Phytochemical Composition and Antioxidant Activity of Manuka Honey and Ohia Lehua Honey
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Honey Products
2.3. Phytochemical Analysisubsection
2.3.1. Total Polyphenol Content
2.3.2. Total Flavonoid Content (TFC)
2.3.3. High-Performance Liquid Chromatography Coupled with Electrospray Ionization Mass Spectrometry (HPLC-ESI MS) Analysis
2.4. In Vitro Antioxidant Activity Analysis
2.4.1. DPPH Radical-Scavenging Activity
2.4.2. Ferric Ion Reducing Antioxidant Power Assay (FRAP)
2.4.3. Hydrogen Peroxide (H2O2) Scavenging Activity
2.4.4. Nitric Oxide (NO) Radical Scavenging Assay
2.5. In Vivo Experimental Design
2.5.1. Animal Subjects
2.5.2. Experimental Protocol
2.5.3. Assessments of Oxidative Stress Markers
- Determination of Total Antioxidant Capacity
- Determination of Total Oxidative Status
- Determination of Oxidative Stress Index
- Determination of Nitric Oxide
- Determination of Malondialdehyde
- Determination of Advanced Oxidation Protein Products
- Determination of Total Thiols
2.6. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.1.1. Total Polyphenols and Flavonoid Content
3.1.2. HPLC-ESI-MS Analysis of Phenolic Compounds
3.2. In Vitro Antioxidant Activity
3.3. In Vivo Antioxidant Activity
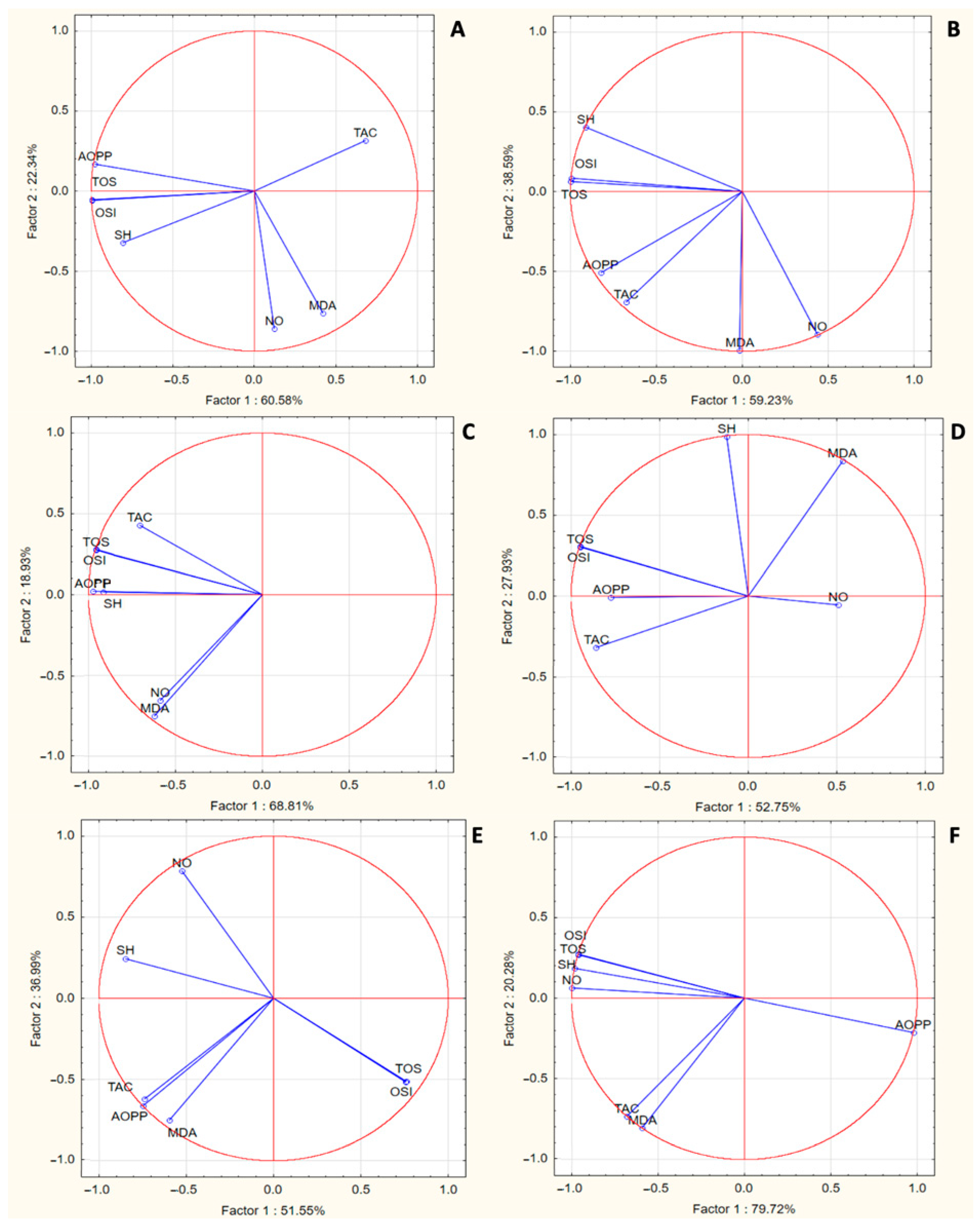
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magoshi, I.B.; Nekhumbe, A.W.; Ibrahim, M.A.; Serem, J.C.; Bester, M.J. Gastrointestinal Effects on the Antioxidant and Immunomodulatory Properties of South African Fynbos Honey. Int. J. Food Sci. 2023, 2023, 2553197. [Google Scholar] [CrossRef]
- Obeidat, B.; Yassin, H. Sugar profile and sensory properties of honey from different geographical zones and botanical origins in Tanzania. Heliyon 2024, 10, e38094. [Google Scholar] [CrossRef]
- Nyarko, K.; Boozer, K.; Greenlief, C.M. Profiling of the Polyphenol Content of Honey from Different Geographical Origins in the United States. Molecules 2023, 28, 5011. [Google Scholar] [CrossRef]
- James, O.O.; Mesubi, M.A.; Usman, L.A.; Yeye, S.O.; Ajanaku, K.O.; Ogunniran, K.O.; Ajani, O.O.; Siyanbola, T.O. Physical characterisation of some honey samples from North-central Nigeria. Int. J. Phys. Sci. 2009, 4, 464–470. [Google Scholar]
- Yayinie, M.; Atlabachew, M.; Tesfaye, A.; Hilluf, W.; Reta, C.; Alemneh, T. Polyphenols, flavonoids, and antioxidant content of honey coupled with chemometric method: Geographical origin classification from Amhara region, Ethiopia. Int. J. Food Prop. 2022, 25, 76–92. [Google Scholar] [CrossRef]
- Shaikh, A.; Ahmad, F.; Teoh, S.L.; Kumar, J.; Yahaya, M.F. Unveiling the Therapeutic Potential of Kelulut (Stingless Bee) Honey in Alzheimer’s Disease: Findings from a Rat Model Study. Antioxidants 2024, 13, 926. [Google Scholar] [CrossRef] [PubMed]
- Benjamaa, R.; Elbouny, H.; Errati, H.; Moujanni, A.; Kaushik, N.; Gupta, R.; Ennibi, O.K.; Nasser, B.; Choi, E.H.; Kaushik, N.K.; et al. Comparative Evaluation of Antioxidant Activity, Total Phenolic Content, Anti-Inflammatory, and Antibacterial Potential of Euphorbia-Derived Functional Products. Front. Pharmacol. 2024, 15, 1345340. [Google Scholar] [CrossRef]
- ApazaTicona, L.; Sánchez Sánchez-Corral, J.; Montoto Lozano, N.; Prieto Ramos, P.; Sánchez, Á.R. Study of Pentacyclic Triterpenes from LyophilisedAguaje: Anti-Inflammatory and Antioxidant Properties. Int. J. Mol. Sci. 2024, 25, 9615. [Google Scholar] [CrossRef]
- Țicolea, M.; Pop, R.M.; Pârvu, M.; Usatiuc, L.O.; Uifălean, A.; Ranga, F.; Pârvu, A.E. Phytochemical Composition Antioxidant and Anti-Inflammatory Activity of Artemisia Dracunculus and Artemisia Abrotanum. Antioxidants 2024, 13, 1016. [Google Scholar] [CrossRef] [PubMed]
- Poulsen-Silva, E.; Gordillo-Fuenzalida, F.; Velásquez, P.; Llancalahuen, F.M.; Carvajal, R.; Cabaña-Brunod, M.; Otero, M.C. Antimicrobial, Antioxidant, and Anti-Inflammatory Properties of Monofloral Honeys from Chile. Antioxidants 2023, 12, 1785. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Shetaia, A.A.; Eid, N.; Abd El-Wahed, A.A.; Abolibda, T.Z.; El Omri, A.; Yu, Q.; Shenashen, M.A.; Hussain, H.; Salem, M.F.; et al. Green Innovation and Synthesis of Honeybee Products-Mediated Nanoparticles: Potential Approaches and Wide Applications. Bioengineering 2024, 11, 829. [Google Scholar] [CrossRef] [PubMed]
- Pleeging, C.C.F.; Wagener, F.A.D.T.G.; de Rooster, H.; Cremers, N.A.J. Revolutionizing non-conventional wound healing using honey by simultaneously targeting multiple molecular mechanisms. Drug Resist. Updates 2022, 62, 100834. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, C.; Stavropoulou, E.; Giorgi, E.; Voidarou, C.C.; Constantinidis, T.C.; Vrioni, G.; Tsakris, A. Antioxidants Honey’s Antioxidant and Antimicrobial Properties: A Bibliometric Study. Antioxidants 2023, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Barańska, J.; Karwowski, B.T. The Antioxidant Potential of Commercial Manuka Honey from New Zealand—Biochemical and Cellular Studies. Curr. Issues Mol. Biol. 2024, 46, 6366–6376. [Google Scholar] [CrossRef]
- Ayele, D.T.; Akele, M.L.; Melese, A.T. Analysis of Total Phenolic Contents, Flavonoids, Antioxidant and Antibacterial Activities of Croton Macrostachyus Root Extracts. BMC Chem. 2022, 16, 30. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Nikolova, M. Screening of Radical Scavenging Activity and Polyphenol Content of Bulgarian Plant Species. Pharmacogn. Res. 2011, 3, 256–259. [Google Scholar] [CrossRef]
- Kiki, G.A.à.; Pop, R.M.; Sabin, O.; Bocsan, I.C.; Chedea, V.S.; Socaci, S.A.; Pârvu, A.E.; Finsia, E.; Francis, T.; Mathieu, Z.; et al. Polyphenols from DichrostachysCinerea Fruits Anti-Inflammatory, Analgesic, and Antioxidant Capacity in Freund’s Adjuvant-Induced Arthritic Rat Model. Molecules 2022, 27, 5445. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Kek, S.P.; Chin, N.L.; Yusof, Y.A.; Tan, S.W.; Chua, L.S. Classification of Entomological Origin of Honey Based on Its Physicochemical and Antioxidant Properties. Int. J. Food Prop. 2018, 20, S2723–S2738. [Google Scholar] [CrossRef]
- Chera, E.I.; Pop, R.M.; Pârvu, M.; Sorițău, O.; Uifălean, A.; Cătoi, F.A.; Cecan, A.; Negoescu, A.G.; Achimaș-Cadariu, P.; Pârvu, A.E. Flaxseed Ethanol Extracts’ Antitumor, Antioxidant, and Anti-Inflammatory Potential. Antioxidants 2022, 11, 892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, P.; Guo, H.F.; Liu, L.; Liu, X.D. Pharmacokinetic-Pharmacodynamic Modeling of Diclofenac in Normal and Freund’s Complete Adjuvant-Induced Arthritic Rats. Acta Pharmacol. Sin. 2012, 33, 1372–1378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diaz, Z.; Laurenzana, A.; Mann, K.K.; Bismar, T.A.; Schipper, H.M.; Miller, W.H. Trolox Enhances the Anti-Lymphoma Effects of Arsenic Trioxide, While Protecting against Liver Toxicity. Leukemia 2007, 21, 2117–2127. [Google Scholar] [CrossRef]
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Ferreira-Santos, P.; Genisheva, Z.; El Ghouizi, A.; Aboulghazi, A.; Teixeira, J.A.; Lyoussi, B. Protective Effect of Honey and Propolis against Gentamicin-Induced Oxidative Stress and Hepatorenal Damages. Oxid. Med. Cell Longev. 2021, 2021, 9719906. [Google Scholar] [CrossRef]
- El-Sherif, M.W. Optimization of Xylazine-Ketamine Anesthetic Dose in Mice Suffering Chronic Liver Injury. J. Anesth. Crit. Care 2019, 11, 6–8. [Google Scholar] [CrossRef]
- Erel, O. A Novel Automated Method to Measure Total Antioxidant Response against Potent Free Radical Reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Erel, O. A New Automated Colorimetric Method for Measuring Total Oxidant Status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Harma, M.; Harma, M.; Erel, O. Increased Oxidative Stress in Patients with Hydatidiform Mole. Swiss Med. Wkly. 2003, 133, 563–566. [Google Scholar] [CrossRef]
- Ghasemi, A.; Hedayati, M.I.; Biabani, H., III. Protein Precipitation Methods Evaluated for Determination of Serum Nitric Oxide End Products by the Griess Assay. J. Med. Sci. Res. 2007, 2, 29–32. [Google Scholar]
- Mitev, D.; Gradeva, H.; Stoyanova, Z.; Petrova, N.; Karova, N.; Dimov, D.; Iliev, V.; Koychev, A.; Prakova, G.; Vlaykova, T. Evaluation of Thiol Compounds and Lipid Peroxidative Products in Plasma of Patients with Copd. Trakia J. Sci. 2010, 8, 306–314. [Google Scholar]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced Oxidation Protein Products as a Novel Marker of Oxidative Stress in Uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Erel, O.; Neselioglu, S. A Novel and Automated Assay for Thiol/Disulphide Homeostasis. Clin. Biochem. 2014, 47, 326–332. [Google Scholar] [CrossRef]
- Valverde, S.; Ares, A.M.; Stephen Elmore, J.; Bernal, J. Recent Trends in the Analysis of Honey Constituents. Food Chem. 2022, 387, 132920. [Google Scholar] [CrossRef]
- Spoiala, A.; Ilie, C.; Ficai, D.; Ficai, A.; Andronescu, E. Synergic Effect of Honey with Other Natural Agents in Developing Efficient Wound Dressings. Antioxidants 2023, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Mieles, J.Y.; Vyas, C.; Aslan, E.; Humphreys, G.; Diver, C.; Bartolo, P. Honey: An Advanced Antimicrobial and Wound Healing Biomaterial for Tissue Engineering Applications. Pharmaceutics 2022, 14, 1663. [Google Scholar] [CrossRef] [PubMed]
- Habryka, C.; Socha, R.; Juszczak, L. The Effect of Enriching Honey with Propolis on the Antioxidant Activity, Sensory Characteristics, and Quality Parameters. Molecules 2020, 25, 1176. [Google Scholar] [CrossRef]
- Zawawi, N.; Chong, P.J.; Mohd Tom, N.N.; SaifulAnuar, N.S.; Mohammad, S.M.; Ismail, N.; Jusoh, A.Z. Establishing Relationship between Vitamins, Total Phenolic and Total Flavonoid Content and Antioxidant Activities in Various Honey Types. Molecules 2021, 26, 4399. [Google Scholar] [CrossRef] [PubMed]
- Dzugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Pięt, M.; Gąsecka, M.; Stroińska, A.; Niedzielski, P.; Mleczek, M.; Rzymski, P.; Wilczak, M. Relation between polyphenols, malondialdehyde, antioxidant capacity, lactate dehydrogenase and toxic elements in human colostrum milk. Chemosphere 2018, 191, 548–554. [Google Scholar] [CrossRef]
- Plasay, M.; Muslimin, L. The Effect of Administration of Honey on Physical Activity in Malondialdehyde, Glutathione, and Superoxide Dismutase Blood Levels in Male Rat. J. Med. Chem. Sci. 2024, 7, 590–597. [Google Scholar] [CrossRef]
- Rob, M.M.; Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic Activity and Identification of Phytotoxic Substances from Schumannianthusdichotomus. Plants 2020, 9, 102. [Google Scholar] [CrossRef]
- Bartel, I.; Mandryk, I.; Horbańczuk, J.O.; Wierzbicka, A.; Koszarska, M. Nutraceutical Properties of Syringic Acid in Civilization Diseases—Review. Nutrients 2024, 16, 10. [Google Scholar] [CrossRef]
- Somade, O.T.; Oyinloye, B.E.; Ajiboye, B.O.; Osukoya, O.A. Syringic Acid Demonstrates an Anti-Inflammatory Effect via Modulation of the NF-ΚB-INOS-COX-2 and JAK-STAT Signaling Pathways in Methyl Cellosolve-Induced Hepato-Testicular Inflammation in Rats. Biochem. Biophys. Rep. 2023, 34, 101484. [Google Scholar] [CrossRef]
- Li, P.; Lu, Y.; Zhao, M.; Chen, L.; Zhang, C.; Cheng, Q.; Chen, C. Effects of Phenyllactic Acid, Lactic Acid Bacteria, and Their Mixture on Fermentation Characteristics and Microbial Community Composition of Timothy Silage. Front. Microbiol. 2021, 12, 743433. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Medhi, B.; Avti, P.K.; Saikia, U.N.; Pandhi, P.; Khanduja, K.L. Effect of Different Doses of Manuka Honey in Experimentally Induced Inflammatory Bowel Disease in Rats. Phytother. Res. 2008, 1519, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.D.; Mandal, S. Honey: Its Medicinal Property and Antibacterial Activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Tashkandi, H. Honey in Wound Healing: An Updated Review. Open Life Sci. 2021, 16, 1091–1100. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, Y.; Cao, W. The Protective Effect of Whole Honey and Phenolic Extract on Oxidative DNA Damage in Mice Lymphocytes Using Comet Assay. Plant Foods Hum. Nutr. 2017, 72, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Ranneh, Y.; Akim, A.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Fadzelly, M.; Bakar, A. Honey and Its Nutritional and Anti-Inflammatory Value. BMC Complement. Med. Ther. 2021, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, A.; Shaheetha, J.; Thangadurai, D.; Rao, D.M. Protective Effect of Indian Honey on Acetaminophen Induced Oxidative Stress and Liver Toxicity in Rat. Biologia 2009, 64, 1225–1231. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxid. Med. Cell Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Y.; Jiang, T.; Liu, B.; Sun, H.; Zhang, Y.; Fan, B.; Li, X.; Qin, X.; Zheng, Q. Enhancing Fatty Acids Oxidation via L-Carnitine Attenuates Obesity-Related Atrial Fibrillation and Structural Remodeling by Activating AMPK Signaling and Alleviating Cardiac Lipotoxicity. Front. Pharmacol. 2021, 12, 771940. [Google Scholar] [CrossRef]
- Vezzoli, A.; Mrakic-Sposta, S.; Dellanoce, C.; Montorsi, M.; Vietti, D.; Ferrero, M.E. Chelation Therapy Associated with Antioxidant Supplementation Can Decrease Oxidative Stress and Inflammation in Multiple Sclerosis: Preliminary Results. Antioxidants 2023, 12, 1338. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Organ Damage: A Current Perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive Oxygen Species (ROS) Scavenging Biomaterials for Anti-Inflammatory Diseases: From Mechanism to Therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Wilczyńska, A.; Żak, N. Polyphenols as the Main Compounds Influencing the Antioxidant Effect of Honey—A Review. Int. J. Mol. Sci. 2024, 25, 10606. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, W.S.; Ritu; Zahoor, I.; Dar, A.H.; Farooq, S.; Mir, T.A.; Ganaie, T.A.; Srivastava, S.; Pandey, V.K.; Altaf, A. Exploiting the Polyphenolic Potential of Honey in the Prevention of Chronic Diseases. Food Chem. Adv. 2023, 3, 100373. [Google Scholar] [CrossRef]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.; Singh Nigam, P. Antibacterial Activity of Manuka Honey and Its Components: An Overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef]
- Puścion-Jakubik, A.; Bielecka, J.; Grabia, M.; Markiewicz-żukowska, R.; Soroczyńska, J.; Teper, D.; Socha, K. Comparative Analysis of Antioxidant Properties of Honey from Poland, Italy, and Spain Based on the Declarations of Producers and Their Results of Melissopalinological Analysis. Nutrients 2022, 14, 2694. [Google Scholar] [CrossRef] [PubMed]
- Patouna, A.; Vardakas, P.; Skaperda, Z.; Spandidos, D.A.; Kouretas, D. Evaluation of the Antioxidant Potency of Greek Honey from the Taygetos and Pindos Mountains Using a Combination of Cellular and Molecular Methods. Mol. Med. Rep. 2023, 27, 54. [Google Scholar] [CrossRef] [PubMed]
- Main, E.N.; Huang, J.C.; Bowlin, G.L. Methyl Syringate: A Primary Driving Factor in Manuka Honeys Ability to Ameliorate Neutrophil Intracellular ROS Activity and NETosis. Front. Biosci.–Landmark 2024, 29, 255. [Google Scholar] [CrossRef] [PubMed]

| Plantextract (100 mg/mL) | Total Polyphenols Content (mgGAE/g) | Total Flavonoids Content (mg QE/g) |
|---|---|---|
| Ohia Lehua honey | 4.826 ± 00.4 | 12.65 ± 1.05 |
| Manuka honey | 5.425 ± 0.09 | 31.65 ± 1.86 |
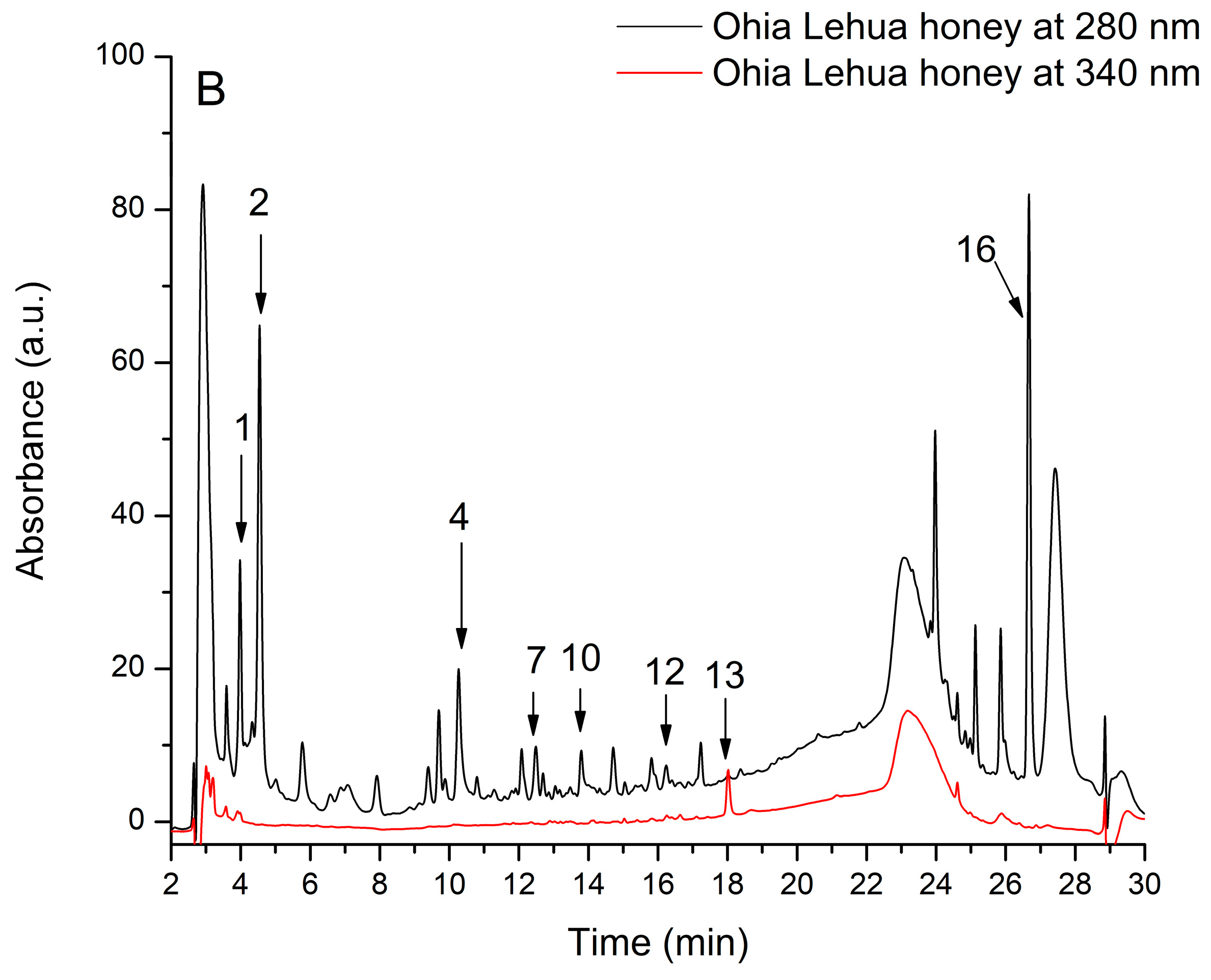
| Peak No | Rt (min) | UV λmax (nm) | [M + H]+ (m/z) | Compound | Subclass | Manuka Honey (μg/mL) | Ohia Lehua Honey (μg/mL) |
|---|---|---|---|---|---|---|---|
| 1 | 4.03 | 270 | 155 | 2,4-Dihydroxybenzoic acid | Hydroxybenzoic acid | 36.65 ± 2.12 | 7.35 ± 0.93 |
| 2 | 4.53 | 270 | 171 | Gallic acid | Hydroxybenzoic acid | 30.04 ± 2.35 | 17.49 ± 1.46 |
| 3 | 10.12 | 320 | 339 | p-Coumaroyquinic acid | Hydroxycinnamic acid | 10.22 ± 0.04 | ND |
| 4 | 10.25 | 280 | 155 | Protocatechuic acid | Hydroxybenzoic acid | 18.98 ± 4.71 | 4.71 ± 0.05 |
| 5 | 11.76 | 330 | 355 | Chlorogenic acid | Hydroxycinnamic acid | 4.50 ± 0.02 | ND |
| 6 | 12.24 | 330 | 343 | Caffeic acid-glucoside | Hydroxycinnamic acid | 11.54 ± 1.30 | ND |
| 7 | 12.49 | 270 | 139 | 4-Hydroxybenzoic acid | Hydroxybenzoic acid | 9.94 ± 0.97 | 0.72 ± 0.22 |
| 8 | 12.90 | 265 | 213 | Trimethoxybenzoic acid | Hydroxybenzoic acid | 106.30 ± 4.20 | ND |
| 9 | 13.42 | 340, 245 | 433, 271 | Apigenin-glucoside | Flavone | 0.72 ± 0.04 | ND |
| 10 | 13.81 | 280 | 169 | Vanillic acid | Hydroxybenzoic acid | 6.14 ± 3.02 | 0.37 ± 0.01 |
| 11 | 15.81 | 360, 250 | 465, 303 | Quercetin-glucoside | Flavonol | 1.92 ± 0.90 | ND |
| 12 | 16.58 | 280 | 199 | Syringic acid | Hydroxybenzoic acid | 80.58 ± 5.68 | 0.28 ± 0.01 |
| 13 | 18.05 | 350, 250 | 419, 257 | Pinocembrin-glucoside | Flavanone | 4.30 ± 2.89 | 0.34 ± 0.05 |
| 14 | 19.69 | 280 | 213, 199 | Methyl-Syringic acid | Hydroxybenzoic acid | 144.37 ± 6.21 | ND |
| 15 | 21.21 | 360, 250 | 303 | Quercetin | Flavonol | 1.74 ± 0.98 | ND |
| 16 | 26.71 | 280 | 167 | Phenyllactic acid | 14.35 ± 2.69 | 14.23 ± 2.24 | |
| Total phenolics | 482.30 ± 34.43 | 45.49 ± 4.95 |
| Samples | DPPH μg TE/g | NO Scavenging Activity mg QE/g | H2O2 mg TE/g | FRAP μg TE/g |
|---|---|---|---|---|
| Ohia Lehua honey (0.1 g/mL) | 108.33 ± 9.20 | 85.79 ± 9.45 | 49.01 ± 4.01 | 148.85 ± 14.09 |
| Manuka honey (0.1 g/mL) | 106.57 ± 13.54 | 78.83 ± 5.83 | 44.71 ± 2.79 | 121.20 ± 11.27 |
| GROUPS | TAC (mmol Trolox Equiv./L) | TOS (µmol H2O2 Equiv./L) | OSI | NO (µmol/L) | MDA (nmol/L) | AOPP (µmol/L) | SH (µmol/L) |
|---|---|---|---|---|---|---|---|
| CONTROL | 1.11 ± 0.00 | 27.56 ± 4.35 | 24.81 ± 3.91 | 52.94 ± 10.48 | 4.61 ± 0.46 | 25.75 ± 2.11 | 458.2 ± 94.95 |
| INFL | 1.10 ± 0.00 a | 45.68 ± 6.39 aaa | 41.24 ± 5.73 aaa | 71.81 ± 16.16 | 5.80 ± 1.13 a | 41.48 ± 3.75 aaa | 299.4 ± 53.72 aa |
| DICLO | 1.10 ± 0.00 | 35.7 ± 3.42 b | 32.23 ± 3.06 b | 66.41 ± 9.27 | 5.15 ± 0.35 | 35.088 ± 4.57 b | 356.2 ± 72.07 b |
| TROLOX | 1.11 ± 0.00 b | 29.32 ± 5.50 bb | 26.40 ± 4.94 bb, c | 45.21 ± 4.54 bbb, cc | 5.71 ± 0.30 | 33.25 ± 0.89 bb | 412.5 ± 45.35 bb, c |
| OLH100 | 1.11 ± 0.00 bb; cc | 24.73 ± 4.61 bbb, cc | 22.19 ± 4.2 bbb, cc | 68.79 ± 11.63 dd | 4.48 ± 0.58 bb, cc, ddd | 40.26 ± 9.47 c, d | 398.6 ± 54.74 bb |
| OLH50 | 1.11 ± 0.00 bb; cc; d | 26.40 ± 4.21 bbb,cc | 22.97 ± 2.68 bbb, cc | 69.01 ± 7.8 dd | 4.32 ± 0.35 bb, cc, ddd | 40.18 ± 7.54 c, d | 373.5 ± 50.79 bb |
| OLH25 | 1.12 ± 0.00 bbb; ccc; ddd | 29.91 ± 5.69 bbb | 26.64 ± 5.05 bb | 60.30 ± 12.68 dd | 3.98 ± 0.43 bb, cc, ddd | 41.62 ± 7.91 c, d | 369.8 ± 59.20 bb |
| MH100 | 1.10 ± 0.00 ddd | 45.2 ± 4.54 cc, ddd | 45.67 ± 3.30 ccc, ddd | 52.49 ± 20.56 bb, c | 5.00 ± 0.74 | 30.11 ± 5.43 bb | 462.6 ± 66.84 bbb, cc |
| MH50 | 1.10 ± 0.00 | 41.12 ± 7.13 c,d | 37.08 ± 6.44 | 51.20 ± 19.24 bb, c | 5.18 ± 0.22 | 29.54 ± 2.29 bb | 424 ± 58.37 bbb, cc |
| MH25 | 1.10 ± 0.00 dd | 33.99 ± 7.26 bb | 30.72 ± 6.55 bb | 54.43 ± 10.22 bb,c | 5.14 ± 0.822 | 32.17 ± 4.94 bb | 362.5 ± 60.71 bbb,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morar, I.I.; Pop, R.M.; Peitzner, E.; Ranga, F.; Orăsan, M.S.; Cecan, A.D.; Chera, E.I.; Bonci, T.I.; Usatiuc, L.O.; Țicolea, M.; et al. Phytochemical Composition and Antioxidant Activity of Manuka Honey and Ohia Lehua Honey. Nutrients 2025, 17, 276. https://doi.org/10.3390/nu17020276
Morar II, Pop RM, Peitzner E, Ranga F, Orăsan MS, Cecan AD, Chera EI, Bonci TI, Usatiuc LO, Țicolea M, et al. Phytochemical Composition and Antioxidant Activity of Manuka Honey and Ohia Lehua Honey. Nutrients. 2025; 17(2):276. https://doi.org/10.3390/nu17020276
Chicago/Turabian StyleMorar, Iulia Ioana, Raluca Maria Pop, Erik Peitzner, Floricuța Ranga, Meda Sandra Orăsan, Andra Diana Cecan, Elisabeta Ioana Chera, Teodora Irina Bonci, Lia Oxana Usatiuc, Mădălina Țicolea, and et al. 2025. "Phytochemical Composition and Antioxidant Activity of Manuka Honey and Ohia Lehua Honey" Nutrients 17, no. 2: 276. https://doi.org/10.3390/nu17020276
APA StyleMorar, I. I., Pop, R. M., Peitzner, E., Ranga, F., Orăsan, M. S., Cecan, A. D., Chera, E. I., Bonci, T. I., Usatiuc, L. O., Țicolea, M., But, A. E., Cătoi, F. A., Pârvu, A. E., & Ghergie, M. C. D. (2025). Phytochemical Composition and Antioxidant Activity of Manuka Honey and Ohia Lehua Honey. Nutrients, 17(2), 276. https://doi.org/10.3390/nu17020276









