Abstract
Background: Non-celiac gluten/wheat sensitivity (NCGWS) is a syndrome for which pathogenesis and management remain debated. It is described as a condition characterized by gastrointestinal and extra-intestinal symptoms rapidly occurring after gluten ingestion in subjects who have had celiac disease or wheat allergy excluded. To date, the diagnosis of NCGWS is challenging as no universally recognized biomarkers have been yet identified, nor has a predisposing genetic profile been described. However, the research is moving fast, and new data regarding pathogenic pathways, patients’ classification, potential candidate biomarkers, and dietary interventions are emerging. Methods: This literature review aims to address the state of the art and summarize the latest updates in this field from 2019 to date. Results and Conclusions: Clinical studies regarding NCGWS in the last five years are reported to shed light on this complex condition and to guide specialists towards a more in-depth, prompt, and objective diagnosis.
1. Introduction
Non-celiac gluten sensitivity (NCGS) is a syndrome for which the pathogenesis and management are still debated. It is described as a condition in which intestinal and extra-intestinal symptoms occur after gluten/wheat ingestion in subjects where the diagnosis of both celiac disease (CD) or IgE-mediated wheat allergy (WA) has been excluded. To date, a genetic background predisposing to NCGS has not been identified, and unlike CD and WA, diagnostic biomarkers for NCGS diagnosis are lacking. Wheat is a complex mixture of proteins, starches, fibers, and micronutrients, cheap and widely available. The term gluten refers to a family of storage proteins (prolamins) contained in wheat, rye, barley and their cross-bred grains [,], and its proline- and glutamine-rich residues, gliadins, are the trigger for CD in genetically predisposed subjects. Meanwhile, for WA, the proteins detected as a trigger for the development of an IgE-mediated response are lipid transfer protein (LTP, Tri a 14), omega-5-gliadin (Tri a 19) and the amylase trypsin inhibitor family (ATI).
The mechanisms involved in NCGS remain only partially understood, with some aspects of immunity likely contributing to its clinical manifestations. Symptoms can be triggered by gluten as well as by other wheat components, such as those contained in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs); ATIs, wheat germ agglutinin (WGA); and wheat glyphosate []. ATIs are highly resistant to intestinal proteolytic degradation and have been identified as strong activators of innate immune responses in human and murine macrophages, monocytes, and DCs, eliciting the release of pro-inflammatory cytokines via the activation of TLR4 []. WGA induces the activation of phlogistic pathways and epithelial barrier disruption [,]. However, more in vivo human studies are needed to better clarify their role in NCGS. Based on this evidence, in this review, we elected to use the more inclusive term “non-celiac gluten/wheat sensitivity (NCGWS)” to encase all of the components leading to symptoms onset.
The innate immune response is believed to play a key role in the pathology of NCGWS through an increased expression of Toll-like receptors and an imbalance between regulatory and pro-inflammatory cytokines. Other factors that may contribute to the development of symptoms are intestinal barrier impairment and microbiota alteration (i.e., dysbiosis). These are known to cause a slight increase in intraepithelial lymphocytes, mast cells, and eosinophils while preserving villous architecture [,].
NCGWS includes a wide array of symptoms; abdominal pain, bloating, reflux, and irregular bowel movements are the most common gastrointestinal (GI) symptoms, but a systemic involvement has often been reported, including fatigue, headache, “foggy mind”, and dermatitis []. Given the significant overlap in symptoms with irritable bowel syndrome (IBS), NCGWS can be difficult to diagnose. The definition of NCGWS has thus far relied on:
- (1)
- Exclusion of CD and wheat allergy;
- (2)
- The patient’s responsiveness to a gluten-free diet (GFD);
- (3)
- Double-blind, placebo-controlled gluten/wheat rechallenge, according to the “Salerno experts’ criteria” [,].
The treatment currently consists of a gluten-free diet (GFD) that resolves both intestinal and extra-intestinal symptoms, albeit without being as strict as required for patients with CD. The only guideline for the management of NCGWS can be found in the “European Society for the Study of Coeliac Disease (ESsCD) guideline for CD and other gluten-related disorders” [].
In this review, recent clinical trials will be discussed to update physicians managing patients with NCGWS. The lack of biomarkers, similarity of symptom profile to other GI conditions, and the limited treatment options will be considered along with directions for future research.
2. Materials and Methods
This narrative review aims to describe the updated state of the art of NCGWS in the last five years. Two independent authors (F.M. and L.L.) performed a comprehensive and independent literature search on PubMed, MEDLINE, and ScienceDirect using the following search terms in the title and abstract: “Non-celiac gluten/wheat sensitivity”; “Non-celiac Wheat Sensitivity”; and “Non-celiac Gluten Sensitivity”, operators “AND” and “OR”. Only articles (1) consistent with the topic; (2) with an original randomized controlled trial (RCT); (3) published in the last five years, from January 2019 to October 2024; (4) written in English; (5) listed as research articles; and (6) available in full text were included in this narrative analysis. Related results fitting the research criteria were also considered. Records were subsequently reviewed by the other authors. Any disagreements between F.M. and L.L. were resolved by consensus with G.C. and R.D.G. Three papers have been excluded because they were not relevant for the aims of this review [,,]. Articles not addressing the inclusion criteria, duplicates, and incomplete articles or those with unclear outcomes were excluded from this review.
3. Latest Clinical Trials (2019 to 2024)
From 2019 to September 2024, twenty-two original clinical trial studies regarding NCGWS were published. Eleven were randomized, double-blind, placebo-controlled design studies [,,,,,,,,,,], one was a randomized single-blind clinical trial [], two were cross-sectional analyses [,], two were prospective, double-blind studies [,], one was a retrospective analysis plus a prospective analysis of a cohort [], one was an interventional trial [], and finally, four were prospective observational trials [,,,,]. The details of studies included in this review have been reported in Section 3.1, Section 3.2, Section 3.3, Section 3.4 and Section 3.5 and summarized in Figure 1.
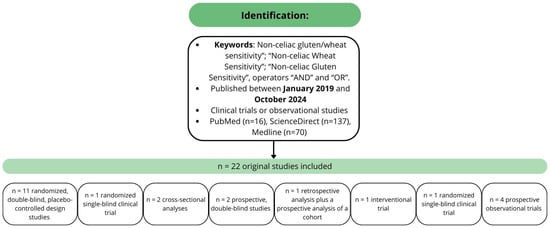
Figure 1.
Methodology and studies characteristics.
3.1. Pathogenetic Mechanisms
To date, the pathogenesis of NCGWS has not been sufficiently investigated. The only study analyzing risk factors for the development of NCGWS is by Gambino et al. []. They analyzed the role of killer immunoglobulin-like receptors (KIRs) genes and haplotypes as predisposing factors for the development of NCGWS. KIRs are surface receptors specific for allelic forms of human leukocyte antigen (HLA) class I molecules, a highly polymorphic family of genes. In CD, KIRs regulate the natural killer cells (NKs) response, and they can be classified as having an activating or inhibitory activity. The authors compared patients with CD, NCGWS, and healthy controls. They found a decreased frequency of KIR2DL1, -2DL3, -2DL5, -2DS2, -2DS3, -2DS4, -2DS5, and -3DS1 genes and an increased frequency of -3DL1 gene in the NCGWS cohort, leading to the authors hypothesizing their involvement in NCGWS susceptibility, with KIR2DL5, -2DS4, and -2DS5 having a protective effect. Using confocal laser endomicroscopy (CLE), Fritscher-Ravens et al. [] showed a non-IgE-mediated mechanism, triggering a mucosal response in patients with IBS symptoms. CLE patients with NCGWS were found to generate a local response (CLE+), characterized by an increase in intraepithelial lymphocytes, mucosal leaks, and intercellular extravasation of fluorescein-labeled plasma fluid into the widening intervillous space upon exposure to wheat. Moreover, the duodenal fluid had significantly higher concentrations of eosinophilic cationic protein (ECP), a higher expression of claudin-2, and lower levels of occludin. The withdrawal of food components causing a CLE+ reaction, including wheat, reduced IBS symptoms in CLE+ patients. Although more research is needed in this field, the study by Fritscher-Ravens et al. paved the way to a better understanding of the mucosal mechanism related to food ingestion and the subsequent generation of symptoms in predisposed patients.
Table 1 briefly summarizes the details of these studies.

Table 1.
Studies regarding pathogenetic hints from 2019 to 2024.
Summary for Pathogenetic Mechanisms
To date, the pathogenesis of NCGWS remains unclear. Therefore, the role of genetic risk factors should be further investigated with larger cohort studies.
The presence of non-IgE-mediated reactions to wheat in patients with IBS symptoms suggests the existence of pathological mechanisms involving a response to wheat proteins (including gluten, non-gluten-related protein, and ATIs), although further research is still needed.
3.2. Clinical Features and Correlation of Symptoms with Specific Food Components’ Ingestions
The spectrum of NCGWS symptoms encompasses both GI (e.g., bloating, abdominal discomfort and pain, diarrhea, and flatulence) and extra-GI manifestations (tiredness, headache, and anxiety) []. Moreover, other sensory symptoms, such as tingling and numbness, have been described by Hadjivassiliou et al. and defined as responsive to gluten withdrawal in a cohort of patients with gluten sensitivity [].
More recently, nine studies analyzed the clinical features and correlation with certain nutrients in patients with NCGWS [,,,,,,,,].
Skodje et al. [] registered the dietary intake of 65 self-reported NCGWS patients and examined clinical symptoms and health-related quality of life (HR-QoL). In this cohort, CD and WA were excluded, and patients had been on a GFD for at least six months. Eighty-eight percent of the population were female, and participants had a lower HR-QoL than the general population. Moreover, the consumption of total and saturated fat was higher and the intake of carbohydrate and dietary fiber was lower than the recommended daily amount. Moreover vitamin D, folic acid, calcium, iodine, and iron assumptions were not in the recommended range, but blood values did not show nutrient deficiencies. The adherence to a GFD and a mean moderate–low intake of FODMAPs (11.6 g) did not significantly reduce intestinal symptoms. These data are part of an RCT carried out by the same authors [], where a challenge with fructans, rather than gluten, showed a significant worsening of GI symptoms. However, there are limitations in this study as the recruited cohort self-reported gluten intolerance and they were not diagnosed with the gold standard method of a double-blind placebo-controlled assessment (DPBC-C). Thus, some of them might represent a sub-population of patients with IBS or small intestinal bacterial overgrowth (SIBO). Also, Skodje et al. did not report other extra-GI symptoms and immune-mediated diseases, apart from thyroiditis.
Moleski et al. [] recruited patients with self-reported gluten intolerance and compared them with a group of healthy subjects on a GFD. Participants received pills containing 0.5 g or 2 g of gluten/day for 7 days or placebo. No significant worsening in symptoms after gluten ingestion was observed in both groups. Of note, the patients with NCGWS did not have a diagnosis confirmed by a clinician, and the consumption of other dietary components was not studied. Furthermore, for the same reasons explained above, we cannot exclude the presence of a selection bias in the study population (inclusion of IBS or SIBO patients).
Barone et al. [] evaluated NCGWS in patients with previous diagnosis of IBS. The authors analyzed the change in visual analog scale (VAS) modification at five timepoints in a FODMAPs-gluten-containing diet (two weeks, t0), after a low FODMAPs-GFD (two weeks, t1), and only patients presenting an improvement in symptoms after a DPBC-C with gluten with wash-out and crossover (t2, t3, t4, one week each). Using the method established by Catassi et al. [], who considered a significant response a reduction of ≥30% in VAS score, 12 out of 26 patients could fit the diagnosis of NCGWS. However, when considering a VAS score variation greater than the mean ∆-VAS score by +2 standard deviations, as performed by Di Sabatino et al. [], only five patients were identified as NCGWS. This study aimed to highlight a possible confounding role of FODMAPs in the identification of NCGWS; the authors concluded that FODMAPs intolerance could hide the response to a challenge test with gluten, and therefore, a low FODMAPs-GFD followed by gluten/placebo challenge could identify better patients with NCGWS.
Focusing on different aspects, Herfindal et al. [] investigated the role of fructo-oligosaccharides (FOSs or fructans) in the intestinal microbial composition and GI symptoms onset in self-reported NCGWS patients. The authors carried out a 7-day-long crossover challenge with gluten-containing, FOS-containing, and placebo bars and collected IBS-related symptoms via the Gastrointestinal Symptom Rating Scale (GSRS-IBS) questionnaire and stool samples. Alpha and beta diversity, fecal metabolites (short-chain fatty acids, SCFAs), and fecal neutrophil gelatinase-associated lipocalin 2 (NGAL/LCN2) did not change across diet challenges, but the relative abundance of certain bacteria taxa were affected. After the FOS-fructans challenge, Fusicatenibacter increased, whereas Eubacterium (E.) coprostanoligenes group, Anaerotruncus, and unknown Ruminococcaceae genera decreased. The gluten challenge was primarily characterized by an increased abundance of Eubacterium (E.) xylanophilum group. However, the statistical association between the variation of certain taxa and GI symptoms showed only a few significant associations. The authors highlighted how the reduction in E. coprostanoligenes group following the FOS-fructans challenge was associated with increased abdominal pain. The heterogeneity of the group of patients included in the study and the short length of the challenges (7 days) are probably the most consistent limitations in this original and innovative study. A longer duration of the crossover challenge could have led to more consistent changes in microbial composition and fecal metabolites []; however, the effort requested of patients would have been higher.
Cotton et al. [] assessed QoL and sleep quality in patients with self-reported NCGWS compared with CD following a GFD. Patients with NCGWS adhered to a GFD less often than those with CD. Furthermore, lower rates of adherence to a GFD in patients with NCGWS were associated with a poorer QoL and worse sleep performance. This study gives interesting insights regarding the application of a GFD in NCGWS patients: patients with NCGWS who adhere to a GFD had better QoL and sleep than those continuing to eat gluten. There is little support available to this group of patients, and there is scarce evidence regarding the benefits/risks of following a lifelong GFD, and while there is evidence to support clinicians in recommending a GFD to NCGWS patients, it is not mandatory for the diet to be as strict as for CD, often identifying a personal threshold for gluten tolerance [].
GI tract dysmotility has been described in CD [,] and to a lesser extent in NCGWS []. Two studies examined NCGWS and functional dyspepsia (FD) [,]. Shahbazkhani et al. [] evaluated the presence of refractory FD after a DPBC-C with gluten. Out of 27 patients, 5 (18.5%) were diagnosed with NCGWS after challenge. Four were female, and a high titer of anti-gliadin antibodies (AGAs) IgG was only found in one subject. Potter et al. [] considered FODMAPs (fructans in particular) in their double-blind challenge. Interestingly, their study design was modified by the Salerno criteria: patients on a normal wheat-containing diet were instructed to follow a low FODMAPs-GFD for 4 weeks (run-in). Subjects with a significant reduction of symptoms underwent a rechallenge using either fructan-containing, gluten-containing, or placebo bars. Unfortunately, due to under-recruitment (only 11 patients enrolled and 5 stopped during run-in phase), a dietary rechallenge was not tried. However, the study highlighted a trend towards the improvement of dyspeptic symptoms on a low FODMAPs-GFD. These two studies are in line with the findings of Elli et al. (2016) [], where a subset of patients with FD positively responded to a GFD approach. Further research is required to establish the role of gluten exclusion in patients with FD and whether it could be used after first-line treatments have failed.
More extensively, Cobos-Quevado et al. [] have studied the whole GI transit time in both newly diagnosed CD and NCGWS patients, diagnosed with a gluten challenge, using a wireless motility and pH capsule (WMC). Patients with NCGWS showed improvements in intestinal transit time and contractility when on GFD, although the colon exhibited no discernible effect. The GFD did not significantly impact intragastric, intestinal, or colonic pH; however, given the improved transit time, similar to in CD, inflammation and epithelial alterations associated with intestinal motor dysfunctions may occur in patients with NCGWS. However, results should be interpreted cautiously as the sample size is small (CD = 12, NCGWS = 12) and there is an absence of a control group.
Mansueto et al. [] analyzed the frequency, severity, and morphologic characteristics of anemia in a cohort of 244 NCGWS patients compared with IBS and CD patients. At the time of diagnosis, patients having IBS-like, dyspepsia-like, and extra-intestinal symptoms were also frequent. Eighty-five participants had anemia (all females; frequency of anemia of 34.8% in NCGWS vs. 17.4% in IBS, p=0.03, vs. 48,3% in CD, not significant). NCGWS patients with anemia showed iron deficiency more frequently than non-anemic patients and higher TSH levels. Of these 85 patients, 31 were re-evaluated after at least 12 months of wheat-free diet. A statistically significant improvement in hemoglobin values, mean corpuscular volume, mean corpuscular hemoglobin, and ferritin levels was found. Different pathogenetic mechanisms may account for anemia in this setting, including: (1) iron plus folic acid and/or vitamin B12 deficiency; (2) hypothyroidism; (3) poly/hypermenorrhea (detected in 43% of female patients); and (4) diagnostic delay that might increase noxious effect of wheat in sensitive patients.
Table 2 summarizes the details of these studies.

Table 2.
Studies regarding the clinical features of NCGWS from 2019 to 2024.
Summary for Clinical Features and Correlation of Symptoms with Specific Food Components’ Ingestions
- Along with classical gastrointestinal symptoms (e.g., bloating, abdominal pain, flatulence) systemic manifestation such as fatigue, tiredness, neurological manifestation, can also be presented symptoms in patients with suspect NCGWS;
- To avoid conflicting results between studies, unambiguous criteria should be used to enroll patients (e.g., Salerno criteria vs. self-reported NCGWS);
- The role of FODMAPs vs. gluten in the development of symptoms is still debated;
- The application of a GFD (regardless of fructans content) seems to be beneficial in the management of symptoms and QoL in NCGWS patients;
- A GFD might be considered an appropriate treatment in a subset of FD patients;
- The presence of an impaired GI motility in NCGWS patients needs to be further assessed;
- NCGWS shares its clinical presentations with several other conditions, including IBS and some neurological/psychiatric conditions. However, the application of a GFD as a treatment option should be considered on a case-by-case basis after discussion with the patient. The effect of the GFD on symptoms should subsequently be reviewed, and the nutritional status of the patient also monitored.
3.3. Diagnostic Tools
To date, a diagnostic biomarker has not been identified yet, and the diagnosis of NCGWS currently relies on a DPBC-C, which is often very difficult to carry out in daily practice. The identification of a reliable diagnostic tool would be of paramount importance for patient identification and for a better understanding of causal agents.
Barbaro et al. [] evaluated serum zonulin as a candidate biomarker. Zonulin is a single-chain protein that is able to reversibly open tight junctions. Higher serum zonulin correlates with increased epithelial permeability, and gliadin can increase its release []. Tight junctions opening increases intestinal permeability, a trigger mechanism involved in the initiation of CD and NCGWS. In Barbaro et al.’s study, CD and NCGWS patients showed significantly increased zonulin levels as compared with patients with diarrhea-predominant IBS (IBS-D) and asymptomatic controls. Zonulin levels were reduced after a 6-month wheat-free diet (WFD) only in HLA-DQ2/8-positive participants with NCGWS. The diagnostic accuracy of zonulin levels in distinguishing NCGWS from IBS-D was 81%. The authors also proposed a diagnostic strategy combining gender, zonulin levels, and symptoms that could improve the accuracy up to 89%. However, a possible limitation of this study is the increased serum zonulin levels that may occur in genetically predisposed, completely asymptomatic individuals.
An alternative method proposed by Bojarski et al. [] tried to develop a diagnostic test for wheat sensitivity in IBS patients. They used CLE with duodenal antigen (wheat, yeast, milk, soy) provocation. CLE generated high-resolution images of the duodenum and was able to detect fluorescein leakage (major criterion) to identify an increase in intraepithelial lymphocytes (IELs) and variations in intervillous spaces (minor criteria). CLE testing was considered positive if at least one major and one minor criterion were documented after wheat administration (CLE+). Patients were then instructed to follow a GFD and register symptoms. Overall, patients with IBS who were CLE+ after application of wheat were approximately twice as likely to have wheat sensitivity compared with those who were CLE-negative after wheat exposure. However, due to its invasiveness and low sensitivity and specificity for wheat sensitivity (less than 80%), CLE is not recommendable as a diagnostic test.
Seidita et al. [] have proposed an already well-established test in the assessment of NCGWS, i.e., measuring fecal calprotectin (FCP) to identify the presence of an inflammatory status in NCGWS as an index differentiating NCGWS from IBS/FD. They enrolled 201 NCGWS patients (diagnosed with a DPBC-C) and 50 IBS/FD patients. Among patients with NCWS, 31.3% (63/201) had increased FCP values (NCGWS FCP+), whereas all IBS/FD patients had values within the normal range. In the prospective phase of the study, the effects of a strict GFD for 6 months in the NCGWS cohort were assessed. With respect to NCGWS FCP+ group, 65.1% of participants had negative values of FCP after 6 months. The authors concluded the presence of two NCGWS subgroups: NCGWS FCP+, characterized by a predominantly inflammatory/immunologic pattern, and NCGWS FCP-, featuring non-immuno-mediated etiopathogenetic mechanisms. It is difficult to establish the significance of this difference, which may be due to multiple pathological processes, or even address separate disease entities.
Another study focused on the assessment of a helpful tool for distinguishing between NCGWS and IBS []. As previously suggested [], AGA IgAs are present in around half of patients with NCGWS. On this assumption, the authors enrolled 492 patients with IBS: those who were AGA (IgA or IgG)-positive (61, 12.4%) were asked to follow a GFD for 6 weeks. Patients who had an improvement in symptoms were rechallenged with gluten. Of the 31 patients who agreed to follow a GFD, 17 (54.8%) had complete (>30% improvement) and 10 (32.2%) had partial (20–30% improvement) responses. Despite the small cohort, AGA might be further explored as a helpful tool for differential diagnosis for NCGWS, at least for identifying a subset of patients.
See Table 3 for a summary of these studies.

Table 3.
Studies analyzing new diagnostic tools for NCGWS from 2019 to 2024.
Summary for Diagnostic Tools
- To date, no candidate biomarkers or diagnostic tool have shown an adequate reliability for diagnosing NCGWS;
- Further research is required to assess the role of additional tests (zonulin, FCP, AGA) in the diagnosis of NCGWS.
3.4. Dietary Interventions
Five studies evaluated different dietary interventions and their consequences on several aspects of NCGWS [,,,,].
Ajamian et al. [] evaluated the effects of FODMAPs and gluten on markers of intestinal epithelial injury (syndecan-1 and intestinal fatty acid-binding protein) and bacterial translocation (lipopolysaccharide-binding protein and soluble CD14). The cohort included patients with IBS and self-reported NCGWS. Only syndecan-1 decreased in a low FODMAPs diet along with a reduction in symptoms, regardless of the presence of gluten. The authors concluded that epithelial integrity and symptoms are not affected by gluten ingestion in their study population; however, patients did not undergo a DPBC-C to confirm a NCGWS diagnosis.
Roncoroni et al. [] exposed NCGWS patients to increasing amounts of gluten in an unblinded fashion: 3.5–4 g/day for week 1, 6.7–8 g/day for week 2, and 10–13 g/day for week 3. Patients were diagnosed according to the Salerno criteria. Among the 24 patients enrolled, 8 did not tolerate even a low gluten content, 6 relapsed on diet containing 6.7/8 g of gluten/day, and 8 tolerated a higher gluten containing diet. This study shows that there is a certain level of tolerance in NCGWS patients, and therefore, a controlled reintroduction of gluten might be helpful for improving the QoL in a specific group of patients.
Ianiro et al. [] tested, in a DPBC-C crossover design study, Senatore Cappelli (a variety of wheat) in a cohort of NCGWS patients. Senatore Cappelli has shown more favorable characteristics, such as higher content of fibers and micronutrients and a reduced gliadin content and pesticide contamination []. Significantly lower overall and GI symptoms scores, as measured by GSRS, were reported in the study group eating Senatore Cappelli pasta compared with the one eating standard pasta. This might support the application of a less strict GFD for patients with NCGWS and the presence of dietary alternatives (such as ancient wheats with a lower content of gliadin and pesticides) with consequent health, economic, and social benefits.
Zimmermann et al. [] evaluated the tolerance of spelt vs. wheat in patients who referred wheat intolerance and simultaneous spelt tolerance, assessed before the beginning of the trial. They used six types of bread: a gluten-free (GF) bread, bread supplemented with gluten or FODMAPs, and spelt or wheat bread both baked accordingly to “traditional” (T) or “current” (C) recipe. The IBS-Severity Scoring System (IBS-SSS) questionnaire was used to assess GI symptoms severity. IBS-SSS scores were higher than expected by the participants after spelt bread consumption and lower for wheat bread consumption, resulting in no difference between spelt and wheat bread tolerance. The results highlighted a high prevalence of a nocebo response (40% of patients). Markers for intestinal permeability (serum zonulin and lipopolysaccharide-binding protein) did not change between the different breads. However, in their study design, a shorter challenge and wash-out between the breads was used (challenge 4 days, wash-out 3 days) compared with other trials that usually performed longer challenges and wash-out periods (most commonly lasting a week) [,].
Another study evaluated the effect of different types of wheat and fermentation techniques []. Patients with self-reported NCGWS were asked to adhere to a “symptom-free diet” (replace or avoid food products that they considered to induce GI symptoms). Subsequently, they received either fermented yeast (FY) bread composed of wheat, spelt, or emmer (group A), or fermented sourdough (FS) bread, also composed of wheat, spelt, or emmer (group B). It is believed that sourdough fermentation leads to fructan degradation and improves digestive tolerance. Moreover, the content of gluten in spelt and emmer is reduced by 20% compared with wheat. Therefore, the authors hypothesized that consumption of FY and FS bread composed of emmer and spelt may cause less GI and extra-GI symptoms than conventional bread. Interestingly, no differences were found when comparing wheat or fermentation type, but the authors highlighted how more than 50% of the participants developed GI symptoms to more than one type of bread. It must be noted that all bread types contained FODMAP, gluten, and ATIs: for these reasons, assigning any of the reported symptoms to one of these components was not possible. However, the study population was very heterogenous, especially in relation to food choices that relieved/relapsed symptoms caused by wheat ingestion.
Lastly, De Graaf et al. [] quantified the presence of a nocebo effect in people with self-reported NCGWS. They investigated the effects of expectancy (E) about gluten intake versus actual (G) gluten intake on GI and extra-intestinal symptoms. Eighty-four patients were randomized into four groups: E+G+ (expectancy to consume gluten-containing bread, combined with actual intake of gluten-containing bread); E+G− (expectancy to consume gluten-containing bread, combined with actual intake of gluten-free bread); E-G+ (expectancy to consume gluten-free bread, combined with actual intake of gluten-containing bread); and E-G− (expectancy to consume gluten-free bread, combined with actual intake of gluten-free bread). Their results showed that the combination of positive expectancy and actual gluten intake had the largest effect on overall GI symptoms; moreover, actual gluten intake did not affect overall or individual symptoms. The fact that the E+G+ group had the highest symptoms score might point out a direct involvement of gluten in the worsening of the VAS scores but also a heterogeneity of mechanisms that lead to the development of symptoms.
See Table 4 for a brief summary of these studies.

Table 4.
Studies regarding dietary interventions for NCGWS from 2019 to 2024.
Summary for Dietary Interventions
- NCGWS patients may be able to tolerate wheat to some degree. However, the role of its components (gluten, FODMAPs) remains unclear; focusing on the uniformity of the patients enrolled among different studies might be pivotal for achieving conclusive results;
- The expectancy of wheat ingestion and the presence of a nocebo effect are emerging but do not help distinguish features in NCGWS. Further assessments are required, which may include differentiating patients into different subsets;
- The dietary approach should be tailored to each patient’s preferences and wheat/FODMAPs tolerance.
3.5. Therapeutic Strategies
Due to the poor understanding of NCGWS, there are limited therapeutic targets. The literature on therapeutic approaches for treating NCGWS is therefore sparse. The only study analyzing a possible therapeutic strategy was the one by Scricciolo et al. []. They assessed the efficacy of a proline-specific endopeptidase enzyme isolated from Aspergillus niger (P1016) with high specificity for the degradation of proline-rich gluten epitopes. Patients assumed either a placebo or capsule containing P1016 in a blinded fashion and were instructed to follow a diet containing increasing amounts of gluten for 21 days. Over the same period, the capsules were administered right before gluten consumption, once a day for the first week, twice a day for the second week, and thrice a day during the third week. Abdominal pain, stool consistency, severity of abdominal swelling, severity of postprandial fullness, severity of early satiety, epigastric burning, state of satisfaction regarding general well-being, and QoL were recorded with VAS score and SF-36 (Short Form health survey-36) scoring. The results did not show any differences in GI and psychological symptoms or QoL using enzyme P1016, despite its ability to break down gluten in vitro. Table 5 briefly summarizes this study.

Table 5.
Details of the study regarding therapeutic strategies.
Summary for Therapeutic Strategies
- To date, there are no effective alternative treatments, rather than a dietary approach, for the improvement of GI symptoms and QoL in patients with NCGWS.
4. Discussion
Several original studies on NCGWS have been published in the past few years, and all of them have clearly indicated that NCGWS is still a debated clinical entity that is difficult to recognize and manage.
An accurate analysis of the currently available literature leads to the following considerations. First, the lack of understanding of the pathogenesis of NCGWS may affect the patients’ homogeneity and recruitment. Despite the Salerno expert criteria published in 2015, only 13 out of 21 trials here listed [,,,,,,,,,,,,] recruited or diagnosed NCGWS patients according to DPBC-C. Indeed, DPBC-C is not practical and easy to use in regular clinical practice. As a result, it currently remains as a research tool. Limitations regarding the availability of identical gluten-containing/gluten-free foods to be used for challenge, the inability to discriminate the harmful component(s) involved, and a role for a nocebo effect are all factors further hampering the progress in understanding NCGWS. Moreover, the application of DPBC-C in clinical studies might imply early discontinuation or screening failure due to the inability of the patients to follow the protocol []. Therefore, the need for a reliable biomarker for diagnosis is needed. At the same time, the lack of a well-defined pathogenetic pathway hinders the findings of a diagnostic tool.
FODMAPs are known for their osmotic and fermentable properties, which can lead to severe bloating, pain, and diarrhea in a subset of patients, and are considered to be one of the possible culprits for symptom generation in NCGWS. FODMAPs are known to have a role in eliciting symptoms in IBS due to their characteristics, and a low FODMAPs containing diet is recommended for the management of IBS in several guidelines [,,]. Moreover, many NCGWS symptoms overlap with the one reported in IBS, specifically bloating and abdominal pain. Because wheat is one of the main sources of dietary fructans, Skodje et al. [] highlighted an increase in symptoms in self-reported gluten-sensitive patients, implying a role for this dietary component rather than gluten (or placebo). Fernandes Dias et al. suggested that a complete low FODMAP diet can further decrease GI symptoms in patients with NCGWS []. Molina-Infante and Carroccio [] summarized the challenges derived from the application of the DPBC-C based on gluten administration only for the diagnosis of NCGWS, stating that less than 20% of patients can be confirmed as gluten/wheat sensitive accordingly to the Salerno criteria.
Based on these considerations, a more complete approach for the diagnosis of NCGWS should be the evaluation of the gluten challenge response after the administration of a low FODMAP/GFD diet. This strategy would not only be able to recognize patients with NCGWS, but also IBS patients responsive to a low FODMAP diet vs. patients not responsive to dietary treatments. Unfortunately, a DPBC-C with three arms may turn out to be actually very difficult for physicians and patients.
The presence of a nocebo response during gluten consumption has been reported in several studies, reaching a prevalence of 40% among the participants [,]. Therefore, sociological aspects should also be considered when describing NCGWS, specifically the perception of the healthiness of GFD nowadays. The self-reported connection between symptoms and gluten ingestion may limit patient recruitment in clinical trials, and as a consequence, their results are often conflicting.
Around 25% of consumers see gluten-free products to be healthier than their gluten containing counterparts []. The lack of appropriate biomarkers has resulted in a self-diagnosed NCGS, and self-reported wheat sensitivity (SRWS) is common in young to middle-aged women who consider wheat-based products to be the culprit for intestinal and extra-GI symptoms. Typical common traits are a previous diagnosis of other GI disorders (i.e., IgE-mediated allergy, multiple food hypersensitivity, IBS) and mood disorders (i.e., anxiety and depression). These patients are usually less receptive to conventional medicine and usually seek alternative treatments in complementary medicine [].
Notably, in the study by S. Golley et al., the decision to avoid wheat-based products was only supported by a formal medical diagnosis in 5.7% of the analyzed sample []. Moreover, more than half of wheat avoiders are also restricting dairy products. Data from Priven et al. [] underline that “free-from” products generate a perception of healthiness, especially in those that, because of different reasons, perceive gluten as a “risky” food component. The assumption of the harmful properties of gluten often comes from misinformation carried on by media and the Internet; therefore, it is falsely claimed that avoiding gluten increases overall health status.
Another misconception that might lead people to pursue a GFD is that eating gluten-free helps lose weight []. However, from the experience of CD patients, studies suggest that body weight is more likely to increase after a GFD regime [,]. Even though this might also be related to an increased ability to absorb nutrients, indeed, GFD products are often lower in protein and fiber content and higher in fats, salt, and sugar []. In addition, maintaining a GFD lifestyle has many challenges, including nutritional deficiencies, high costs, and social and psychological barriers [].
Therefore, the term NCGWS does not always seem to include a homogeneous group of patients. As highlighted by De Graaf et al. [], the combination of expectancy and actual gluten intake had the largest effect on overall GI symptoms. This suggests that a role for gluten itself cannot be excluded but might imply at the same time the involvement of a central processing of external pieces of information (the expectancy) influencing gastrointestinal sensory and vice versa. This bidirectional connection between the GI tract and the nervous system is a distinguishing feature of the disorders of the gut–brain interaction (DGBI), a definition that includes both FD and IBS []. Some studies analyzed in this review [,,] suggest an overlap between DGBIs and NCGWS, while others stress more distinctive and organic features of NCGWS [,,]. However, in clinical practice, an accurate distinction between DGBIs and NCGWS can be challenging due to the presence of shared symptoms and the absence of a reliable biomarker. Moreover, the majority of IBS patients believe that certain food items are important triggers of their GI symptoms, specifically high carbohydrate-containing products or histamine-releasing, amine-rich food items [,]. Ultimately, a low FODMAPs-containing diet has shown a reduction in clinical and psychological symptoms in NCGWS in several studies [,].
What has been summarized here might suggest the presence of at least three subgroups of patients: one with a specific reaction to gluten (antibodies positivity—AGA, genetic background [,]), another sensitive to other wheat’s components (ATIs, WGA, FODMAPs, and wheat), and a third belonging to a IBS/FD/DGBI group of patients, implying a multifactorial etiology of NCGWS. However, this hypothesis needs further validation.
5. Conclusions
Over the past five years, several original and significant papers on NCGWS have been published, and the most relevant data focus on pathogenesis, clinical features, candidate diagnostic tools, and dietary and therapeutic strategies. The intense research in this field has improved our understanding of NCGWS, although further studies are necessary to improve the knowledge on this debated clinical entity and develop a reliable biomarker for diagnosis and management.
Author Contributions
Conceptualization F.M.; original draft preparation, F.M. and L.L.; writing F.M. and L.L.; review and editing F.M., L.L., A.C. (Anna Costanzini), F.C., A.C. (Antonio Carroccio), P.M., A.S., S.A.R., U.V., D.S.S., R.D.G. and G.C.; supervision, R.D.G., D.S.S. and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
R.D.G. and G.C. receive constant support from the University of Ferrara (FAR2024 and FIR funds).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ATIs | Amylase trypsin inhibitor |
| AGAs | Anti-gliadin antibodies |
| AUC | Area under the curve |
| CI | Confidential interval |
| CLE | Confocal laser endomicroscopy |
| CD | Celiac disease |
| DGBI | Disorders of the gut–brain interaction |
| DPBC-C | Double blind placebo-controlled challenge |
| ECP | Eosinophil cationic protein |
| FCP | Fecal calprotectin |
| FD | Functional dyspepsia |
| FODMAPs | Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols |
| FS | Fermented sourdough |
| FY | Fermented yeast |
| GFD | Gluten-free diet |
| GI | Gastrointestinal |
| GIPs | Gluten immunogenic peptides |
| GSRS | Gastrointestinal symptom rating scale |
| HCs | Healthy controls |
| HLA | Human leukocyte antigen |
| HR-QoL | Health-related quality of life |
| IBS | Irritable bowel syndrome |
| IBS-D | Diarrhea-predominant IBS |
| IBS-SSS | IBS Severity Scoring System |
| IEL | Intraepithelial lymphocytes |
| IQR | Interquartile range |
| KIRs | Killer immunoglobulin-like receptors |
| NCGS | Non-celiac gluten sensitivity |
| NCWS | Non-celiac wheat sensitivity |
| NCGWS | Non-celiac gluten/wheat sensitivity |
| NDI | Nepean Dyspepsia Index |
| NGAL/LCN2 | Neutrophil gelatinase-associated lipocalin 2 |
| QoL | Quality of life |
| RCT | Randomized controlled trial |
| RFD | Refractory functional dyspepsia |
| SCFA | Short-chain fatty acid |
| SCI | Sleep condition indicator |
| SCL-90 | Symptom CheckList-90 |
| SF-36 | Short Form health survey-36 |
| VAS | Visual analog scale |
| WA | Wheat allergy |
| WFD | Wheat-free diet |
| WGA | Wheat germ agglutinins |
| WMC | Wireless motility and pH capsule |
References
- Mumolo, M.G.; Rettura, F.; Melissari, S.; Costa, F.; Ricchiuti, A.; Ceccarelli, L.; de Bortoli, N.; Marchi, S.; Bellini, M. Is Gluten the Only Culprit for Non-Celiac Gluten/Wheat Sensitivity? Nutrients 2020, 12, 3785. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328.e3. [Google Scholar] [CrossRef]
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Rüssel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional Wheat Amylase-Trypsin Inhibitors Promote Intestinal Inflammation via Activation of Myeloid Cells. Gastroenterology 2017, 152, 1100–1113.e12. [Google Scholar] [CrossRef] [PubMed]
- Pellegrina, C.D.; Perbellini, O.; Scupoli, M.T.; Tomelleri, C.; Zanetti, C.; Zoccatelli, G.; Fusi, M.; Peruffo, A.; Rizzi, C.; Chignola, R. Effects of wheat germ agglutinin on human gastrointestinal epithelium: Insights from an experimental model of immune/epithelial cell interaction. Toxicol. Appl. Pharmacol. 2009, 237, 146–153. [Google Scholar] [CrossRef]
- Cárdenas-Torres, F.I.; Cabrera-Chávez, F.; Figueroa-Salcido, O.G.; Ontiveros, N. Non-Celiac Gluten Sensitivity: An Update. Medicina 2021, 57, 526. [Google Scholar] [CrossRef]
- Giancola, F.; Volta, U.; Repossi, R.; Latorre, R.; Beeckmans, D.; Carbone, F.; Van den Houte, K.; Bianco, F.; Bonora, E.; Gori, A.; et al. Mast cell-nerve interactions correlate with bloating and abdominal pain severity in patients with non-celiac gluten/wheat sensitivity. Neurogastroenterol. Motil. 2020, 32, e13814. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Bardella, M.T.; Calabrò, A.; Troncone, R.; Corazza, G.R.; Study Group for Non-Celiac Gluten Sensitivity. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014, 12, 85. [Google Scholar] [CrossRef]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Mansueto, P.; Soresi, M.; Peralta, S.; Perricone, S.; La Blasca, F.; Sichera, R.; Giambalvo, O.; Carroccio, A. Self-reported nonceliac wheat sensitivity in an outpatient digestive endoscopy center: High frequency but insufficient medical approach. Eur. J. Gastroenterol. Hepatol. 2021, 33, e789–e795. [Google Scholar] [CrossRef]
- Martín-Cardona, A.; Carrasco, A.; Arau, B.; Vidal, J.; Tristán, E.; Ferrer, C.; Gonzalez-Puglia, G.; Pallarès, N.; Tebé, C.; Farrais, S.; et al. γδ+ T-Cells Is a Useful Biomarker for the Differential Diagnosis between Celiac Disease and Non-Celiac Gluten Sensitivity in Patients under Gluten Free Diet. Nutrients 2024, 16, 2294. [Google Scholar] [CrossRef] [PubMed]
- Saadati, S.; Sadeghi, A.; Mohaghegh-Shalmani, H.; Rostami-Nejad, M.; Elli, L.; Asadzadeh-Aghdaei, H.; Rodrigo, L.; Zali, M.R. Effects of a gluten challenge in patients with irritable bowel syndrome: A randomized single-blind controlled clinical trial. Sci. Rep. 2022, 12, 4960. [Google Scholar] [CrossRef] [PubMed]
- Scricciolo, A.; Lombardo, V.; Elli, L.; Bascuñán, K.A.; Doneda, L.; Rinaldi, F.; Pinto, D.; Araya, M.; Costantino, A.; Vecchi, M.; et al. Use of a proline-specific endopeptidase to reintroduce gluten in patients with non-coeliac gluten sensitivity: A randomized trial. Clin. Nutr. 2022, 41, 2025–2030. [Google Scholar] [CrossRef] [PubMed]
- Moleski, S.M.; Shah, A.; Durney, P.; Matthews, M.; Kaushal, G.; Smith, C.; Koons, K.C.; Rubin, E.; Casey, P.; Miller, R.; et al. Symptoms of gluten ingestion in patients with non-celiac gluten sensitivity: A randomized clinical trial. Nutrition 2021, 81, 110944. [Google Scholar] [CrossRef] [PubMed]
- Ajamian, M.; Rosella, G.; Newnham, E.D.; Biesiekierski, J.R.; Muir, J.G.; Gibson, P.R. Effect of Gluten Ingestion and FODMAP Restriction on Intestinal Epithelial Integrity in Patients with Irritable Bowel Syndrome and Self-Reported Non-Coeliac Gluten Sensitivity. Mol. Nutr. Food Res. 2021, 65, e1901275. [Google Scholar] [CrossRef] [PubMed]
- Fritscher-Ravens, A.; Pflaum, T.; Mösinger, M.; Ruchay, Z.; Röcken, C.; Milla, P.J.; Das, M.; Böttner, M.; Wedel, T.; Schuppan, D. Many Patients with Irritable Bowel Syndrome Have Atypical Food Allergies Not Associated with Immunoglobulin E. Gastroenterology 2019, 157, 109–118.e5. [Google Scholar] [CrossRef]
- Barone, M.; Gemello, E.; Viggiani, M.T.; Cristofori, F.; Renna, C.; Iannone, A.; Di Leo, A.; Francavilla, R. Evaluation of Non-Celiac Gluten Sensitivity in Patients with Previous Diagnosis of Irritable Bowel Syndrome: A Randomized Double-Blind Placebo-Controlled Crossover Trial. Nutrients 2020, 12, 705. [Google Scholar] [CrossRef]
- Shahbazkhani, B.; Fanaeian, M.M.; Farahvash, M.J.; Aletaha, N.; Alborzi, F.; Elli, L.; Shahbazkhani, A.; Zebardast, J.; Rostami-Nejad, M. Prevalence of Non-Celiac Gluten Sensitivity in Patients with Refractory Functional Dyspepsia: A Randomized Double-blind Placebo Controlled Trial. Sci. Rep. 2020, 10, 2401. [Google Scholar] [CrossRef]
- Ianiro, G.; Rizzatti, G.; Napoli, M.; Matteo, M.V.; Rinninella, E.; Mora, V.; Fanali, C.; Leonetti, A.; Benedettelli, S.; Mele, M.C.; et al. A Durum Wheat Variety-Based Product Is Effective in Reducing Symptoms in Patients with Non-Celiac Gluten Sensitivity: A Double-Blind Randomized Cross-Over Trial. Nutrients 2019, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.D.E.; Duncanson, K.; Jones, M.P.; Walker, M.M.; Keely, S.; Talley, N.J. Wheat Sensitivity and Functional Dyspepsia: A Pilot, Double-Blind, Randomized, Placebo-Controlled Dietary Crossover Trial with Novel Challenge Protocol. Nutrients 2020, 12, 1947. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, M.C.G.; Lawton, C.L.; Croden, F.; Smolinska, A.; Winkens, B.; Hesselink, M.A.M.; van Rooy, G.; Weegels, P.L.; Shewry, P.R.; Houghton, L.A.; et al. The effect of expectancy versus actual gluten intake on gastrointestinal and extra-intestinal symptoms in non-coeliac gluten sensitivity: A randomised, double-blind, placebo-controlled, international, multicentre study. Lancet Gastroenterol. Hepatol. 2024, 9, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Herfindal, A.M.; Nilsen, M.; Aspholm, T.E.; Schultz, G.I.G.; Valeur, J.; Rudi, K.; Thoresen, M.; Lundin, K.E.A.; Henriksen, C.; Bøhn, S.K. Effects of fructan and gluten on gut microbiota in individuals with self-reported non-celiac gluten/wheat sensitivity-a randomised controlled crossover trial. BMC Med. 2024, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, M.C.; Timmers, E.; Bonekamp, B.; van Rooy, G.; Witteman, B.J.; Shewry, P.R.; Lovegrove, A.; America, A.H.; Gilissen, L.J.; Keszthelyi, D.; et al. Two randomized crossover multicenter studies investigating gastrointestinal symptoms after bread consumption in individuals with noncoeliac wheat sensitivity: Do wheat species and fermentation type matter? Am. J. Clin. Nutr. 2024, 119, 896–907. [Google Scholar] [CrossRef]
- Zimmermann, J.; Longin, F.H.; Schweinlin, A.; Basrai, M.; Bischoff, S.C. No Difference in Tolerance between Wheat and Spelt Bread in Patients with Suspected Non-Celiac Wheat Sensitivity. Nutrients 2022, 14, 2800. [Google Scholar] [CrossRef] [PubMed]
- Gambino, C.M.; Agnello, L.; Vidali, M.; Lo Sasso, B.; Mansueto, P.; Seidita, A.; Giuliano, A.; Scazzone, C.; Massa, D.; Masucci, A.; et al. The role of Killer immunoglobulin-like receptors (KIRs) in the genetic susceptibility to non-celiac wheat sensitivity (NCWS). Clin. Chem. Lab. Med. 2024, 62, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Skodje, G.I.; Minelle, I.H.; Rolfsen, K.L.; Iacovou, M.; Lundin, K.E.A.; Veierød, M.B.; Henriksen, C. Dietary and symptom assessment in adults with self-reported non-coeliac gluten sensitivity. Clin. Nutr. ESPEN 2019, 31, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Bojarski, C.; Tangermann, P.; Barmeyer, C.; Buchkremer, J.; Kiesslich, R.; Ellrichmann, M.; Schreiber, S.; Schmidt, C.; Stallmach, A.; Roehle, R.; et al. Prospective, double-blind diagnostic multicentre study of confocal laser endomicroscopy for wheat sensitivity in patients with irritable bowel syndrome. Gut 2022, 71, 1567–1576. [Google Scholar] [CrossRef]
- Seidita, A.; Giuliano, A.; Soresi, M.; Chiavetta, M.; Nardi, E.; Mogavero, G.; Giannone, G.; Carroccio, A.; Mansueto, P. Fecal calprotectin levels in patients with non-celiac wheat sensitivity: A proof of concept. Intern. Emerg. Med. 2024, 19, 1255. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, P.; Seidita, A.; Soresi, M.; Giuliano, A.; Riccio, G.; Volta, U.; Caio, G.; La Blasca, F.; Disclafani, R.; De Giorgio, R.; et al. Anemia in non-celiac wheat sensitivity: Prevalence and associated clinical and laboratory features. Dig. Liver Dis. 2023, 55, 735–742. [Google Scholar] [CrossRef]
- Roncoroni, L.; Bascuñán, K.A.; Vecchi, M.; Doneda, L.; Bardella, M.T.; Lombardo, V.; Scricciolo, A.; Branchi, F.; Elli, L. Exposure to Different Amounts of Dietary Gluten in Patients with Non-Celiac Gluten Sensitivity (NCGS): An Exploratory Study. Nutrients 2019, 11, 136. [Google Scholar] [CrossRef]
- Cotton, C.; Raju, S.A.; Ahmed, H.; Webster, G.; Hallam, R.; Croall, I.; Coleman, S.; Trott, N.; Rej, A.; Shiha, M.G.; et al. Does a Gluten-Free Diet Improve Quality of Life and Sleep in Patients with Non-Coeliac Gluten/Wheat Sensitivity? Nutrients 2023, 15, 3461. [Google Scholar] [CrossRef]
- Barbaro, M.R.; Cremon, C.; Morselli-Labate, A.M.; Di Sabatino, A.; Giuffrida, P.; Corazza, G.R.; Di Stefano, M.; Caio, G.; Latella, G.; Ciacci, C.; et al. Serum zonulin and its diagnostic performance in non-coeliac gluten sensitivity. Gut 2020, 69, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Dixit, K.; Singh, A.; Agarwal, A.; Mehtab, W.; Prasad, S.; Rajput, M.S.; Chauhan, A.; Agarwal, A.; Mehta, S.; et al. Sieving out non-celiac gluten sensitivity amongst patients with irritable bowel syndrome. Dig. Liver Dis. 2024, 56, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Cobos-Quevedo, O.; Hernández, G.A.; Rivera-Gutiérrez, X.J.; Grube-Pagola, P.; Remes-Troche, J.M. Effect of a Gluten-Free Diet on Whole Gut Transit Time in Celiac Disease (CD) and Non-Celiac Gluten Sensitivity (NCGS) Patients: A Study Using the Wireless Motility Capsule (WMC). J. Clin. Med. 2024, 13, 1716. [Google Scholar] [CrossRef]
- Cha, R.R.; Kim, J.H.; Koo, H.S.; Jung, K.W.; Min, Y.W.; Choi, C.H.; Ryu, H.S.; Kwon, Y.H.; Cho, D.H.; Kwon, J.G.; et al. Self-reported Non-celiac Gluten Sensitivity in the Korean Population: Demographic and Clinical Characteristics. J. Neurogastroenterol. Motil. 2022, 28, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Trott, N.; Hoggard, N.; Sanders, D.S. Sensory Symptoms without Structural Pathology in Patients with Gluten Sensitivity. Nutrients 2024, 16, 1209. [Google Scholar] [CrossRef]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierød, M.B.; Henriksen, C.; Lundin, K.E.A. Fructan, Rather Than Gluten, Induces Symptoms in Patients with Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology 2018, 154, 529–539.e2. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.D.; Volta, U.; Salvatore, C.; Biancheri, P.; Caio, G.; Giorgio, R.D.; Stefano, M.D.; Corazza, G.R. Small Amounts of Gluten in Subjects with Suspected Nonceliac Gluten Sensitivity: A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1604–1612.e3. [Google Scholar] [CrossRef] [PubMed]
- Birkeland, E.; Gharagozlian, S.; Birkeland, K.I.; Valeur, J.; Måge, I.; Rud, I.; Aas, A.-M. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: A randomised controlled trial. Eur. J. Nutr. 2020, 59, 3325. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Castellucci, G.; Betti, C.; Fusaro, C.; Cavalletti, M.L.; Bertotto, A.; Spinozzi, F.; Morelli, A.; Pelli, M.A. Abnormal gastrointestinal motility in patients with celiac sprue. Dig. Dis. Sci. 1994, 39, 1947–1954. [Google Scholar] [CrossRef]
- Cucchiara, S.; Bassotti, G.; Castellucci, G.; Minella, R.; Betti, C.; Fusaro, C.; Morelli, A.; Bertotto, A.; Auricchio, S. Upper gastrointestinal motor abnormalities in children with active celiac disease. J. Pediatr. Gastroenterol. Nutr. 1995, 21, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sanchez, M.I.; Bercik, P.; Verdu, E.F. Motility alterations in celiac disease and non-celiac gluten sensitivity. Dig. Dis. 2015, 33, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Tomba, C.; Branchi, F.; Roncoroni, L.; Lombardo, V.; Bardella, M.T.; Ferretti, F.; Conte, D.; Valiante, F.; Fini, L.; et al. Evidence for the Presence of Non-Celiac Gluten Sensitivity in Patients with Functional Gastrointestinal Symptoms: Results from a Multicenter Randomized Double-Blind Placebo-Controlled Gluten Challenge. Nutrients 2016, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020, 9, 69. [Google Scholar] [CrossRef]
- Volta, U.; Tovoli, F.; Cicola, R.; Parisi, C.; Fabbri, A.; Piscaglia, M.; Fiorini, E.; Caio, G. Serological tests in gluten sensitivity (nonceliac gluten intolerance). J. Clin. Gastroenterol. 2012, 46, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Graziano, S.; Marando, S.; Prandi, B.; Boukid, F.; Marmiroli, N.; Francia, E.; Pecchioni, N.; Sforza, S.; Visioli, G.; Gullì, M. Technological Quality and Nutritional Value of Two Durum Wheat Varieties Depend on Both Genetic and Environmental Factors. J. Agric. Food Chem. 2019, 67, 2384–2395. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Cremon, C.; Bellini, M.; Corsetti, M.; Nardo, G.D.; Falangone, F.; Fuccio, L.; Galeazzi, F.; Iovino, P.; Sarnelli, G.; et al. Italian guidelines for the management of irritable bowel syndrome: Joint Consensus from the Italian Societies of: Gastroenterology and Endoscopy (SIGE), Neurogastroenterology and Motility (SINGEM), Hospital Gastroenterologists and Endoscopists (AIGO), Digestive Endoscopy (SIED), General Medicine (SIMG), Gastroenterology, Hepatology and Pediatric Nutrition (SIGENP) and Pediatrics (SIP). Dig. Liver Dis. 2023, 55, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Savarino, E.; Zingone, F.; Barberio, B.; Marasco, G.; Akyuz, F.; Akpinar, H.; Barboi, O.; Bodini, G.; Bor, S.; Chiarioni, G.; et al. Functional bowel disorders with diarrhoea: Clinical guidelines of the United European Gastroenterology and European Society for Neurogastroenterology and Motility. United Eur. Gastroenterol. J. 2022, 10, 556–584. [Google Scholar] [CrossRef]
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef]
- de Aquino Fernandes Dias, L.B.; Kobus, R.A.; do Nascimento, A.B. Effectiveness of the low-FODMAP diet in improving non-celiac gluten sensitivity: A systematic review. Br. J. Nutr. 2022, 129, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Carroccio, A. Suspected Nonceliac Gluten Sensitivity Confirmed in Few Patients After Gluten Challenge in Double-Blind, Placebo-Controlled Trials. Clin. Gastroenterol. Hepatol. 2017, 15, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Priven, M.; Baum, J.; Vieira, E.; Fung, T.; Herbold, N. The Influence of a Factitious Free-From Food Product Label on Consumer Perceptions of Healthfulness. J. Acad. Nutr. Diet. 2015, 115, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Golley, S.; Corsini, N.; Topping, D.; Morell, M.; Mohr, P. Motivations for avoiding wheat consumption in Australia: Results from a population survey. Public Health Nutr. 2015, 18, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Sabença, C.; Ribeiro, M.; de Sousa, T.; Poeta, P.; Bagulho, A.S.; Igrejas, G. Wheat/Gluten-Related Disorders and Gluten-Free Diet Misconceptions: A Review. Foods 2021, 10, 1765. [Google Scholar] [CrossRef] [PubMed]
- Dickey, W.; Kearney, N. Overweight in celiac disease: Prevalence, clinical characteristics, and effect of a gluten-free diet. Am. J. Gastroenterol. 2006, 101, 2356–2359. [Google Scholar] [CrossRef]
- Valletta, E.; Fornaro, M.; Cipolli, M.; Conte, S.; Bissolo, F.; Danchielli, C. Celiac disease and obesity: Need for nutritional follow-up after diagnosis. Eur. J. Clin. Nutr. 2010, 64, 1371–1372. [Google Scholar] [CrossRef] [PubMed]
- Melini, V.; Melini, F. Gluten-Free Diet: Gaps and Needs for a Healthier Diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef]
- Aljada, B.; Zohni, A.; El-Matary, W. The Gluten-Free Diet for Celiac Disease and Beyond. Nutrients 2021, 13, 3993. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, M.R.; Cremon, C.; Wrona, D.; Fuschi, D.; Marasco, G.; Stanghellini, V.; Barbara, G. Non-Celiac Gluten Sensitivity in the Context of Functional Gastrointestinal Disorders. Nutrients 2020, 12, 3735. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Törnblom, H.; Bengtsson, U.; Simrén, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Monsbakken, K.W.; Vandvik, P.O.; Farup, P.G. Perceived food intolerance in subjects with irritable bowel syndrome—Etiology, prevalence and consequences. Eur. J. Clin. Nutr. 2006, 60, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, W.; Schuppan, D.; Schink, M.; Schwappacher, R.; Wirtz, S.; Agaimy, A.; Neurath, M.F.; Zopf, Y. Influence of low FODMAP and gluten-free diets on disease activity and intestinal microbiota in patients with non-celiac gluten sensitivity. Clin. Nutr. 2019, 38, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sanchez, M.I.; Nardelli, A.; Borojevic, R.; Palma, G.D.; Calo, N.C.; McCarville, J.; Caminero, A.; Basra, D.; Mordhorst, A.; Ignatova, E.; et al. Gluten-Free Diet Reduces Symptoms, Particularly Diarrhea, in Patients with Irritable Bowel Syndrome and Antigliadin IgG. Clin. Gastroenterol. Hepatol. 2021, 19, 2343–2352.e8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).