Efficacy of N-Acetylcysteine in Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Methods for Identification of Studies
2.3. Data Extraction and Data Items
2.4. Assessment of Risk of Bias in Included Studies
2.5. Assessment of Results
2.6. Risk of Bias Across the Studies
2.7. Additional Analyses
3. Result
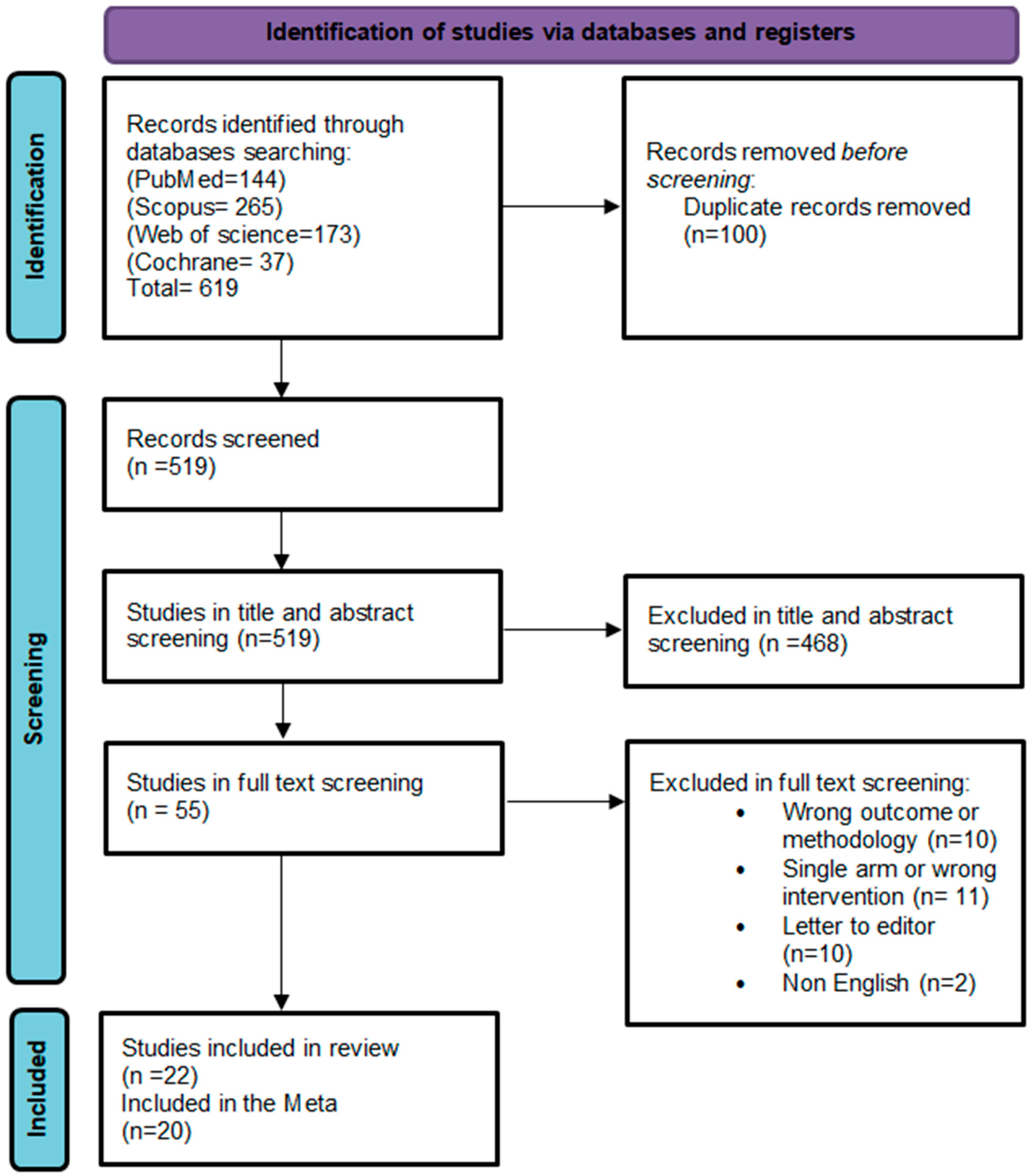
3.1. Study Selection
3.2. Study Characteristics
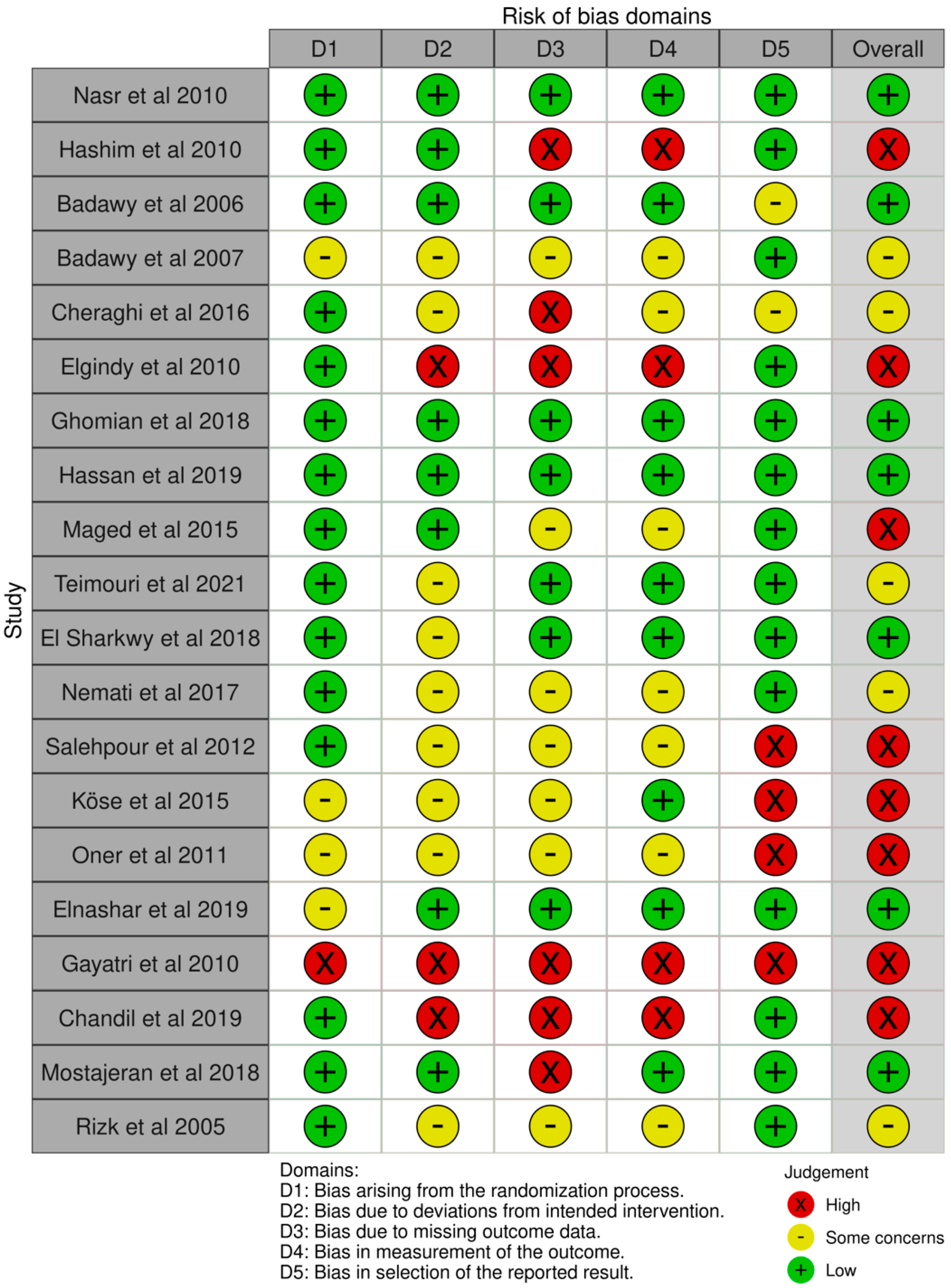
3.3. Risk of Bias Assessment
3.4. Serum Estradiol (E2) Levels
3.5. Sex Hormone-Binding Globulin (SHBG) Levels
3.6. Follicle-Stimulating Hormone (FSH) Levels
3.7. Luteinizing Hormone (LH) Levels
3.8. Serum Progesterone Levels
3.9. Total Testosterone (TT)
3.10. Female Uterine Endometrial Thickness
3.11. Number of Follicles
3.12. Sensitivity Analysis
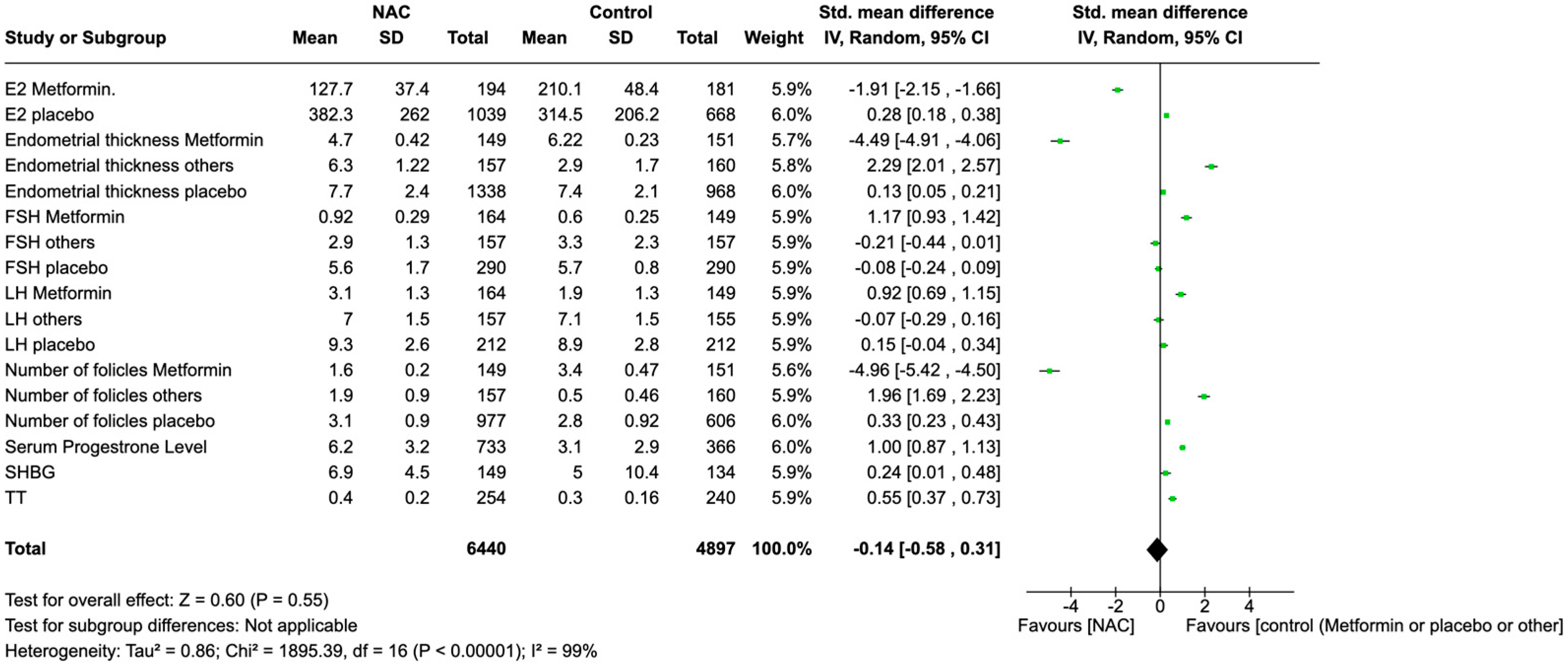
3.13. Global Effects of NAC Supplementation
3.14. Publication Bias
3.15. GRADE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dilliyappan, S.; Kumar, A.S.; Venkatesalu, S.; Palaniyandi, T.; Baskar, G.; Sivaji, A.; Rab, S.O.; Saeed, M.; Shivaranjani, K.S. Polycystic ovary syndrome: Recent research and therapeutic advancements. Life Sci. 2024, 359, 123221. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.G.; Jeon, M.; Ryu, K.J.; Kim, J.; Choe, B.Y.; Joo, Y.Y.; Park, H. Similar but Distinct Comorbidity Patterns Between Polycystic Ovary Syndrome and Endometriosis in Korean Women: A Nationwide Cohort Study. J. Korean Med. Sci. 2024, 39, e284. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, A.; Ebadinejad, A.; Rahmati, M.; Momenan, A.A.; Niroomand, M.; Valizadeh, M.; Azizi, F.; Tehrani, F.R.; Hosseinpanah, F. Body composition analysis in women with polycystic ovary syndrome: A cross-sectional study from the Tehran Lipid and Glucose Study (TLGS). BMC Endocr. Disord. 2024, 24, 251. [Google Scholar] [CrossRef]
- Murdoch, A.P.; Diggle, P.J.; White, M.C.; Kendall-Taylor, P.; Dunlop, W. LH in polycystic ovary syndrome: Reproducibility and pulsatile secretion. J. Endocrinol. 1989, 121, 185–191. [Google Scholar] [CrossRef]
- Fang, Y.Q.; Ding, H.; Li, T.; Zhao, X.J.; Luo, D.; Liu, Y.; Li, Y. N-acetylcysteine supplementation improves endocrine-metabolism profiles and ovulation induction efficacy in polycystic ovary syndrome. J. Ovarian Res. 2024, 17, 205. [Google Scholar] [CrossRef]
- Dunaif, A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr. Rev. 1997, 18, 774–800. [Google Scholar] [CrossRef]
- Purwar, A.; Nagpure, S. Insulin resistance in polycystic ovarian syndrome. Cureus 2022, 14, e31119. [Google Scholar] [CrossRef]
- Collée, J.; Mawet, M.; Tebache, L.; Nisolle, M.; Brichant, G. Polycystic ovarian syndrome and infertility: Overview and insights of the putative treatments. Gynecol. Endocrinol. 2021, 37, 869–874. [Google Scholar] [CrossRef]
- Singh, S.; Pal, N.; Shubham, S.; Sarma, D.K.; Verma, V.; Marotta, F.; Kumar, M. Polycystic ovary syndrome: Etiology, current management, and future therapeutics. J. Clin. Med. 2023, 12, 1454. [Google Scholar] [CrossRef]
- Jayasena, C.N.; Franks, S. The management of patients with polycystic ovary syndrome. Nat. Rev. Endocrinol. 2014, 10, 624–636. [Google Scholar] [CrossRef]
- Panda, S.R.; Sharmila, V.; Kalidoss, V.K.; Hota, S. A triple-blind, randomized controlled trial, comparing combined letrozole and clomiphene versus only letrozole for ovulation induction in women with polycystic ovarian syndrome. Int. J. Gynaecol. Obstet. 2023, 161, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Yifu, P. A review of antioxidant N-acetylcysteine in addressing polycystic ovary syndrome. Gynecol. Endocrinol. 2024, in press. [CrossRef]
- Fulghesu, A.M.; Ciampelli, M.; Muzj, G.; Belosi, C.; Selvaggi, L.; Ayala, G.F.; Lanzone, A. N-acetyl-cysteine treatment improves insulin sensitivity in women with polycystic ovary syndrome. Fertil. Steril. 2002, 77, 1128–1135. [Google Scholar] [CrossRef]
- Pingarrón Santofimia, C.; Poyo Torcal, S.; López Verdú, H.; Henríquez Linares, A.; Calvente Aguilar, V.; Terol Sánchez, P.; Martínez García, M.S.; Lafuente González, P. Evaluation of the efficacy of an antioxidant combination for the modulation of metabolic, endocrine, and clinical parameters in patients with polycystic ovary syndrome. Gynecol. Endocrinol. 2023, 39, 2227277. [Google Scholar] [CrossRef]
- Asl, Z.S.; Parastouei, K.; Eskandari, E. The effects of N-acetylcysteine on ovulation and sex hormones profile in women with polycystic ovary syndrome: A systematic review and meta-analysis. Br. J. Nutr. 2023, 130, 202–210. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.J. Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Guyatt, G.H.; Thorlund, K.; Oxman, A.D.; Walter, S.D.; Patrick, D.; Furukawa, T.A.; Johnston, B.C.; Karanicolas, P.; Akl, E.A.; Vist, G.; et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles—Continuous outcomes. J. Clin. Epidemiol. 2013, 66, 173–183. [Google Scholar] [CrossRef]
- Mostajeran, F.; Tehrani, H.G.; Rahbary, B. N-acetylcysteine as an adjuvant to letrozole for induction of ovulation in infertile patients with polycystic ovary syndrome. Adv. Biomed. Res. 2018, 7, 100. [Google Scholar] [CrossRef]
- Nasr, A. Effect of N-acetyl-cysteine after ovarian drilling in clomiphene citrate-resistant PCOS women: A pilot study. Reprod. Biomed. Online 2010, 20, 403–409. [Google Scholar] [CrossRef]
- Badawy, A.; State, O.; Abdelgawad, S. N-Acetyl cysteine and clomiphene citrate for induction of ovulation in polycystic ovary syndrome: A cross-over trial. Acta Obstet. Gynecol. Scand. 2007, 86, 218–222. [Google Scholar] [CrossRef]
- Köse, S.A.; Nazıroğlu, M. N-acetyl cysteine reduces oxidative toxicity, apoptosis, and calcium entry through TRPV1 channels in the neutrophils of patients with polycystic ovary syndrome. Free Radic. Res. 2015, 49, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Oner, G.; Muderris, I.I. Clinical, endocrine and metabolic effects of metformin vs N-acetyl-cysteine in women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Kilic-Okman, T.; Guldiken, S.; Kucuk, M. Relationship between homocysteine and insulin resistance in women with polycystic ovary syndrome. Endocr. J. 2004, 51, 505–508. [Google Scholar] [CrossRef] [PubMed]
- El Sharkwy, I.; Sharaf El-Din, M. l-Carnitine plus metformin in clomiphene-resistant obese PCOS women, reproductive and metabolic effects: A randomized clinical trial. Gynecol. Endocrinol. 2019, 35, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Cheraghi, E.; Mehranjani, M.S.; Shariatzadeh, M.A.; Esfahani, M.H.; Ebrahimi, Z. N-Acetylcysteine improves oocyte and embryo quality in polycystic ovary syndrome patients undergoing intracytoplasmic sperm injection: An alternative to metformin. Reprod. Fertil. Dev. 2016, 28, 723–731. [Google Scholar] [CrossRef]
- Maged, A.M.; Elsawah, H.; Abdelhafez, A.; Bakry, A.; Mostafa, W.A. The adjuvant effect of metformin and N-acetylcysteine to clomiphene citrate in induction of ovulation in patients with Polycystic Ovary Syndrome. Gynecol. Endocrinol. 2015, 31, 635–638. [Google Scholar] [CrossRef]
- Hashim, H.A.; Shokeir, T.; Badawy, A. Retracted: Letrozole versus combined metformin and clomiphene citrate for ovulation induction in clomiphene-resistant women with polycystic ovary syndrome: A randomized controlled trial. Fertil. Steril. 2020, 114, 667–668. [Google Scholar] [CrossRef]
- Rizk, A.Y.; Bedaiwy, M.A.; Al-Inany, H.G. N-acetyl-cysteine is a novel adjuvant to clomipbene citrate in clomiphene citrate-resistant patients with polycystic ovary syndrome. Altern. Med. Rev. 2005, 10, 152–153. [Google Scholar] [CrossRef]
- Salehpour, S.; Akbari Sene, A.; Saharkhiz, N.; Sohrabi, M.R.; Moghimian, F. N-acetylcysteine as an adjuvant to clomiphene citrate for successful induction of ovulation in infertile patients with polycystic ovary syndrome. J. Obstet. Gynaecol. Res. 2012, 38, 1182–1186. [Google Scholar] [CrossRef]
- Javanmanesh, F.; Kashanian, M.; Rahimi, M.; Sheikhansari, N. A comparison between the effects of metformin and N-acetyl cysteine (NAC) on some metabolic and endocrine characteristics of women with polycystic ovary syndrome. Gynecol. Endocrinol. 2016, 32, 285–289. [Google Scholar] [CrossRef]
- Ghomian, N.; Khadem, N.; Moeindarbari, S.; Abdolrazagh, A. Comparison of pregnancy rate in patients with polycystic ovary syndrome treated with clomiphene alone and in combination with N-acetyl cysteine: A randomized clinical trial. Int. J. Womens Health Reprod. Sci. 2019, 7, 185–189. [Google Scholar] [CrossRef]
- Nemati, M.; Nemati, S.; Taheri, A.M.; Heidari, B. Comparison of metformin and N-acetyl cysteine, as an adjuvant to clomiphene citrate, in clomiphene-resistant women with polycystic ovary syndrome. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Elgindy, E.A.; El-Huseiny, A.M.; Mostafa, M.I.; Gaballah, A.M.; Ahmed, T.A. N-acetyl cysteine: Could it be an effective adjuvant therapy in ICSI cycles? A preliminary study. Reprod. Biomed. Online 2010, 20, 789–796. [Google Scholar] [CrossRef]
- Teimouri, B.; Mollashahi, S.; Paracheh, M.; Farzaneh, F. Comparison of the effect of letrozole alone with letrozole plus n-acetylcysteine on pregnancy rate in patients with polycystic ovarian syndrome: A randomized clinical trial. Int. J. Womens Health Reprod. Sci. 2021, 9, 75–79. [Google Scholar] [CrossRef]
- Gayatri, K.; Kumar, J.S.; Kumar, B.B. Metformin and N-acetyl cysteine in polycystic ovarian syndrome–-a comparative study. Indian J. Clin. Med. 2010, 1, 2. [Google Scholar]
- Chandil, N.; Pande, S.; Sen, S.S.; Gupta, D. Comparison of metformin and N acetylcysteine on clinical, metabolic parameter and hormonal profile in women with polycystic ovarian syndrome. J. Obstet. Gynaecol. India 2019, 69, 77–81. [Google Scholar] [CrossRef]
- Elnashar, A.; Fahmy, M.; Mansour, A.; Ibrahim, K. N-acetyl cysteine vs. metformin in treatment of clomiphene citrate–resistant polycystic ovary syndrome: A prospective randomized controlled study. Fertil. Steril. 2007, 88, 406–409. [Google Scholar] [CrossRef]
- Hassan, M.; Alalfy, M.; Hassan, H.; Ogila, A. Combined n-acetylcysteine and clomiphene citrate for ovulation induction in polycystic ovary syndrome, a Double Blind Randomized Controlled Trial. Austin J. Obstet. Gynecol. 2019, 6, 1134. [Google Scholar]
- Moran, L.J.; Misso, M.L.; Wild, R.A.; Norman, R.J. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2010, 16, 347–363. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations From the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Eur. J. Endocrinol. 2023, 108, G43–G64. [Google Scholar] [CrossRef]
| Study ID | Country | Study Design | Interventions | Sample Size | Age Mean ± SD | BMI Mean ± SD |
|---|---|---|---|---|---|---|
| Mostajeran et al., 2018 [19] | Iran | RCT | Letrozole + NAC | 65 | 29.1 ± 3.7 | 26.6 ± 4.7 |
| Letrozole + placebo | 61 | 30.3 ± 3.9 | 26.1 ± 4.7 | |||
| Nasr et al., 2010 [20] | Egypt | RCT | LOD + NAC | 30 | 28.4 ± 4.2 | 28.6 ± 3.7 |
| LOD + placebo | 30 | 29.2 ± 3.7 | 29.1 ± 4.2 | |||
| Badawy et al., 2007 [21] | Egypt | cross-over trial | CC | 260 | 27.2 ± 3.2 | 28.2 ± 3.2 |
| CC + NAC | 210 | |||||
| Köse et al., 2015 [22] | Turkey | clincl trial | PCOS + NAC | 17 | 24.5 ± 5.9 | 26.0 ± 4.5 |
| PCOS | 17 | 24.5 ± 5.9 | 26.0 ± 4.6 | |||
| Oner et al., 2011 [23] | Turkey | RCT | NAC | 45 | 23.7 ± 4.4 | 23.0 ± 4.6 |
| metformin | 30 | 22.6 ± 4.0 | 24.3 ± 6.2 | |||
| Kilic-Okman et al., 2004 [24] | Turkey | clincl trial | NAC | 20 | 26.7 ± 4.3 | 25.1 ± 5.6 |
| El Sharkwy et al., 2019 [25] | Egypt | RCT | CC + NAC | 82 | 26.6 ± 1.5 | 29.5 ± 3.3 |
| CC + l-carnitine | 80 | 26.6 ± 1.5 | 29.7 ± 2.4 | |||
| Cheraghi et al., 2016 [26] | Iran | RCT | NAC | 15 | 29.7 ± 3.4 | 27.7 ± 4.5 |
| placebo | 15 | 27.9 ± 2.8 | 26.9 ± 2.3 | |||
| Fulghesu et al., 2002 [13] | Italy | Prospective data analysis | NAC | 37 | NA | 32.4 ± 7.3 |
| Maged et al., 2015 [27] | Egypt | RCT | CC | 40 | 26.0 ± 3.6 | 27.3 ± 3.2 |
| CC + NAC | 40 | 25.8 ± 3.5 | 27.4 ± 3.1 | |||
| Hashim et al., 2010 [28] | Egypt | RCT | NAC + CC | 95 | 27.3 ± 2.6 | 26.6 ± 2.2 |
| metformin + CC | 97 | 26.8 ± 2.2 | 26.3 ± 2.3 | |||
| Rizk et al., 2005 [29] | Egypt | RCT | NAC | 75 | 28.9 ± 4.7 | 30.5 ± 2.6 |
| CC + placebo | 75 | 28.4 ± 5.7 | 30.1 ± 3.1 | |||
| Salehpour et al., 2012 [30] | Iran | RCT | CC + NAC | 82 | 27.2 ± 3.3 | 26.8 ± 2.2 |
| CC + placebo | 85 | 27.4 ± 3.4 | 26.7 ± 2.0 | |||
| Javanmanesh et al., 2015 [31] | UK | RCT | NAC | 46 | 29.0 ± 4.4 | 28.1 ± 5.5 |
| Metformin | 48 | 29.8 ± 4.9 | 29.1 ± 2.8 | |||
| Ghomian et al., 2019 [32] | Iran | RCT | Clomiphene + NAC | 33 | 28.7 ± 6.9 | 24.5 ± 3.0 |
| Clomiphene + NAC | 33 | 28.5 ± 6.2 | 25.3 ± 5.0 | |||
| Nemati et al., 2017 [33] | Iran | RCT | CC + NAC | 54 | NA | 33.1 ± 6.3 |
| CC + metformin | 54 | NA | 29.0 ± 7.1 | |||
| Elgindy et al., 2010 [34] | Egypt | RCT | long protocol + NAC | 38 | 26.4 ± 4.1 | 27.2 ± 1.9 |
| long protocol | 38 | 28.0 ± 3.9 | 27.2 ± 1.3 | |||
| Teimouri et al., 2021 [35] | Iran | RCT | Letrozole + NAC | 158 | 28.2 ± 5.0 | 25.9 ± 4.3 |
| Letrozole | 159 | 28.7 ± 4.8 | 26.6 ± 5.7 | |||
| Gayatri et al., 2010 [36] | India | RCT | NAC | 50 | 23.2 ± 4.1 | 27.3 ± 3.3 |
| Metformin | 50 | 22.6 ± 3.8 | 27.5 ± 2.4 | |||
| Chandil et al., 2019 [37] | India | RCT | NAC | 45 | 26.8 ± 5.4 | 24.2 ± 2.4 |
| Metformin | 45 | 27.6 ± 5.1 | 24.5 ± 2.6 | |||
| Elnashar et al., 2007 [38] | Egypt | RCT | NAC | 30 | 27.3 ± 3.4 | 25.8 ± 0.9 |
| Metformin | 31 | 26.7 ± 5.4 | 26.8 ± 1.5 | |||
| Hassan et al., 2019 [39] | Egypt | RCT | CC + NAC | 150 | 26.0 ± 5.2 | 27.8 ± 3.1 |
| CC + placebo | 150 | 26.2 ± 4.9 | 28.1 ± 3.2 |
| Effect Size | Omitted Study | n Studies | n Participants | Random Effects Model (OR 95% CI) | I2 (%) | p-Value |
|---|---|---|---|---|---|---|
| Placebo | ||||||
| E2 | Badawy et al., 2007 [21] | 3 | 700 | −0.10 [−0.30, 0.10] | 70% | 0.05 |
| FSH | Kose et al., 2015 [22] | 4 | 100 | −0.05 [−0.25, 0.15] | 10% | 0.70 |
| LH | Hassan et al., 2019 [39] | 3 | 62 | 0.10 [−0.20, 0.40] | 30% | 0.60 |
| Endometrial thickness | Badawy et al., 2007 [21] | 8 | 934 | 0.65 [0.20, 1.10] | 95% | 0.01 |
| Number of follicles | Badawy et al., 2007 [21] | 4 | 573 | 0.80 [0.20, 1.40] | 95% | 0.01 |
| Metformin | ||||||
| E2 | Hashim et al., 2010 [28] | 2 | 150 | 0.20 [−0.10, 0.50] | 55% | 0.07 |
| FSH | Gayatri et al., 2010 [36] | 3 | 114 | 2.50 [0.50, 4.50] | 95% | 0.08 |
| LH | Nemati et al., 2017 [33] | 3 | 110 | 0.70 [0.20, 1.20] | 65% | 0.02 |
| SHBG, serum progesterone level, and TT | ||||||
| SHBG | Gayatri et al., 2010 [36] | 2 | 84 | 0.15 [−0.20, 0.50] | 40% | 0.30 |
| Serum progesterone level | Badawy et al., 2007 [21] | 3 | 263 | 0.60 [0.20, 1.00] | 90% | 0.01 |
| TT | Nemati et al., 2017 [33] | 6 | 200 | 0.30 [−0.10, 0.70] | 85% | 0.15 |
| Certainty Assessment | No of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | [NAC] | [Other] | Relative (95% CI) | Absolute (95% CI) | ||
| E2 | ||||||||||||
| 7 | randomized trials | not serious | serious a | not serious | not serious | None | 1233 | 849 | - | SMD −0.00 (higher −0.66 lower to 0.66 higher) | ⨁◯◯◯ Very low | CRITICAL |
| SHBG | ||||||||||||
| 3 | randomized trials | not serious | not serious | not serious | not serious | None | 149 | 134 | - | SMD 0.27 higher (−0.12 lower to 0.66 higher) | ⨁⨁⨁◯ Moderate | CRITICAL |
| FSH | ||||||||||||
| 11 | randomized trials | not serious | serious a | not serious | not serious | publication bias strongly suspected c | 611 | 596 | - | SMD 0.73 (higher −0.01 lower to 1.47 higher) | ⨁◯◯◯ Very low | IMPORTANT |
| LH | ||||||||||||
| 10 | randomized trials | not serious | not serious | not serious | not serious | publication bias strongly suspected c | 533 | 516 | - | SMD 0.29 lower (0.02 lower to 0.55 higher) | ⨁⨁⨁◯ Moderate | IMPORTANT |
| Progesterone | ||||||||||||
| 4 | randomized trials | not serious | not serious | not serious | not serious | none | 733 | 366 | - | SMD 0.95 higher (0.13 lower to 1.77 higher) | ⨁⨁⨁⨁ High | CRITICAL |
| TT | ||||||||||||
| 7 | randomized trials | not serious | not serious | not serious | not serious | None | 254 | 240 | - | SMD 0.43 (higher −0.20 lower to 1.07 higher) | ⨁⨁⨁◯ Moderate | CRITICAL |
| Endometrial thickness | ||||||||||||
| 13 | randomized trials | not serious | serious a | not serious | not serious | publication bias strongly suspected c | 1644 | 1279 | - | SMD −0.07 higher (−0.67 lower to 0.53 higher) | ⨁⨁◯◯ low | CRITICAL |
| Number of follicles | ||||||||||||
| not serious | serious | not serious | not serious | None | 1283 | 917 | - | SMD 0.07 higher (−0.93 lower to 1.07 higher) | ⨁⨁◯◯ low | CRITICAL | ||
| Outcome Measure | Comparison Groups | Fixed Effect Model SMDs (95% CI) | I2 (%) | p-Value |
|---|---|---|---|---|
| FSH | NAC vs. Placebo (FSH subgroup) | −0.04 (−0.2, 0.13) | 0% | 0.65 |
| NAC vs. Metformin (FSH subgroup) | 2.98 (−0.09, 6.05) | 99% | 0.06 | |
| NAC vs. Other (FSH subgroup) | −0.69 (−1.8, 0.41) | 96% | 0.23 | |
| NAC vs. Control (FSH global) | 0.75 (−0.01, 1.47) | 97% | 0.08 | |
| LH | NAC vs. Placebo (LH subgroup) | 0.12 (−0.2, 0.43) | 41% | 0.47 |
| NAC vs. Metformin (LH subgroup) | 0.67 (0.23, 1.12) | 71% | 0.003 | |
| NAC vs. Other (LH subgroup) | −0.01 (−0.32, 0.29) | 48% | 0.93 | |
| NAC vs. Control (LH global) | 0.29 (0.02, 0.55) | 74% | 0.03 | |
| E2 | NAC vs. Placebo (E2 subgroup) | 0.36 (−0.4, 1.11) | 98% | 0.35 |
| NAC vs. Metformin (E2 subgroup) | −0.47 (−1.78, 0.83) | 97% | 0.48 | |
| NAC vs. Control (E2 global) | 0 (−0.66, 0.66) | 98% | 1 | |
| SP | NAC vs. Other | 0.05 (0.13, 1.77) | 97% | 0.02 |
| TT | NAC vs. Other | 0.43 (−0.2, 1.07) | 91% | 0.18 |
| Endometrial thickness | NAC vs. Placebo (ET subgroup) | 0.58 (0.1, 1.06) | 96% | 0.02 |
| NAC vs. Other (ET subgroup) | 0.71 (0.48, 0.94) | 0% | 0.00001 | |
| NAC vs. Metformin (ET subgroup) | −4.71 (−15.33, 5.91) | 100% | 0.38 | |
| NAC vs. Control (ET global) | −0.07 (−0.67, 0.53) | 98% | 0.81 | |
| Number of follicles | NAC vs. Placebo (NF subgroup) | 0.7 (−0.01, 1.4) | 96% | 0.05 |
| NAC vs. Others (NF subgroup) | 2.01 (−0.77, 4.8) | 99% | 0.16 | |
| NAC vs. Metformin (NF subgroup) | −3.51 (−7.29, 0.27) | 99% | 0.07 | |
| NAC vs. Control (NF global) | 0.07(−0.93, 1.07) | 99% | 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viña, I.; Viña, J.R.; Carranza, M.; Mariscal, G. Efficacy of N-Acetylcysteine in Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis. Nutrients 2025, 17, 284. https://doi.org/10.3390/nu17020284
Viña I, Viña JR, Carranza M, Mariscal G. Efficacy of N-Acetylcysteine in Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis. Nutrients. 2025; 17(2):284. https://doi.org/10.3390/nu17020284
Chicago/Turabian StyleViña, Isabel, Juan R. Viña, Macarena Carranza, and Gonzalo Mariscal. 2025. "Efficacy of N-Acetylcysteine in Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis" Nutrients 17, no. 2: 284. https://doi.org/10.3390/nu17020284
APA StyleViña, I., Viña, J. R., Carranza, M., & Mariscal, G. (2025). Efficacy of N-Acetylcysteine in Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis. Nutrients, 17(2), 284. https://doi.org/10.3390/nu17020284







