopathy. Front. Pharmacol. 2022, 13, 971164. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Colette, C.; Wojtusciszyn, A.; Dejager, S.; Renard, E.; Molinari, N.; Owens, D.R. Toward Defining the Threshold Between Low and High Glucose Variability in Diabetes. Diabetes Care 2016, 40, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Lachin, J.M.; Bebu, I.; Bergenstal, R.M.; Pop-Busui, R.; Service, F.J.; Zinman, B.; Nathan, D.M.; DCCT/EDIC Research Group. Association of Glycemic Variability in Type 1 Diabetes with Progression of Microvascular Outcomes in the Diabetes Control and Complications Trial. Diabetes Care 2017, 40, 777–783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. Med. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. Erratum in: J. Clin. Endocrinol. Metab. 2011, 96, 3908; Erratum in: J. Clin. Endocrinol. Metab. 2024, 109, e1991. https://doi.org/10.1210/clinem/dgae373. [CrossRef] [PubMed]
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin. Proc. 2010, 85, 752–758. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanas, R.; John, G.; International HBA1c Consensus Committee. 2010 consensus statement on the worldwide standardization of the hemoglobin A1C measurement. Diabetes Care 2010, 33, 1903–1904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. Erratum in: J. Am. Coll. Cardiol. 2019, 73, 3237–3241. https://doi.org/10.1016/j.jacc.2019.05.013. Erratum in: J. Am. Coll. Cardiol. 2024, 84, 1772. https://doi.org/10.1016/j.jacc.2024.09.026. [CrossRef] [PubMed]
- Wilkinson, C.; Ferris, F.L.; E Klein, R.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T. Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.I.; Kim, S.J.; Bailey, S.T.; Kovach, J.L.; Vemulakonda, G.A.; Ying, G.-S.; Flaxel, C.J. American Academy of Ophthalmology Preferred Practice Pattern Retina/Vitreous Committee. Diabetic Retinopathy Preferred Practice Pattern®. Ophthalmology 2025, 132, P75–P162. [Google Scholar] [CrossRef] [PubMed]
- Wakasugi, S.; Mita, T.; Katakami, N.; Okada, Y.; Yoshii, H.; Osonoi, T.; Nishida, K.; Shiraiwa, T.; Torimoto, K.; Kurozumi, A.; et al. Associations between continuous glucose monitoring-derived metrics and diabetic retinopathy and albuminuria in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2021, 9, e001923. [Google Scholar] [CrossRef] [PubMed]
- Totolici, G.; Tiutiuca, C.; Jurja, S.; Tutunaru, D.; Pătrașcu, A.-M. The role of vitamin D in the onset and progression of diabetic retinopathy. Rom. J. Ophthalmol. 2022, 66, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, M.; Motahari, M.M.; Fakhri, F.; Aphshari, N.M.; Poursharif, S.; Jahed, R.; Nikpayam, O. Is vitamin D deficiency associated with retinopathy in type 2 diabetes mellitus? A case-control study. Clin. Nutr. ESPEN 2023, 59, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Jee, D.; Han, K.D.; Kim, E.C. Inverse association between high blood 25-hydroxy-vitamin D levels and diabetic retinopathy in a representative Korean population. PLoS ONE 2014, 9, e115199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, W.; Li, L.; Wang, W.; Liang, Y.; Bai, X.; Xu, Y.; Guo, Q.; Ge, L.; Liang, J.; Lu, B.; et al. Association of circulating 25-hydroxy-vitamin D with time in range and insulin secretion in type 2 diabetes. Front. Endocrinol. 2025, 16, 1573963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, L.; Lu, Q.; Chen, W.; Li, J.; Li, C.; Zheng, Z. Vitamin D3 Protects against Diabetic Retinopathy by Inhibiting High-Glucose-Induced Activation of the ROS/TXNIP/NLRP3 Inflammasome Pathway. J. Diabetes Res. 2018, 2018, 8193523. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-S.; Jamali, N.; Sorenson, C.M.; Sheibani, N. Vitamin D Receptor Expression Limits the Angiogenic and Inflammatory Properties of Retinal Endothelial Cells. Cells 2023, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Meza, C.A.; Clarke, H.; Kim, J.-S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Fekri, S.; Soheilian, M.; Roozdar, S.; Abtahi, S.-H.; Nouri, H. The effect of vitamin D supplementation on the outcome of treatment with bevacizumab in diabetic macular edema: A randomized clinical trial. Int. Ophthalmol. 2022, 42, 3345–3356. [Google Scholar] [CrossRef] [PubMed]
- Antunica, A.G.; Znaor, L.; Ivanković, M.; Puzović, V.; Marković, I.; Kaštelan, S. Vitamin D and Diabetic Retinopathy. Int. J. Mol. Sci. 2023, 24, 12014. [Google Scholar] [CrossRef] [PubMed]
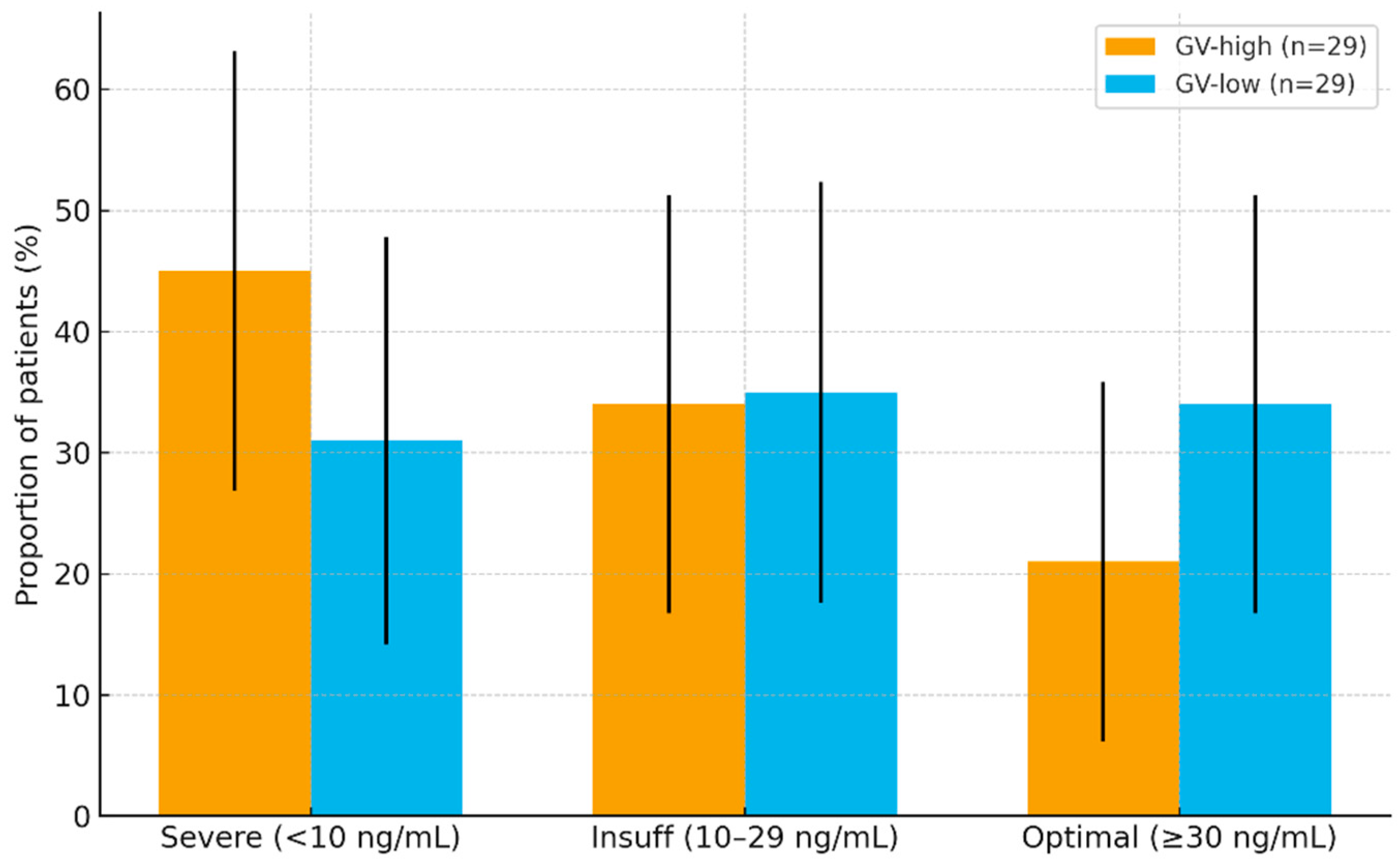
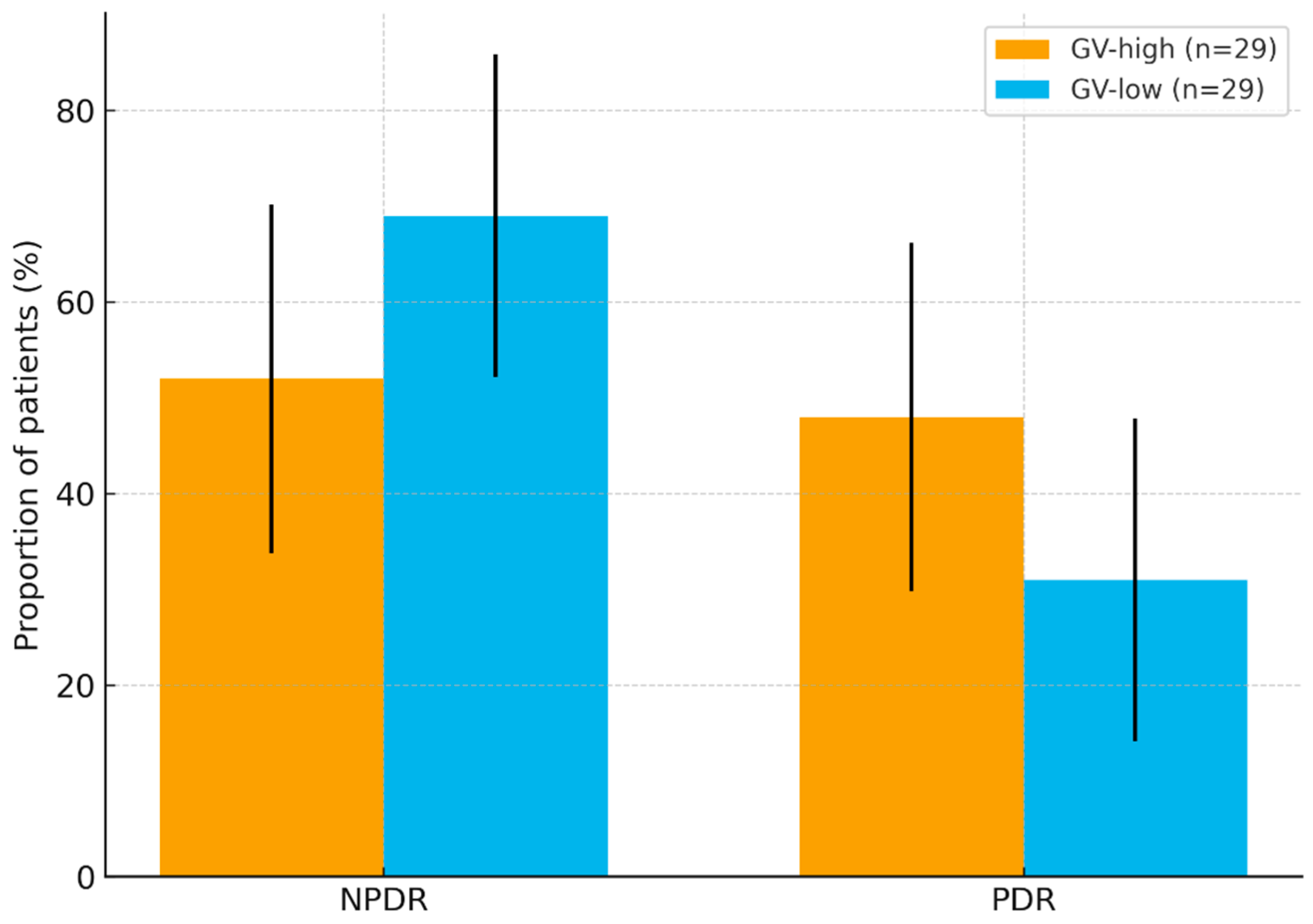


| Predictor. | Adjusted OR | 95% CI | p |
|---|---|---|---|
| High GV (vs. low) | 2.31 | 1.05–5.09 | 0.04 |
| Severe vitamin D deficiency (vs. non-severe) | 2.04 | 0.98–4.25 | 0.06 |
| Both risk factors present (vs. neither) | 3.88 | 1.35–11.1 | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dervis, N.; Carniciu, S.; Spinean, A.; Jurja, S. Synergistic Association of Glycemic Variability and Severe Vitamin D Deficiency with Proliferative Diabetic Retinopathy. Nutrients 2025, 17, 3210. https://doi.org/10.3390/nu17203210
Dervis N, Carniciu S, Spinean A, Jurja S. Synergistic Association of Glycemic Variability and Severe Vitamin D Deficiency with Proliferative Diabetic Retinopathy. Nutrients. 2025; 17(20):3210. https://doi.org/10.3390/nu17203210
Chicago/Turabian StyleDervis, Nejla, Simona Carniciu, Alina Spinean, and Sanda Jurja. 2025. "Synergistic Association of Glycemic Variability and Severe Vitamin D Deficiency with Proliferative Diabetic Retinopathy" Nutrients 17, no. 20: 3210. https://doi.org/10.3390/nu17203210
APA StyleDervis, N., Carniciu, S., Spinean, A., & Jurja, S. (2025). Synergistic Association of Glycemic Variability and Severe Vitamin D Deficiency with Proliferative Diabetic Retinopathy. Nutrients, 17(20), 3210. https://doi.org/10.3390/nu17203210






