Association Between Protein-Rich Foods, Nutritional Supplements, and Age of Natural Menopause and Its Symptoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
Inclusion and Exclusion Criteria
2.2. Questionnaire Survey
2.3. Statistical Analysis
3. Results
3.1. General Demographic Characteristics
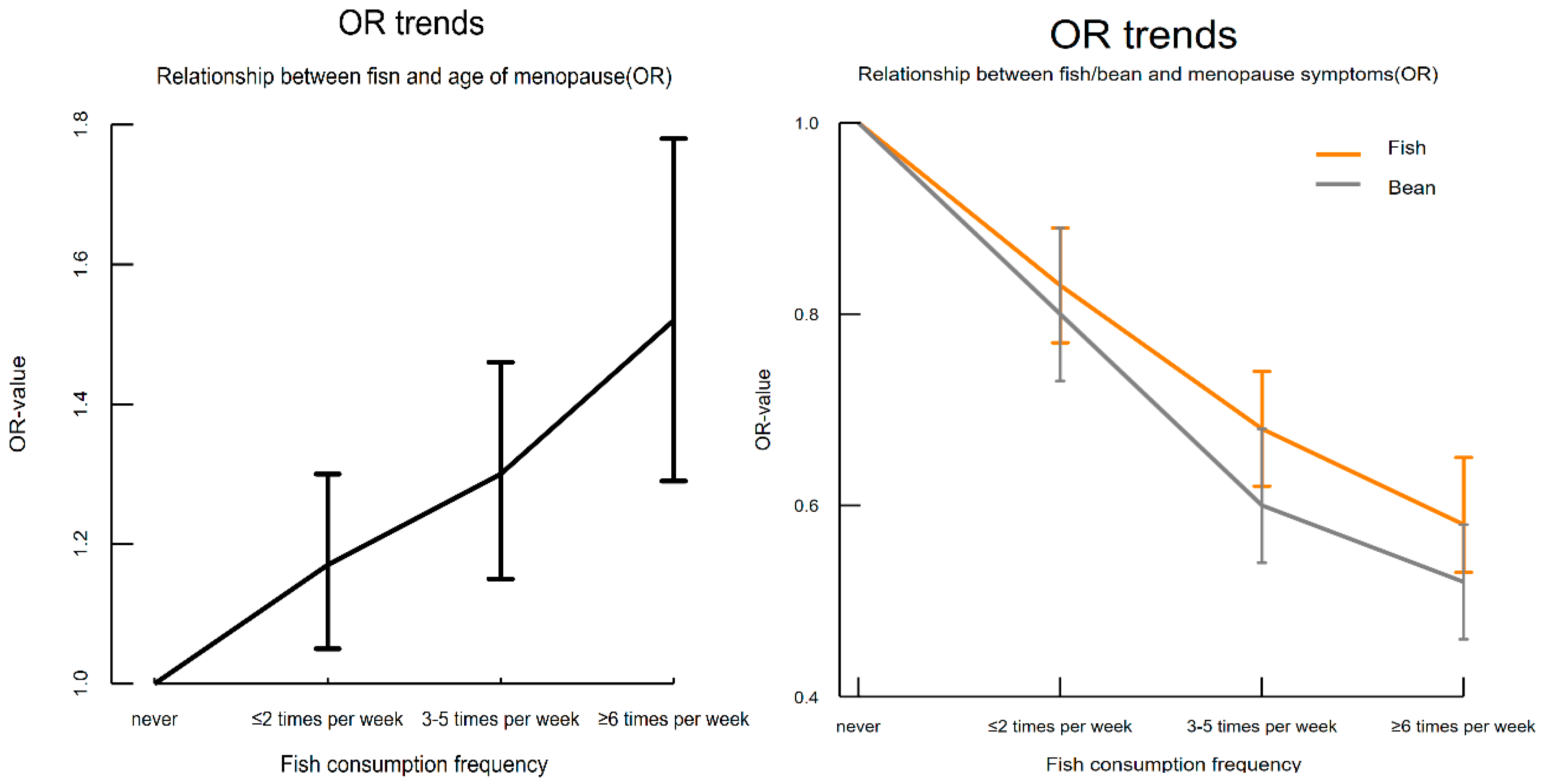
3.2. Relationship Between Protein-Rich Foods and Nutrient Supplements and Natural Menopause Age
3.3. Relationship Between Protein-Rich Foods and Nutrient Supplements and Menopausal Symptoms
3.4. Relationship Between Protein-Rich Foods and Nutrient Supplements and Different Dimensions of Menopausal Symptoms
3.5. Relationship Between Protein-Rich Foods and Nutrient Supplements and Menopausal Symptoms Across Different Age Groups
3.6. Relationship Between Protein-Rich Foods and Nutrient Supplements and Menopausal Symptoms Under Different Menopausal Statuses
3.7. Relationship Between Protein-Rich Foods and Nutrient Supplements and Menopausal Symptoms Across Different BMI Categories
4. Discussion
4.1. Association of Fish Consumption with Menopause Age and Its Symptoms
4.2. Association of Milk Consumption with Menopause Age and Its Symptoms
4.3. Association of Soy Products with Menopause Age and Its Symptoms
4.4. Association of Eggs with Menopause Age and Its Symptoms
4.5. Association of Nutrient Supplements with Menopause Age and Its Symptoms
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Utian, W.H. The International Menopause menopause-related terminology definitions. Climacteric 1999, 2, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kartsonaki, C.; Guo, Y.; Lv, J.; Gan, W.; Chen, Z.-M.; Li, L.-M.; Hu, C.-G.; Yang, L.; Yu, M. Factors related to age at natural menopause in China: Results from the China Kadoorie Biobank. Menopause 2021, 28, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Talaulikar, V. Menopause transition: Physiology and symptoms. Best. Pr. Res. Clin. Obs. Gynaecol. 2022, 81, 3–7. [Google Scholar] [CrossRef] [PubMed]
- La Marca, A.; Diamanti, M. Factors affecting age at menopause and their relationship with ovarian reserve: A comprehensive review. Eur. J. Contracept. Reprod. Health Care 2024, 29, 245–255. [Google Scholar] [CrossRef]
- Davis, S.R.; Lambrinoudaki, I.; Lumsden, M.; Mishra, G.D.; Pal, L.; Rees, M.; Santoro, N.; Simoncini, T. Menopause. Nat. Rev. Dis. Primers 2015, 1, 15004. [Google Scholar] [CrossRef]
- Monteleone, P.; Mascagni, G.; Giannini, A.; Genazzani, A.R.; Simoncini, T. Symptoms of menopause—Global prevalence, physiology and implications. Nat. Rev. Endocrinol. 2018, 14, 199–215. [Google Scholar] [CrossRef]
- Barnard, N.D.; Kahleova, H.; Holtz, D.N.; Znayenko-Miller, T.; Sutton, M.; Holubkov, R.; Zhao, X.; Galandi, S.; Setchell, K.D. A dietary intervention for vasomotor symptoms of menopause: A randomized, controlled trial. Menopause 2023, 30, 80–87. [Google Scholar] [CrossRef]
- Vetrani, C.; Barrea, L.; Rispoli, R.; Verde, L.; De Alteriis, G.; Docimo, A.; Auriemma, R.S.; Colao, A.; Savastano, S.; Muscogiuri, G. Mediterranean diet: What are the consequences for menopause? Front. Endocrinol. 2022, 13, 886824. [Google Scholar] [CrossRef]
- Byrne-Kirk, M.; Mantzioris, E.; Scannell, N.; Villani, A. Adherence to a Mediterranean-style diet and severity of menopausal symptoms in perimenopausal and menopausal women from Australia: A cross-sectional analysis. Eur. J. Nutr. 2024, 63, 2743–2751. [Google Scholar] [CrossRef]
- Abshirini, M.; Siassi, F.; Koohdani, F.; Qorbani, M.; Khosravi, S.; Aslani, Z.; Pak, N.; Sotoudeh, G. Higher intake of dietary n-3 PUFA and lower MUFA are associated with fewer menopausal symptoms. Climacteric 2019, 22, 195–201. [Google Scholar] [CrossRef]
- Nordin, B.C. Calcium and osteoporosis. Nutrition 1997, 13, 664–686. [Google Scholar] [CrossRef]
- Li, G.; Pan, Y.; Sirois, P.; Li, K.; Xu, Y. Iron homeostasis in osteoporosis and its clinical implications. Osteoporos. Int. 2012, 23, 2403–2408. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, M.; Siassi, F.; Qorbani, M.; Khosravi, S.; Aslany, Z.; Abshirini, M.; Zolfaghari, G.; Sotoudeh, G. Dietary patterns and their association with menopausal symptoms: A cross-sectional study. Menopause 2019, 26, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Grisotto, G.; Langton, C.R.; Li, Y.; Bertone-Johnson, E.R.; Baden, M.Y.; Franco, O.H.; Hu, F.B.; Muka, T.; Eliassen, A.H. Association of plant-based diet and early onset of natural menopause. Menopause 2022, 29, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Boutot, M.E.; Purdue-Smithe, A.; Whitcomb, B.W.; Szegda, K.L.; Manson, J.E.; Hankinson, S.E.; Rosner, B.A.; Bertone-Johnson, E.R. Dietary Protein Intake and Early Menopause in the Nurses’ Health Study II. Am. J. Epidemiol. 2018, 187, 270–277. [Google Scholar] [CrossRef]
- Grisotto, G.; Farago, J.S.; Taneri, P.E.; Wehrli, F.; Roa-Diaz, Z.M.; Minder, B.; Glisic, M.; Gonzalez-Jaramillo, V.; Voortman, T.; Marques-Vidal, P.; et al. Dietary factors and onset of natural menopause: A systematic review and meta-analysis. Maturitas 2022, 159, 15–32. [Google Scholar] [CrossRef]
- Tao, M.; Shao, H.; Li, C.; Teng, Y. Correlation between the modified Kupperman Index and the Menopause Rating Scale in Chinese women. Patient Prefer. Adherence 2013, 7, 223–229. [Google Scholar]
- Li, L.; Wu, J.; Pu, D.; Zhao, Y.; Wan, C.; Sun, L.; Shen, C.-e.; Sun, W.; Yuan, Z.; Shen, Q. Factors associated with the age of natural menopause and menopausal symptoms in Chinese women. Maturitas 2012, 73, 354–360. [Google Scholar] [CrossRef]
- Dunneram, Y.; Greenwood, D.C.; Burley, V.J.; Cade, J.E. Dietary intake and age at natural menopause: Results from the UK Women’s Cohort Study. J. Epidemiol. Community Health 2018, 72, 733–740. [Google Scholar] [CrossRef]
- Barrea, L.; Pugliese, G.; Laudisio, D.; Savastano, S.; Colao, A.; Muscogiuri, G. Does mediterranean diet could have a role on age at menopause and in the management of vasomotor menopausal symptoms? The viewpoint of the endocrinological nutritionist. Curr. Opin. Food Sci. 2021, 39, 171–181. [Google Scholar] [CrossRef]
- Purzand, B.; Rokhgireh, S.; Shabani Zanjani, M.; Eshraghi, N.; Mohamadianamiri, M.; Esmailzadeh, A.; Alkatout, I.; Gitas, G.; Allahqoli, L. The comparison of the effect of soybean and fish oil on supplementation on menopausal symptoms in postmenopausal women: A randomized, double-blind, placebo-controlled trial. Complement. Ther. Clin. Pract. 2020, 41, 101239. [Google Scholar] [CrossRef] [PubMed]
- Saimin, J.; Ridwan, S.; Yohanis, M.V.; Lianawati, L.; Arimaswati, A.; Hamliati, H. Menopausal symptoms and fish consuption of menopausal women in the costal areas of Southest Sulawesi, Indonesia. Public. Health Indones. 2020, 6, 57–62. [Google Scholar] [CrossRef]
- Chae, M.; Park, K. Association between dietary omega-3 fatty acid intake and depression in postmenopausal women. Nutr. Res. Pract. 2021, 15, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.K.; Chen, W.J.; Chang, J.P.; Guu, T.W.; Hsin, M.C.; Huang, C.K.; Mischoulon, D.; Capuron, L.; Su, K.P. Personalized Medicine of Omega-3 Fatty Acids in Depression Treatment in Obese and Metabolically Dysregulated Patients. J. Pers. Med. 2023, 13, 1003. [Google Scholar] [CrossRef]
- Li, H.; Ruan, X.Z.; Powis, S.H.; Fernando, R.; Mon, W.Y.; Wheeler, D.C.; Moorhead, J.F.; Varghese, Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: Evidence for a PPAR-γ–dependent mechanism. Kidney Int. 2005, 67, 867–874. [Google Scholar] [CrossRef]
- Bercea, C.I.; Cottrell, G.S.; Tamagnini, F.; McNeish, A.J. Omega-3 polyunsaturated fatty acids and hypertension: A review of vasodilatory mechanisms of docosahexaenoic acid and eicosapentaenoic acid. Br. J. Pharmacol. 2021, 178, 860–877. [Google Scholar] [CrossRef]
- Shibabaw, T. Omega-3 polyunsaturated fatty acids: Anti-inflammatory and anti-hypertriglyceridemia mechanisms in cardiovascular disease. Mol. Cell Biochem. 2021, 476, 993–1003. [Google Scholar] [CrossRef]
- Lucas, M.; Asselin, G.; Mérette, C.; Poulin, M.-J.; Dodin, S. Effects of ethyl-eicosapentaenoic acid omega-3 fatty acid supplementation on hot flashes and quality of life among middle-aged women: A double-blind, placebo-controlled, randomized clinical trial. Menopause 2009, 16, 357–366. [Google Scholar] [CrossRef]
- Berendsen, H.H. The role of serotonin in hot flushes. Maturitas 2000, 36, 155–164. [Google Scholar] [CrossRef]
- Frank, S.M.; Raja, S.N.; Wu, P.K.; el-Gamal, N. Alpha-adrenergic mechanisms of thermoregulation in humans. Ann. N. Y Acad. Sci. 1997, 813, 101–110. [Google Scholar] [CrossRef]
- Carwile, J.L.; Willett, W.C.; Michels, K.B. Consumption of low-fat dairy products may delay natural menopause. J. Nutr. 2013, 143, 1642–1650. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abshirini, M.; Siassi, F.; Koohdani, F.; Qorbani, M.; Golpour-Hamedani, S.; Khosravi, S.; Aslani, Z.; Soleymani, M.; Sotoudeh, G. Association between dairy consumption and menopausal symptoms: A cross-sectional study among Iranian postmenopausal women. Int. Dairy. J. 2020, 105, 104688. [Google Scholar] [CrossRef]
- Flor-Alemany, M.; Marin-Jimenez, N.; Coll-Risco, I.; Aranda, P.; Aparicio, V.A. Influence of dietary habits and Mediterranean diet adherence on menopausal symptoms. The FLAMENCO project. Menopause 2020, 27, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, J.; Davoodi, A.; Khosravi, M.; Sedaghat, M.; Abedi, V.; Hosseinverdi, S.; Ehrampoush, E.; Homayounfar, R.; Shojaie, L. Nutrition and osteoporosis prevention and treatment. Biomed. Res. Ther. 2020, 7, 3709–3720. [Google Scholar] [CrossRef]
- Purdue-Smithe, A.C.; Whitcomb, B.W.; Szegda, K.L.; Boutot, M.E.; Manson, J.E.; Hankinson, S.E.; Rosner, B.A.; Troy, L.M.; Michels, K.B.; Bertone-Johnson, E.R. Vitamin D and calcium intake and risk of early menopause. Am. J. Clin. Nutr. 2017, 105, 1493–1501. [Google Scholar] [CrossRef]
- Dokoh, S.; Donaldson, C.A.; Marion, S.L.; Pike, J.W.; Haussler, M.R. The ovary: A target organ for 1,25-dihydroxyvitamin D3. Endocrinology 1983, 112, 200–206. [Google Scholar] [CrossRef]
- Markus, C.R.; Olivier, B.; de Haan, E.H. Whey protein rich in alpha-lactalbumin increases the ratio of plasma tryptophan to the sum of the other large neutral amino acids and improves cognitive performance in stress-vulnerable subjects. Am. J. Clin. Nutr. 2002, 75, 1051–1056. [Google Scholar] [CrossRef]
- Nagel, G.; Altenburg, H.-P.; Nieters, A.; Boffetta, P.; Linseisen, J. Reproductive and dietary determinants of the age at menopause in EPIC-Heidelberg. Maturitas 2005, 52, 337–347. [Google Scholar] [CrossRef]
- Dorjgochoo, T.; Kallianpur, A.; Gao, Y.-T.; Cai, H.; Yang, G.; Li, H.; Zheng, W.; Shu, X.O. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women’s Health Study. Menopause 2008, 15, 924–933. [Google Scholar] [CrossRef]
- Júnior, L.; Silva, K.; Oliveira, F.; Nisar, S. The most abundant isoflavone contained in soy beans and its effects on menopausal symptoms and related pathophysiologies: A review. Int. J. Chem. Biochem. Sci. 2022, 21, 22–35. [Google Scholar]
- Carmignani, L.O.; Pedro, A.O.; Costa-Paiva, L.H.; Pinto-Neto, A.M. The effect of dietary soy supplementation compared to estrogen and placebo on menopausal symptoms: A randomized controlled trial. Maturitas 2010, 67, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.R.; Chen, K.H. Utilization of Isoflavones in Soybeans for Women with Menopausal Syndrome: An Overview. Int. J. Mol. Sci. 2021, 22, 3212. [Google Scholar] [CrossRef]
- Kim, I.-S. Current perspectives on the beneficial effects of soybean isoflavones and their metabolites for humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef]
- Wei, P.; Liu, M.; Chen, Y.; Chen, D.C. Systematic review of soy isoflavone supplements on osteoporosis in women. Asian Pac. J. Trop. Med. 2012, 5, 243–248. [Google Scholar] [CrossRef]
- Atmaca, A.; Kleerekoper, M.; Bayraktar, M.; Kucuk, O. Soy isoflavones in the management of postmenopausal osteoporosis. Menopause 2008, 15, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gong, W.-W.; Hu, R.-Y.; Wang, H.; Guo, Y.; Bian, Z.; Lv, J.; Chen, Z.-M.; Li, L.-M.; Yu, M. Age at natural menopause and associated factors in adult women: Findings from the China Kadoorie Biobank study in Zhejiang rural area. PLoS ONE 2018, 13, e0195658. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.C.; Chen, L.H.; Mossavar-Rahmani, Y.; Kamensky, V.; Shadyab, A.H.; Haring, B.; Wild, R.A.; Silver, B.; Kuller, L.H.; Sun, Y.; et al. Dietary cholesterol and egg intake in relation to incident cardiovascular disease and all-cause and cause-specific mortality in postmenopausal women. Am. J. Clin. Nutr. 2021, 113, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Zhong, V.W.; Van Horn, L.; Cornelis, M.C.; Wilkins, J.T.; Ning, H.; Carnethon, M.R.; Greenland, P.; Mentz, R.J.; Tucker, K.L.; Zhao, L.; et al. Associations of Dietary Cholesterol or Egg Consumption With Incident Cardiovascular Disease and Mortality. JAMA 2019, 321, 1081–1095. [Google Scholar] [CrossRef]
- Blesso, C.N.; Fernandez, M.L. Dietary Cholesterol, Serum Lipids, and Heart Disease: Are Eggs Working for or Against You? Nutrients 2018, 10, 426. [Google Scholar] [CrossRef]
- Li, M.-Y.; Chen, J.-H.; Chen, C.; Kang, Y.-N. Association between egg consumption and cholesterol concentration: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2020, 12, 1995. [Google Scholar] [CrossRef]
- Gordon, J.L.; Rubinow, D.R.; Thurston, R.C.; Paulson, J.; Schmidt, P.J.; Girdler, S.S. Cardiovascular, hemodynamic, neuroendocrine, and inflammatory markers in women with and without vasomotor symptoms. Menopause 2016, 23, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Imbelloni, L.E.; Gouveia, M. Paresthesia; BoD–Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Miller, R.E.; Miller, R.J.; Malfait, A.-M. Osteoarthritis joint pain: The cytokine connection. Cytokine 2014, 70, 185–193. [Google Scholar] [CrossRef]
- Sharif-Alhoseini, M.; Rahimi-Movaghar, V.; Vaccaro, A.R. Underlying causes of paresthesia. Paresthesia 2012, 71–90. [Google Scholar]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Graf, E.; Mahoney, J.R.; Bryant, R.G.; Eaton, J.W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J. Biol. Chem. 1984, 259, 3620–3624. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Naguro, I.; Ichijo, H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1398–1409. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Rizwan, S.; ReddySekhar, P.; MalikAsrar, B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar]
- Den Tonkelaar, I. Validity and reproducibility of self-reported age at menopause in women participating in the DOM-project. Maturitas 1997, 27, 117–123. [Google Scholar] [CrossRef]


| Variable | Total No. | Percentage% | |
|---|---|---|---|
| Overall | 52,347 | ||
| Age stage | 35–39 | 10,356 | 19.78% |
| 40–44 | 9340 | 17.84% | |
| 45–49 | 10,389 | 19.85% | |
| 50–54 | 11,840 | 22.62% | |
| 55–60 | 10,422 | 19.91% | |
| Menopause | Yes | 18,528 | 35.39% |
| No | 33,819 | 64.61% | |
| Age of menopause | <40 | 142 | 0.77% |
| 40–44 | 1130 | 6.10% | |
| 45–49 | 7249 | 39.12% | |
| 50–54 | 9186 | 49.58% | |
| 55–60 | 821 | 4.43% | |
| Total | 18,528 | 100% | |
| Kupperman | ≤6 | 28,494 | 54.43% |
| 7–15 | 16,229 | 31.00% | |
| 16–30 | 7131 | 13.62% | |
| >30 | 493 | 0.95% | |
| BMI | <18.5 | 2003 | 3.83% |
| 18.5–23.9 | 30,252 | 57.79% | |
| 24.0–27.9 | 16,301 | 31.14% | |
| ≥28.0 | 3791 | 7.24% | |
| Marriage | Married | 49,951 | 95.42% |
| Divorced | 1514 | 2.89% | |
| Widowed | 480 | 0.92% | |
| Single | 402 | 0.77% | |
| Education | ≤9 years | 26,422 | 50.47% |
| 9–12 years | 10,178 | 19.44% | |
| >12 years | 15,747 | 30.09% | |
| OR(crude) | 95%CI | p-Value | OR(adjust) | 95%CI | p-Value | |
|---|---|---|---|---|---|---|
| Fish | ||||||
| 2 | 1.26 | 1.15–1.39 | 0.00 | 1.17 | 1.05–1.30 | 0.00 |
| 3 | 1.47 | 1.33–1.63 | 0.00 | 1.30 | 1.15–1.46 | 0.00 |
| 4 | 1.60 | 1.40–1.83 | 0.00 | 1.52 | 1.29–1.78 | 0.00 |
| Milk | ||||||
| 2 | 1.11 | 1.01–1.21 | 0.03 | 0.97 | 0.87–1.07 | 0.53 |
| 3 | 1.22 | 1.11–1.34 | 0.00 | 0.97 | 0.87–1.09 | 0.63 |
| 4 | 1.13 | 1.02- 1.26 | 0.02 | 0.92 | 0.81–1.06 | 0.25 |
| Egg | ||||||
| 2 | 1.17 | 0.96–1.42 | 0.13 | 1.00 | 0.79–1.27 | 0.96 |
| 3 | 1.28 | 1.06–1.60 | 0.01 | 1.04 | 0.82–1.32 | 0.77 |
| 4 | 1.26 | 1.03–1.54 | 0.02 | 1.04 | 0.81–1.33 | 0.75 |
| Bean | ||||||
| 2 | 1.25 | 1.08–1.44 | 0.00 | 1.13 | 0.96–1.32 | 0.15 |
| 3 | 1.35 | 1.17–1.56 | 0.00 | 1.14 | 0.97–1.35 | 0.12 |
| 4 | 1.23 | 1.04–1.44 | 0.01 | 1.02 | 0.85–1.24 | 0.81 |
| Vitamin | 1.07 | 1.00–1.15 | 0.04 | 1.02 | 0.95–1.10 | 0.59 |
| Ca | 1.08 | 1.01–1.14 | 0.01 | 1.04 | 0.98–1.11 | 0.23 |
| Fe | 1.07 | 0.94–1.22 | 0.28 |
| OR(crude) | 95%CI | p-Value | OR(adjust) | 95%CI | p-Value | |
|---|---|---|---|---|---|---|
| Fish | ||||||
| 2 | 0.99 | 0.93–1.06 | 0.81 | 0.83 | 0.77–0.89 | 0.00 |
| 3 | 0.78 | 0.73–0.83 | 0.00 | 0.68 | 0.62–0.74 | 0.00 |
| 4 | 0.64 | 0.59–0.70 | 0.00 | 0.58 | 0.53–0.65 | 0.00 |
| Milk | ||||||
| 2 | 0.90 | 0.85–0.95 | 0.00 | 0.85 | 0.80–0.91 | 0.00 |
| 3 | 0.66 | 0.62–0.70 | 0.00 | 0.69 | 0.65–0.74 | 0.00 |
| 4 | 0.68 | 0.64–0.73 | 0.00 | 0.72 | 0.66–0.78 | 0.00 |
| Egg | ||||||
| 2 | 0.95 | 0.84–1.07 | 0.38 | 0.94 | 0.80–1.10 | 0.40 |
| 3 | 0.81 | 0.72–0.91 | 0.00 | 0.94 | 0.81–1.10 | 0.44 |
| 4 | 0.85 | 0.75–0.96 | 0.01 | 0.99 | 0.84–1.16 | 0.89 |
| Bean | ||||||
| 2 | 0.84 | 0.77–0.91 | 0.00 | 0.80 | 0.73–0.89 | 0.00 |
| 3 | 0.55 | 0.51–0.61 | 0.00 | 0.60 | 0.54–0.68 | 0.00 |
| 4 | 0.47 | 0.42–0.52 | 0.00 | 0.52 | 0.46–0.58 | 0.00 |
| Vitamin | 1.14 | 1.09–1.18 | 0.00 | 1.00 | 0.96–1.04 | 0.93 |
| Ca | 1.61 | 1.55–1.67 | 0.00 | 1.48 | 1.43–1.54 | 0.00 |
| Fe | 1.41 | 1.31–1.51 | 0.00 | 1.35 | 1.25–1.45 | 0.00 |
| Vasomotor | Somatic | Psychological | Urogenital | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95%CI | p-Value | β | 95%CI | p-Value | β | 95%CI | p-Value | β | 95%CI | p-Value | |
| Fish | ||||||||||||
| 2 | 0.01 | −0.06–0.08 | 0.87 | −0.32 | −0.41–−0.23 | 0.00 | −0.30 | −0.40–−0.21 | 0.00 | −0.06 | −0.12–−0.01 | 0.00 |
| 3 | −0.03 | −0.11–0.05 | 0.52 | −0.59 | −0.69–−0.49 | 0.00 | −0.59 | −0.69–−0.48 | 0.00 | −0.20 | −0.27–−0.13 | 0.00 |
| 4 | −0.10 | −0.20–0.01 | 0.07 | −0.83 | −0.96–−0.70 | 0.00 | −0.83 | −0.97–−0.69 | 0.00 | −0.10 | −0.18–−0.01 | 0.02 |
| Milk | ||||||||||||
| 2 | −0.06 | −0.13–0.01 | 0.08 | −0.28 | −0.37–−0.20 | 0.00 | −0.28 | −0.37–−0.20 | 0.00 | −0.09 | −0.15–−0.04 | 0.00 |
| 3 | −0.16 | −0.23–−0.09 | 0.00 | −0.56 | −0.64–−0.47 | 0.00 | −0.58 | −0.67–−0.48 | 0.00 | −0.12 | −0.18–−0.06 | 0.00 |
| 4 | −0.16 | −0.25–−0.07 | 0.00 | −0.49 | −0.60–−0.38 | 0.00 | −0.47 | −0.59–−0.36 | 0.00 | −0.05 | −0.12–0.02 | 0.17 |
| Egg | ||||||||||||
| 2 | −0.11 | −0.26–0.05 | 0.18 | 0.02 | −0.18–0.22 | 0.84 | 0.12 | −0.08–0.33 | 0.24 | −0.09 | −0.22–0.04 | 0.15 |
| 3 | −0.21 | −0.37–−0.05 | 0.00 | −0.01 | −0.21–0.19 | 0.94 | 0.19 | −0.02–0.40 | 0.08 | −0.07 | −0.20–0.06 | 0.28 |
| 4 | −0.13 | −0.30–0.03 | 0.11 | 0.01 | −0.19–0.22 | 0.91 | 0.30 | 0.09–0.52 | 0.01 | −0.05 | −0.19–0.08 | 0.43 |
| Bean | ||||||||||||
| 2 | −0.21 | −0.32–−0.11 | 0.00 | −0.52 | −0.66–−0.39 | 0.00 | −0.29 | −0.43–−0.15 | 0.00 | −0.16 | −0.25–−0.07 | 0.00 |
| 3 | −0.33 | −0.44–−0.22 | 0.00 | −0.81 | −0.95–−0.68 | 0.00 | −0.73 | −0.88–−0.59 | 0.00 | −0.33 | −0.42–−0.24 | 0.00 |
| 4 | −0.35 | −0.48–−0.22 | 0.00 | −0.96 | −1.12–−0.80 | 0.00 | −1.07 | −1.24–−0.91 | 0.00 | −0.30 | −0.41–−0.20 | 0.00 |
| Vitamin | 0.04 | −0.01–0.08 | 0.07 | −0.01 | −0.06–0.05 | 0.92 | 0.06 | 0.01–−0.12 | 0.03 | −0.10 | −0.13–−0.06 | 0.00 |
| Ca | 0.30 | 0.26–0.34 | 0.00 | 0.48 | 0.43–0.53 | 0.00 | 0.44 | 0.39–0.49 | 0.00 | 0.20 | 0.17–0.24 | 0.00 |
| Fe | 0.38 | 0.30–0.46 | 0.00 | 0.53 | 0.02–0.03 | 0.00 | 0.25 | 0.15–0.36 | 0.00 | 0.22 | 0.15–0.28 | 0.00 |
| Age Stage | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 35–39 | 40–44 | 45–49 | 50–54 | 55–60 | |||||||||||
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | OR | 95%CI | p-Value | OR | 95%CI | p-Value | OR | 95%CI | p-Value | |
| Fish | |||||||||||||||
| 2 | 0.90 | 0.73–1.11 | 0.32 | 0.78 | 0.65–0.93 | 0.00 | 0.76 | 0.65–0.90 | 0.00 | 0.80 | 0.70–0.92 | 0.00 | 0.88 | 0.77–1.01 | 0.07 |
| 3 | 0.67 | 0.54–0.83 | 0.00 | 0.59 | 0.48–0.72 | 0.00 | 0.65 | 0.54–0.77 | 0.00 | 0.68 | 0.58–0.79 | 0.00 | 0.79 | 0.68–0.92 | 0.00 |
| 4 | 0.55 | 0.42–0.73 | 0.00 | 0.41 | 0.32–0.54 | 0.00 | 0.54 | 0.43–0.69 | 0.00 | 0.62 | 0.51–0.76 | 0.00 | 0.77 | 0.62–0.95 | 0.01 |
| Milk | |||||||||||||||
| 2 | 0.79 | 0.67–0.94 | 0.01 | 0.81 | 0.69–0.94 | 0.01 | 0.90 | 0.78–1.04 | 0.15 | 0.87 | 0.76–0.98 | 0.03 | 0.89 | 0.78–1.02 | 0.08 |
| 3 | 0.63 | 0.52–0.75 | 0.00 | 0.59 | 0.50–0.70 | 0.00 | 0.74 | 0.64–0.86 | 0.00 | 0.68 | 0.60–0.78 | 0.00 | 0.84 | 0.73–0.97 | 0.02 |
| 4 | 0.53 | 0.43–0.66 | 0.00 | 0.63 | 0.52–0.78 | 0.00 | 0.73 | 0.61–0.88 | 0.00 | 0.81 | 0.68–0.95 | 0.01 | 0.94 | 0.78–1.11 | 0.45 |
| Egg | |||||||||||||||
| 2 | 0.62 | 0.04–0.90 | 0.01 | 0.81 | 0.53–1.21 | 0.30 | 1.37 | 0.95–1.97 | 0.09 | 0.94 | 0.70–1.27 | 0.70 | 1.04 | 0.77–1.40 | 0.80 |
| 3 | 0.71 | 0.48–1.03 | 0.07 | 0.88 | 0.58–1.33 | 0.55 | 1.31 | 0.91–1.89 | 0.15 | 0.92 | 0.68–1.24 | 0.58 | 0.94 | 0.70–1.28 | 0.70 |
| 4 | 0.77 | 0.52–1.14 | 0.19 | 0.98 | 0.64–1.50 | 0.94 | 1.44 | 0.99–2.10 | 0.06 | 0.95 | 0.69–1.30 | 0.75 | 0.83 | 0.61–1.14 | 0.25 |
| Bean | |||||||||||||||
| 2 | 0.68 | 0.52–0.87 | 0.00 | 0.96 | 0.75–1.23 | 0.74 | 0.73 | 0.58–0.92 | 0.01 | 0.84 | 0.68–1.03 | 0.09 | 0.81 | 0.66–0.99 | 0.04 |
| 3 | 0.54 | 0.41–0.71 | 0.00 | 0.75 | 0.58–0.97 | 0.03 | 0.53 | 0.42–0.67 | 0.00 | 0.63 | 0.51–0.78 | 0.00 | 0.60 | 0.49–0.75 | 0.00 |
| 4 | 0.51 | 0.38–0.70 | 0.00 | 0.60 | 0.44–0.82 | 0.00 | 0.39 | 0.29–0.51 | 0.00 | 0.48 | 0.38–0.62 | 0.00 | 0.63 | 0.49–0.81 | 0.00 |
| Vitamin | 1.04 | 0.94–1.14 | 0.47 | 1.04 | 0.95–1.15 | 0.35 | 0.99 | 0.90–1.09 | 0.85 | 0.99 | 0.90–1.08 | 0.75 | 1.00 | 0.91–1.10 | 0.99 |
| Ca | 1.26 | 1.13–1.39 | 0.00 | 1.33 | 1.20–1.47 | 0.00 | 1.44 | 1.33–1.57 | 0.00 | 1.75 | 1.43–1.66 | 0.00 | 1.62 | 1.49–1.76 | 0.00 |
| Fe | 1.44 | 1.22–1.69 | 0.00 | 1.43 | 1.20–1.71 | 0.00 | 1.38 | 1.17–1.63 | 0.00 | 1.18 | 1.10–1.52 | 0.00 | 1.38 | 1.16–1.64 | 0.00 |
| Menopause | ||||||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | |
| Fish | ||||||
| 2 | 0.82 | 0.74–0.91 | 0.00 | 0.83 | 0.76–0.92 | 0.00 |
| 3 | 0.69 | 0.62–0.78 | 0.00 | 0.67 | 0.60–0.75 | 0.00 |
| 4 | 0.66 | 0.56–0.77 | 0.00 | 0.55 | 0.48–0.63 | 0.00 |
| Milk | ||||||
| 2 | 0.86 | 0.78–0.95 | 0.00 | 0.86 | 0.79–0.94 | 0.00 |
| 3 | 0.73 | 0.65–0.80 | 0.00 | 0.68 | 0.62–0.75 | 0.00 |
| 4 | 0.87 | 0.76–0.99 | 0.03 | 0.66 | 0.59–0.74 | 0.00 |
| Egg | ||||||
| 2 | 0.99 | 0.79–1.24 | 0.96 | 0.91 | 0.74–1.11 | 0.36 |
| 3 | 0.94 | 0.75–1.18 | 0.60 | 0.95 | 0.77–1.17 | 0.62 |
| 4 | 0.84 | 0.67–1.07 | 0.16 | 1.08 | 0.88–1.34 | 0.47 |
| Bean | ||||||
| 2 | 0.79 | 0.68–0.92 | 0.00 | 0.81 | 0.71–0.92 | 0.00 |
| 3 | 0.61 | 0.52–0.72 | 0.00 | 0.61 | 0.53–0.70 | 0.00 |
| 4 | 0.54 | 0.44–0.65 | 0.00 | 0.49 | 0.42–0.58 | 0.00 |
| Vitamin | 1.00 | 0.93–1.07 | 0.95 | 1.01 | 0.96–1.06 | 0.66 |
| Ca | 1.58 | 1.48–1.68 | 0.00 | 1.39 | 1.32–1.46 | 0.00 |
| Fe | 1.34 | 1.18–1.53 | 0.00 | 1.38 | 1.26–1.51 | 0.00 |
| Underweight | Normal Weight | Overweight | Obesity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | OR | 95%CI | p-Value | OR | 95%CI | p-Value | |
| Fish | ||||||||||||
| 2 | 1.35 | 0.93–1.97 | 0.11 | 0.86 | 0.78–0.94 | 0.00 | 0.78 | 0.70–0.88 | 0.00 | 0.77 | 0.61–0.97 | 0.03 |
| 3 | 1.17 | 0.78–1.76 | 0.45 | 0.68 | 0.61–0.76 | 0.00 | 0.67 | 0.59–0.76 | 0.00 | 0.62 | 0.47–0.80 | 0.00 |
| 4 | 0.83 | 0.49–1.40 | 0.48 | 0.59 | 0.51–0.67 | 0.00 | 0.57 | 0.47–0.69 | 0.00 | 0.61 | 0.42–0.90 | 0.01 |
| Milk | ||||||||||||
| 2 | 0.74 | 0.53–1.04 | 0.08 | 0.86 | 0.79–0.94 | 0.00 | 0.83 | 0.75–0.92 | 0.00 | 0.92 | 0.75–1.14 | 0.44 |
| 3 | 0.65 | 0.45–0.93 | 0.02 | 0.67 | 0.61–0.74 | 0.00 | 0.70 | 0.62–0.79 | 0.00 | 0.73 | 0.58–0.92 | 0.00 |
| 4 | 0.78 | 0.51–1.19 | 0.25 | 0.64 | 0.58–0.72 | 0.00 | 0.82 | 0.71–0.94 | 0.01 | 1.00 | 0.74–1.36 | 0.98 |
| Egg | ||||||||||||
| 2 | 1.59 | 0.74–3.46 | 0.24 | 0.97 | 0.78–1.21 | 0.82 | 0.78 | 0.61–1.00 | 0.06 | 1.22 | 0.76–1.95 | 0.41 |
| 3 | 1.27 | 0.59–2.76 | 0.54 | 1.07 | 0.86–1.32 | 0.57 | 0.74 | 0.57–0.95 | 0.02 | 0.99 | 0.62–1.60 | 0.97 |
| 4 | 1.32 | 0.59–2.93 | 0.50 | 1.17 | 0.93–1.46 | 0.18 | 0.71 | 0.55–0.93 | 0.01 | 1.05 | 0.64–1.72 | 0.84 |
| Bean | ||||||||||||
| 2 | 0.74 | 0.43–1.27 | 0.28 | 0.84 | 0.73–0.97 | 0.02 | 0.80 | 0.67–0.94 | 0.01 | 0.62 | 0.44–0.88 | 0.01 |
| 3 | 0.50 | 0.29–0.87 | 0.01 | 0.62 | 0.53–0.71 | 0.00 | 0.66 | 0.55–0.78 | 0.00 | 0.46 | 0.32–0.65 | 0.00 |
| 4 | 0.34 | 0.18–0.64 | 0.00 | 0.49 | 0.42–0.58 | 0.00 | 0.60 | 0.49–0.74 | 0.00 | 0.45 | 0.29–0.70 | 0.00 |
| Vitamin | 0.81 | 0.66–1.00 | 0.05 | 1.04 | 0.99–1.10 | 0.11 | 0.94 | 0.87–1.02 | 0.15 | 1.06 | 0.90–1.25 | 0.49 |
| Ca | 1.61 | 1.31–1.99 | 0.00 | 1.43 | 1.36–1.51 | 0.00 | 1.54 | 1.44–1.66 | 0.00 | 1.58 | 1.37–1.83 | 0.00 |
| Fe | 1.44 | 1.00–2.05 | 0.05 | 1.30 | 1.18–1.44 | 0.00 | 1.38 | 1.20–1.58 | 0.00 | 1.57 | 1.16–2.12 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Yang, Y.; Yong, Z.; Yang, L.; Zhao, Y.; Yan, M.; Zheng, R.; Luo, X. Association Between Protein-Rich Foods, Nutritional Supplements, and Age of Natural Menopause and Its Symptoms. Nutrients 2025, 17, 356. https://doi.org/10.3390/nu17020356
Yang Y, Yang Y, Yong Z, Yang L, Zhao Y, Yan M, Zheng R, Luo X. Association Between Protein-Rich Foods, Nutritional Supplements, and Age of Natural Menopause and Its Symptoms. Nutrients. 2025; 17(2):356. https://doi.org/10.3390/nu17020356
Chicago/Turabian StyleYang, Yilin, Yehuan Yang, Zhenghua Yong, Li Yang, Yanxia Zhao, Mengke Yan, Ruimin Zheng, and Xiaomin Luo. 2025. "Association Between Protein-Rich Foods, Nutritional Supplements, and Age of Natural Menopause and Its Symptoms" Nutrients 17, no. 2: 356. https://doi.org/10.3390/nu17020356
APA StyleYang, Y., Yang, Y., Yong, Z., Yang, L., Zhao, Y., Yan, M., Zheng, R., & Luo, X. (2025). Association Between Protein-Rich Foods, Nutritional Supplements, and Age of Natural Menopause and Its Symptoms. Nutrients, 17(2), 356. https://doi.org/10.3390/nu17020356





