Amino Acid Patterns in Children with Autistic Spectrum Disorder: A Preliminary Biochemical Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Control Group
2.2. Ethical Statement
2.3. AA Profiles in Plasma
2.4. Statistical Methods
3. Results
3.1. Univariate and Correlation Analysis
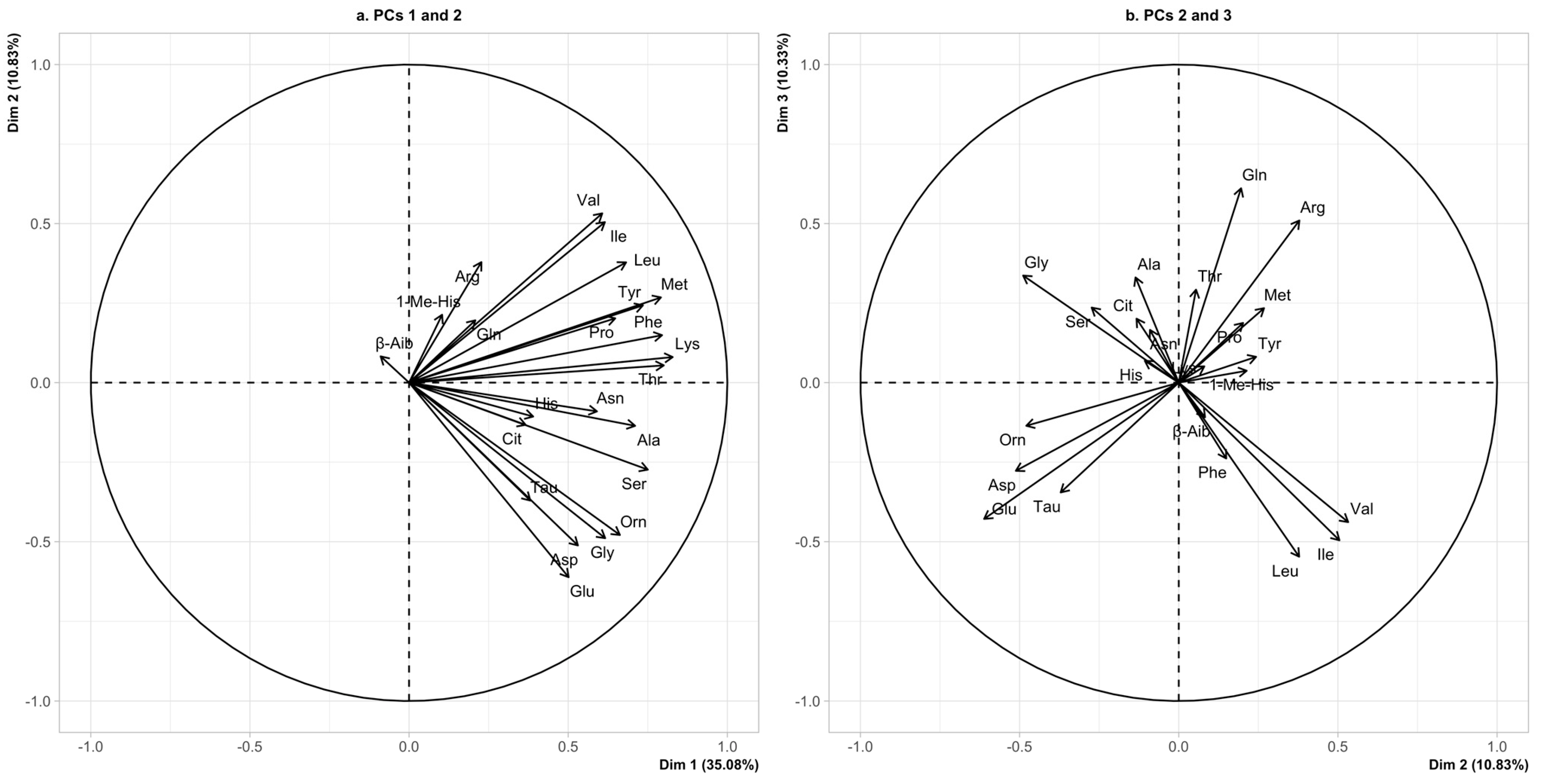
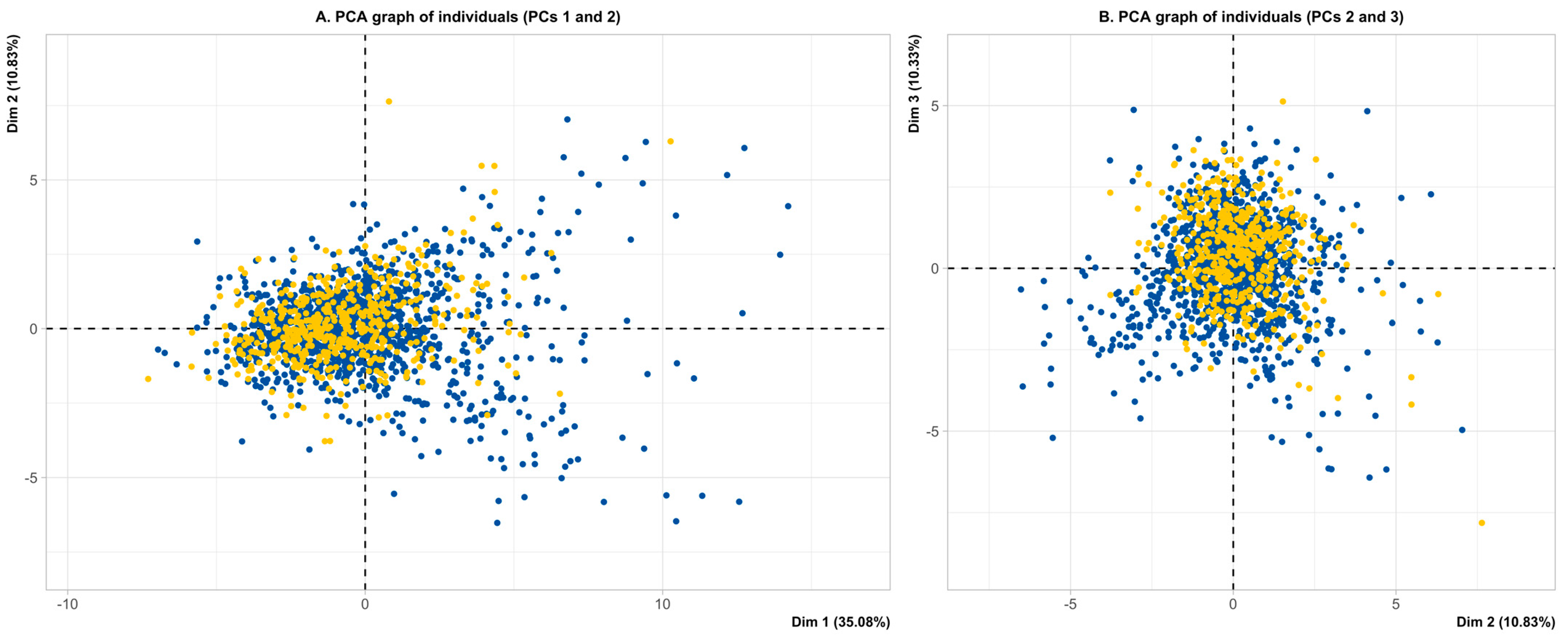
3.2. Multivariate Analysis—Principal Component Analysis
Autism Spectrum
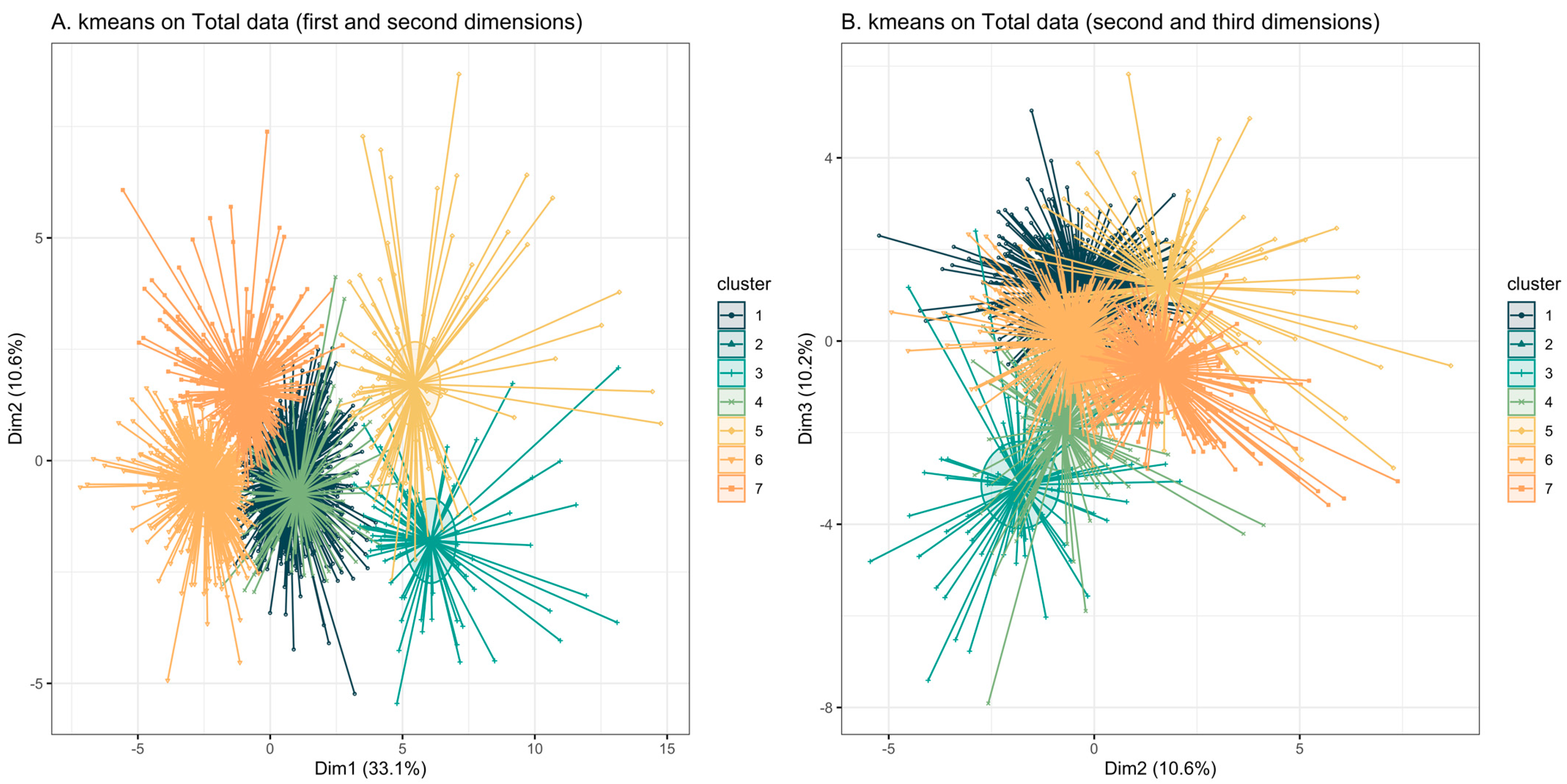
3.3. K-Means Clustering
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lai, M.C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Žigman, T.; Petković Ramadža, D.; Šimić, G.; Barić, I. Inborn Errors of Metabolism Associated with Autism Spectrum Disorders: Approaches to Intervention. Front. Neurosci. 2021, 15, 673600. [Google Scholar] [CrossRef] [PubMed]
- Waterval, W.A.; Scheijen, J.L.; Ortmans-Ploemen, M.M.; Habets-van der Poel, C.D.; Bierau, J. Quantitative UPLC-MS/MS analysis of underivatised amino acids in body fluids is a reliable tool for the diagnosis and follow-up of patients with inborn errors of metabolism. Clin. Chim. Acta 2009, 407, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Smon, A.; Cuk, V.; Brecelj, J.; Murko, S.; Groselj, U.; Tansek, M.Z.; Battelino, T.; Lampret, B.R. Comparison of liquid chromatography with tandem mass spectrometry and ion-exchange chromatography by post-column ninhydrin derivatization for amino acid monitoring. Clin. Chim. Acta 2019, 495, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Arnold, G.L.; Hyman, S.L.; Mooney, R.A.; Kirby, R.S. Plasma amino acids profiles in children with autism: Potential risk of nutritional deficiencies. J. Autism Dev. Disord. 2003, 33, 449–454. [Google Scholar] [CrossRef]
- Moreno-Fuenmayor, H.; Borjas, L.; Arrieta, A.; Valera, V.; Socorro-Candanoza, L. Plasma excitatory amino acids in autism. Investig. Clin. 1996, 37, 113–128. [Google Scholar]
- Aldred, S.; Moore, K.M.; Fitzgerald, M.; Waring, R.H. Plasma amino acid levels in children with autism and their families. J. Autism Dev. Disord. 2003, 33, 93–97. [Google Scholar] [CrossRef]
- Yu, X.; Qian-Qian, L.; Cong, Y.; Xiao-Bing, Z.; Hong-Zhu, D. Reduction of essential amino acid levels and sex-specific alterations in serum amino acid concentration profiles in children with autism spectrum disorder. Psychiatry Res. 2021, 297, 113675. [Google Scholar] [CrossRef]
- Zheng, H.F.; Wang, W.Q.; Li, X.M.; Rauw, G.; Baker, G.B. Body fluid levels of neuroactive amino acids in autism spectrum disorders: A review of the literature. Amino Acids 2017, 49, 57–65. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Could Candida Overgrowth Be Involved in the Pathophysiology of Autism? J. Clin. Med. 2022, 11, 442. [Google Scholar] [CrossRef]
- Cascio, L.; Chen, C.F.; Pauly, R.; Srikanth, S.; Jones, K.; Skinner, C.D.; Stevenson, R.E.; Schwartz, C.E.; Boccuto, L. Abnormalities in the genes that encode Large Amino Acid Transporters increase the risk of Autism Spectrum Disorder. Mol. Genet. Genom. Med. 2020, 8, e1036. [Google Scholar] [CrossRef]
- Tărlungeanu, D.C.; Deliu, E.; Dotter, C.P.; Kara, M.; Janiesch, P.C.; Scalise, M.; Galluccio, M.; Tesulov, M.; Morelli, E.; Sonmez, F.M.; et al. Impaired Amino Acid Transport at the Blood Brain Barrier Is a Cause of Autism Spectrum Disorder. Cell 2016, 167, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Novarino, G.; El-Fishawy, P.; Kayserili, H.; Meguid, N.A.; Scott, E.M.; Schroth, J.; Silhavy, J.L.; Kara, M.; Khalil, R.O.; Ben-Omran, T.; et al. Mutations in BCKD-kinase lead to a potentially treatable form of autism with epilepsy. Science 2012, 338, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.; Buxbaum, J.D. The promise of precision medicine in autism. Neuron 2021, 109, 2212–2215. [Google Scholar] [CrossRef] [PubMed]
- The American Psychiatric Association. Diagnostic And Statistical Manual of Mental Disorders, 5th ed.; Text Revision (DSM-5-TR); The American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar]
- Duran, M. Amino acids. In Laboratory Guide to the Methods in Biochemical Genetics; Blau, N., Duran, M., Gibson, K.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 53–90. [Google Scholar]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–245. [Google Scholar] [CrossRef]
- Forgy, E.W. Cluster analysis of multivariate data: Efficiency vs interpretability of classifications. Biometrics 1965, 21, 768–769. [Google Scholar]
- Salceda, R. Glycine neurotransmission: Its role in development. Front. Neurosci. 2022, 16, 947563. [Google Scholar] [CrossRef]
- Bala, K.A.; Doğan, M.; Mutluer, T.; Kaba, S.; Aslan, O.; Balahoroğlu, R.; Çokluk, E.; Üstyol, L.; Kocaman, S. Plasma amino acid profile in autism spectrum disorder (ASD). Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 923–929. [Google Scholar]
- Vilela, J.; Martiniano, H.; Marques, A.R.; Santos, J.X.; Asif, M.; Rasga, C.; Oliveira, G.; Vicente, A.M. Identification of Neurotransmission and Synaptic Biological Processes Disrupted in Autism Spectrum Disorder Using Interaction Networks and Community Detection Analysis. Biomedicines 2023, 11, 2971. [Google Scholar] [CrossRef]
- Tu, W.J.; Chen, H.; He, J. Application of LC-MS/MS analysis of plasma amino acids profiles in children with autism. J. Clin. Biochem. Nutr. 2012, 51, 248–249. [Google Scholar] [CrossRef]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr. Metab. 2011, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Lobanova, Y.N.; Korobeinikova, T.V.; Ajsuvakova, O.P.; Notova, S.V.; Burtseva, T.I.; Skalnaya, M.G.; Tinkov, A.A. Serum amino acid spectrum in children with autism spectrum disorder (ASD). Res. Autism Spectr. Disord. 2020, 77, 101605. [Google Scholar] [CrossRef]
- Kuwabara, H.; Yamasue, H.; Koike, S.; Inoue, H.; Kawakubo, Y.; Kuroda, M.; Takano, Y.; Iwashiro, N.; Natsubori, T.; Aoki, Y.; et al. Altered metabolites in the plasma of autism spectrum disorder: A capillary electrophoresis time-of-flight mass spectroscopy study. PLoS ONE 2013, 8, e73814. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, A.; Murgia, F.; Capodiferro, A.M.; Tanca, M.G.; Hendren, A.; Falqui, S.G.; Aresti, M.; Comini, M.; Carucci, S.; Cocco, E.; et al. NMR-Based Metabolomics in Autism Spectrum Disorder and Pediatric Acute-Onset Neuropsychiatric Syndrome. J. Clin. Med. 2022, 11, 6493. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Sweeten, T.L.; Posey, D.J.; Shankar, S.; McDougle, C.J. High nitric oxide production in autistic disorder: A possible role for interferon-gamma. Biol. Psychiatry 2004, 55, 434–437. [Google Scholar] [CrossRef]
- Andersen, J.V.; Markussen, K.H.; Jakobsen, E.; Schousboe, A.; Waagepetersen, H.S.; Rosenberg, P.A.; Aldana, B.I. Glutamate metabolism and recycling at the excitatory synapse in health and neurodegeneration. Neuropharmacology 2021, 196, 108719. [Google Scholar] [CrossRef]
- Essa, M.M.; Braidy, N.; Vijayan, K.R.; Subash, S.; Guillemin, G.J. Excitotoxicity in the pathogenesis of autism. Neurotox. Res. 2013, 23, 393–400. [Google Scholar] [CrossRef]
- Montanari, M.; Martella, G.; Bonsi, P.; Meringolo, M. Autism Spectrum Disorder: Focus on Glutamatergic Neurotransmission. Int. J. Mol. Sci. 2022, 23, 3861. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhu, T.; Qu, Y.; Mu, D. Blood Glutamate Levels in Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158688. [Google Scholar] [CrossRef]
- Shimmura, C.; Suda, S.; Tsuchiya, K.J.; Hashimoto, K.; Ohno, K.; Matsuzaki, H.; Iwata, K.; Matsumoto, K.; Wakuda, T.; Kameno, Y.; et al. Alteration of plasma glutamate and glutamine levels in children with high-functioning autism. PLoS ONE 2011, 6, e25340. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef] [PubMed]
- Piras, C.; Mussap, M.; Noto, A.; De Giacomo, A.; Cristofori, F.; Spada, M.; Fanos, V.; Atzori, L.; Francavilla, R. Alterations of the Intestinal Permeability are Reflected by Changes in the Urine Metabolome of Young Autistic Children: Preliminary Results. Metabolites 2022, 12, 104. [Google Scholar] [CrossRef]
- Usui, N.; Kobayashi, H.; Shimada, S. Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder. Int. J. Mol. Sci. 2023, 24, 5487. [Google Scholar] [CrossRef]
- Heberling, C.; Dhurjati, P. Novel systems modeling methodology in comparative microbial metabolomics: Identifying key enzymes and metabolites implicated in autism spectrum disorders. Int. J. Mol. Sci. 2015, 16, 8949–8967. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C. A metabolome study on 90 autism spectrum disorder patients by mass spectrometry. Med. Mass. Spectrom. 2017, 1, 14–19. [Google Scholar]
- Vargason, T.; Kruger, U.; McGuinness, D.L.; Adams, J.B.; Geis, E.; Gehn, E.; Coleman, D.; Hahn, J. Investigating Plasma Amino Acids for Differentiating Individuals with Autism Spectrum Disorder and Typically Developing Peers. Res. Autism Spectr. Disord. 2018, 50, 60–72. [Google Scholar] [CrossRef]
- Neul, J.L.; Skinner, S.A.; Annese, F.; Lane, J.; Heydemann, P.; Jones, M.; Kaufmann, W.E.; Glaze, D.G.; Percy, A.K. Metabolic Signatures Differentiate Rett Syndrome from Unaffected Siblings. Front. Integr. Neurosci. 2020, 14, 7. [Google Scholar] [CrossRef]
- Ritz, B.; Yan, Q.; Uppal, K.; Liew, Z.; Cui, X.; Ling, C.; Inoue, K.; von Ehrenstein, O.; Walker, D.I.; Jones, D.P. Untargeted Metabolomics Screen of Mid-pregnancy Maternal Serum and Autism in Offspring. Autism Res. 2020, 13, 1258–1269. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, R.; Xu, Y.; Zhou, Z.; Guan, P.; Wu, Y.; Zhou, J.; Cheng, Z.; Zhang, L. Altered Metabolic Characteristics in Plasma of Young Boys with Autism Spectrum Disorder. J. Autism Dev. Disord. 2022, 52, 4897–4907. [Google Scholar] [CrossRef]
- Sotelo-Orozco, J.; Abbeduto, L.; Hertz-Picciotto, I.; Slupsky, C.M. Association Between Plasma Metabolites and Psychometric Scores Among Children with Developmental Disabilities: Investigating Sex-Differences. Front. Psychiatry 2020, 11, 579538. [Google Scholar] [CrossRef] [PubMed]
- Herring, B.E.; Silm, K.; Edwards, R.H.; Nicoll, R.A. Is Aspartate an Excitatory Neurotransmitter? J. Neurosci. 2015, 35, 10168–10171. [Google Scholar] [CrossRef] [PubMed]
- West, P.R.; Amaral, D.G.; Bais, P.; Smith, A.M.; Egnash, L.A.; Ross, M.E.; Palmer, J.A.; Fontaine, B.R.; Conard, K.R.; Corbett, B.A.; et al. Metabolomics as a tool for discovery of biomarkers of autism spectrum disorder in the blood plasma of children. PLoS ONE 2014, 9, e112445. [Google Scholar] [CrossRef] [PubMed]
- Davison, A.N.; Kaczmarek, L.K. Taurine—A possible neurotransmitter? Nature 1971, 234, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-J.; Cai, X.-E.; Meng, F.-C.; Song, T.-J.; Wang, X.-X.; Wei, Y.-Z.; Zhai, F.-J.; Long, B.; Wang, J.; You, X.; et al. Comparison of the Metabolic Profiles in the Plasma and Urine Samples Between Autistic and Typically Developing Boys: A Preliminary Study. Front. Psychiatry 2021, 12, 657105. [Google Scholar] [CrossRef]
- Park, E.; Cohen, I.; Gonzalez, M.; Castellano, M.R.; Flory, M.; Jenkins, E.C.; Brown, W.T.; Schuller-Levis, G. Is Taurine a Biomarker in Autistic Spectrum Disorder? Adv. Exp. Med. Biol. 2017, 975 Pt 1, 3–16. [Google Scholar]
- Dickinson, A.; Jones, M.; Milne, E. Measuring neural excitation and inhibition in autism: Different approaches, different findings and different interpretations. Brain Res. 2016, 1648, 277–289. [Google Scholar] [CrossRef]
| Amino Acid | ASD (n = 1242) (mmol/L) | Non-ASD (n = 488) (mmol/L) | p-Value |
|---|---|---|---|
| Essential amino acids | |||
| Histidine | 77 (69–85) | 75 (67–83) | 0.002 |
| Isoleucine | 58 (50–69) | 55 (47–66) | <0.001 |
| Leucine | 117 (102–137) | 110 (92–127) | <0.001 |
| Lysine | 147 (127–171) | 142 (124–167) | 0.024 |
| Methionine | 21 (18–25) | 21 (18–26) | >0.9 |
| Phenylalanine | 54 (48–61) | 51 (45–57) | <0.001 |
| Threonine | 110 (90–131) | 107 (89–136) | 0.7 |
| Tyrosine | 64 (54–76) | 62 (52–73) | 0.005 |
| Valine | 218 (192–253) | 205 (168–237) | <0.001 |
| Conditionally essential amino acids | |||
| Arginine | 56 (39–73) | 59 (43–76) | 0.064 |
| Glutamine | 462 (413–512) | 500 (444–556) | <0.001 |
| Glycine | 207 (178–240) | 203 (175–232) | 0.065 |
| Ornithine | 66 (51–87) | 61 (49–79) | <0.001 |
| Proline | 151 (123–194) | 149 (122–181) | 0.2 |
| Taurine | 78 (61–107) | 70 (56–95) | <0.001 |
| Non-essential amino acids | |||
| Alanine | 285 (234–360) | 271 (225–321) | <0.001 |
| Asparagine | 57 (49–67) | 57 (49–65) | 0.5 |
| Aspartic acid | 6.0 (4.0–8.0) | 5.0 (4.0–7.0) | <0.001 |
| Citrulline | 31 (25–37) | 26 (21–31) | <0.001 |
| Glutamic acid | 56 (41–78) | 53 (37–79) | 0.018 |
| Serine | 131 (115–150) | 128 (112–144) | 0.004 |
| Amino Acid | NEU (n = 103) (mmol/L) | NUT (n = 78) (mmol/L) | NON-ASD (n = 488) (mmol/L) |
|---|---|---|---|
| Essential amino acids | |||
| Histidine | 97 (87–108) ^ | 87 (79–98) # | 75 (67–83) |
| Isoleucine | 71 (62–80) ^ | 96 (82–114) # | 55 (47–66) |
| Leucine | 154 (136–171) ^ | 177 (160–214) # | 110 (92–127) |
| Lysine | 192 (170–220) ^ | 220 (201–248) # | 142 (124–167) |
| Methionine | 24 (21–29) ^ | 36 (31–42) # | 21 (18–26) |
| Phenylalanine | 70 (63–77) ^ | 74 (67–81) # | 51 (45–57) |
| Threonine | 139 (123–159) ^ | 166 (143–205) # | 107 (89–136) |
| Tyrosine | 78 (67–88) ^ | 110 (88–129) # | 62 (52–73) |
| Valine | 249 (224–278) ^ | 310 (281–383) # | 205 (168–237) |
| Conditionally essential amino acids | |||
| Arginine | 30 (17–47) ^ | 86 (65–106) # | 59 (43–76) |
| Glutamine | 427 (378–467) ^ | 514 (455–568) § | 500 (444–556) |
| Glycine | 296 (256–343) ^ | 221 (196–270) # | 203 (175–232) |
| Ornithine | 148 (123–180) ^ | 90 (75–110) # | 61 (49–79) |
| Proline | 197 (161–228) ^ | 274 (223–349) # | 149 (122–181) |
| Taurine | 156 (127–211) ^ | 83 (62–114) # | 70 (56–95) |
| Non-essential amino acids | |||
| Alanine | 376 (327–462) ^ | 437 (370–497) # | 271 (225–321) |
| Asparagine | 74 (66–93) ^ | 77 (66–88) # | 57 (49–65) |
| Aspartic acid | 17 (14–22) ^ | 7.0 (5.0–9.0) # | 5.0 (4.0–7.0) |
| Citrulline | 37 (30–43) ^ | 31 (24–40) $ | 26 (21–31) |
| Glutamic acid | 177 (140–214) ^ | 67 (49–88) £ | 53 (37–79) |
| Serine | 173 (154–197) ^ | 161 (143–185) # | 128 (112–144) |
| Amino Acid | NEU vs. non-ASD | NEU vs. ASD | ||
|---|---|---|---|---|
| Fold Change | % | Fold Change | % | |
| Arginine | 0.50 | −96.6% | 0.53 | −86.6% |
| Ornithine | 2.42 | +58.8% | 2.24 | +55.4% |
| Taurine | 2.22 | +55.1% | 2.00 | +50.0% |
| Aspartic acid | 3.40 | +70.6% | 2.83 | +64.7% |
| Glutamic acid | 3.34 | +70.1% | 3.16 | +64.4% |
| Amino Acid | NUT vs. non-ASD | NUT vs. ASD | ||
| Fold Change | % | Fold Change | % | |
| Lysine | 1.55 | +35.5% | 1.49 | +33.2% |
| Methionine | 1.71 | +41.7% | 1.71 | +41.7% |
| Threonine | 1.55 | +35.6% | 1.51 | +33.8% |
| Tyrosine | 1.77 | +43.6% | 1.72 | +41.8% |
| Isoleucine | 1.74 | +42.8% | 1.65 | +39.6% |
| Leucine | 1.60 | +37.9% | 1.51 | +33.9% |
| Valine | 1.51 | +33.9% | 1.42 | +29.7% |
| Proline | 1.83 | +45.7% | 1.81 | +44.9% |
| Alanine | 1.61 | +38.0% | 1.53 | +34.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraro, S.; Saielli, L.; Biganzoli, D.; Tosi, M.; Guidi, L.; Longo, R.; Severino, F.; Carelli, S.; Rossi, M.; Pisciotta, L.; et al. Amino Acid Patterns in Children with Autistic Spectrum Disorder: A Preliminary Biochemical Evaluation. Nutrients 2025, 17, 274. https://doi.org/10.3390/nu17020274
Ferraro S, Saielli L, Biganzoli D, Tosi M, Guidi L, Longo R, Severino F, Carelli S, Rossi M, Pisciotta L, et al. Amino Acid Patterns in Children with Autistic Spectrum Disorder: A Preliminary Biochemical Evaluation. Nutrients. 2025; 17(2):274. https://doi.org/10.3390/nu17020274
Chicago/Turabian StyleFerraro, Simona, Laura Saielli, Davide Biganzoli, Martina Tosi, Laura Guidi, Roberto Longo, Francesca Severino, Stephana Carelli, Maura Rossi, Livia Pisciotta, and et al. 2025. "Amino Acid Patterns in Children with Autistic Spectrum Disorder: A Preliminary Biochemical Evaluation" Nutrients 17, no. 2: 274. https://doi.org/10.3390/nu17020274
APA StyleFerraro, S., Saielli, L., Biganzoli, D., Tosi, M., Guidi, L., Longo, R., Severino, F., Carelli, S., Rossi, M., Pisciotta, L., Ricci, E., Brustia, F., Verduci, E., Zuccotti, G., Mussap, M., & Cereda, C. (2025). Amino Acid Patterns in Children with Autistic Spectrum Disorder: A Preliminary Biochemical Evaluation. Nutrients, 17(2), 274. https://doi.org/10.3390/nu17020274









