A High-Fat Diet Induces Epigenetic 1-Carbon Metabolism, Homocystinuria, and Renal-Dependent HFpEF
Abstract
1. Introduction
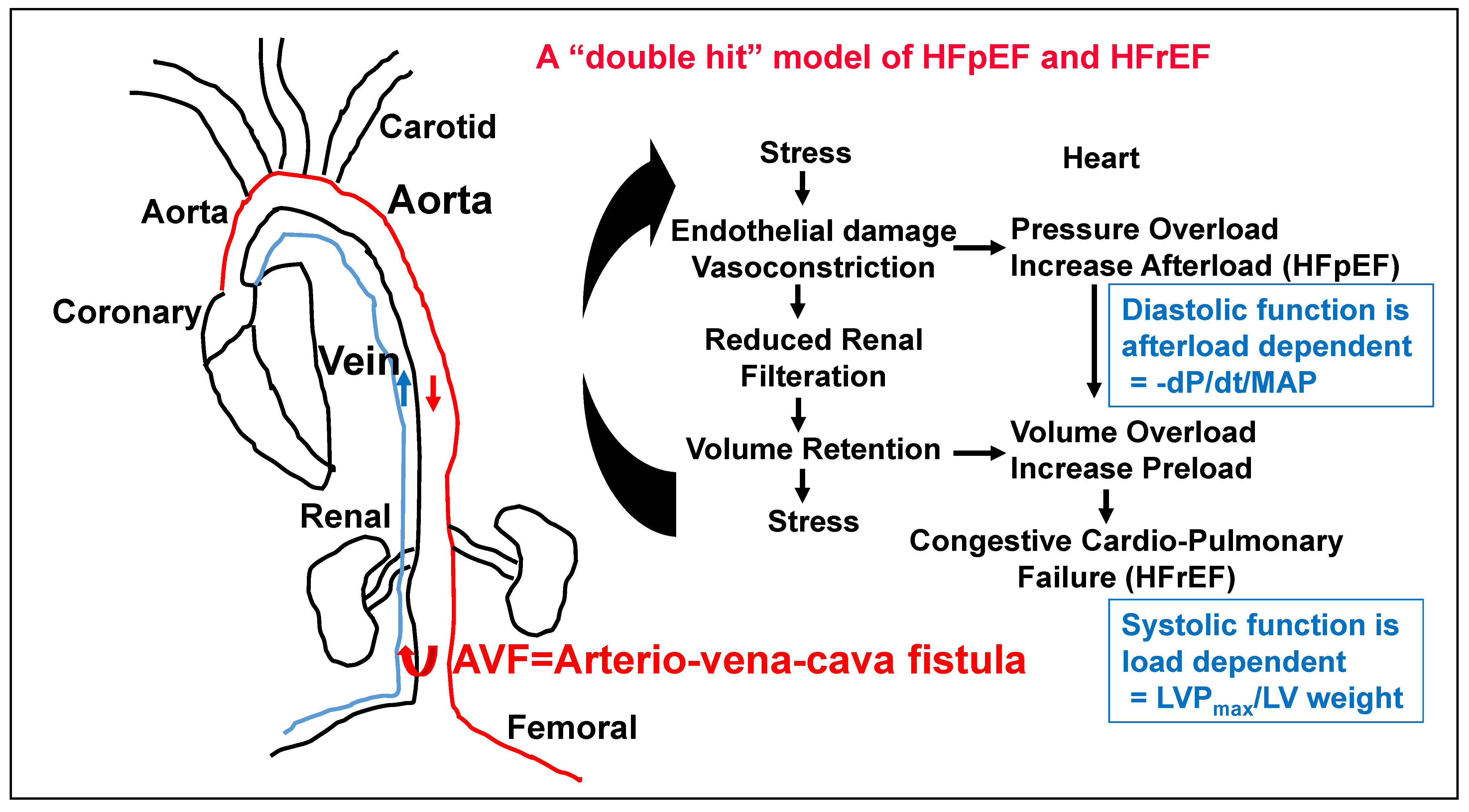
2. The Role of Kidney in HFpEF
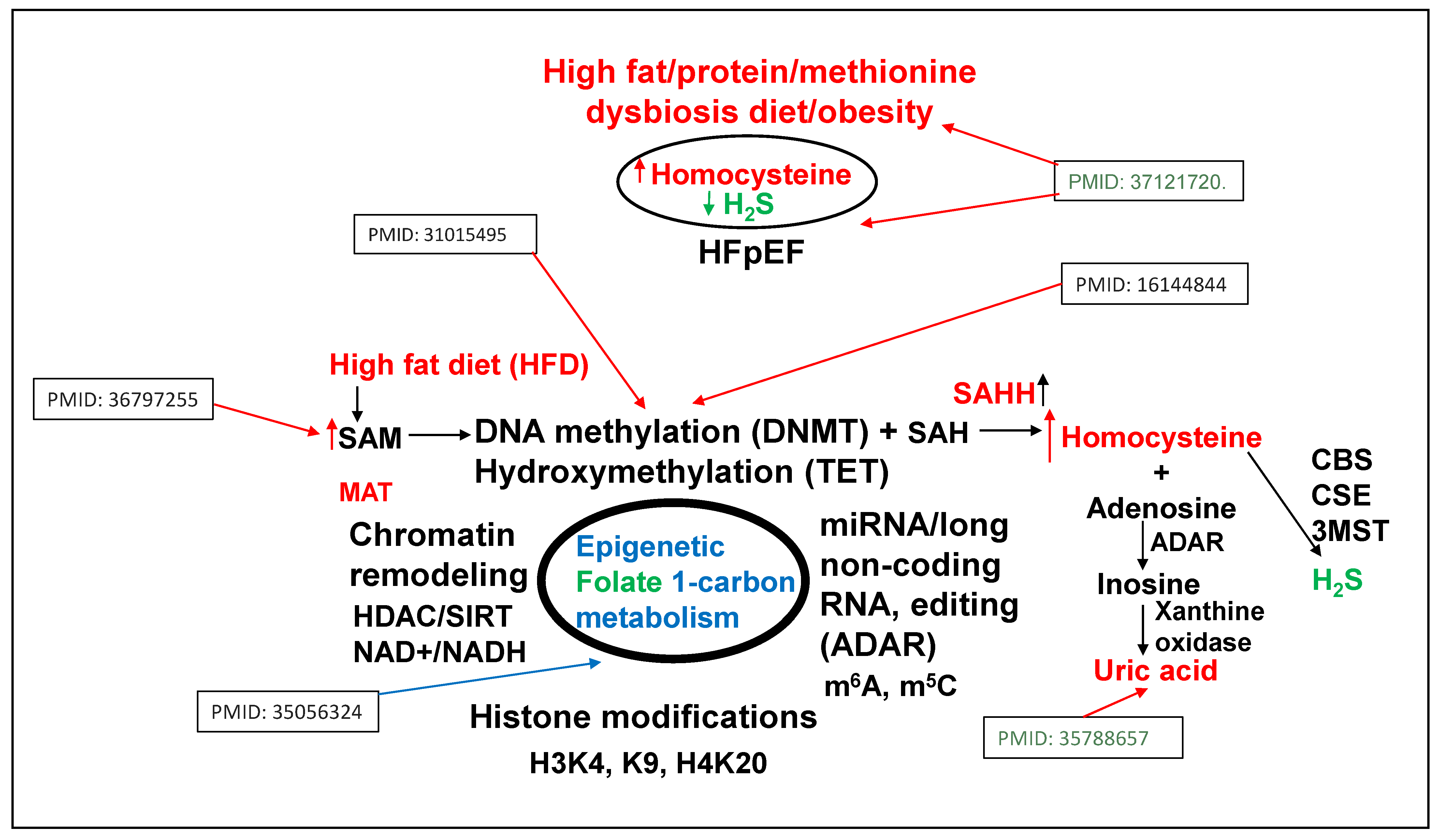
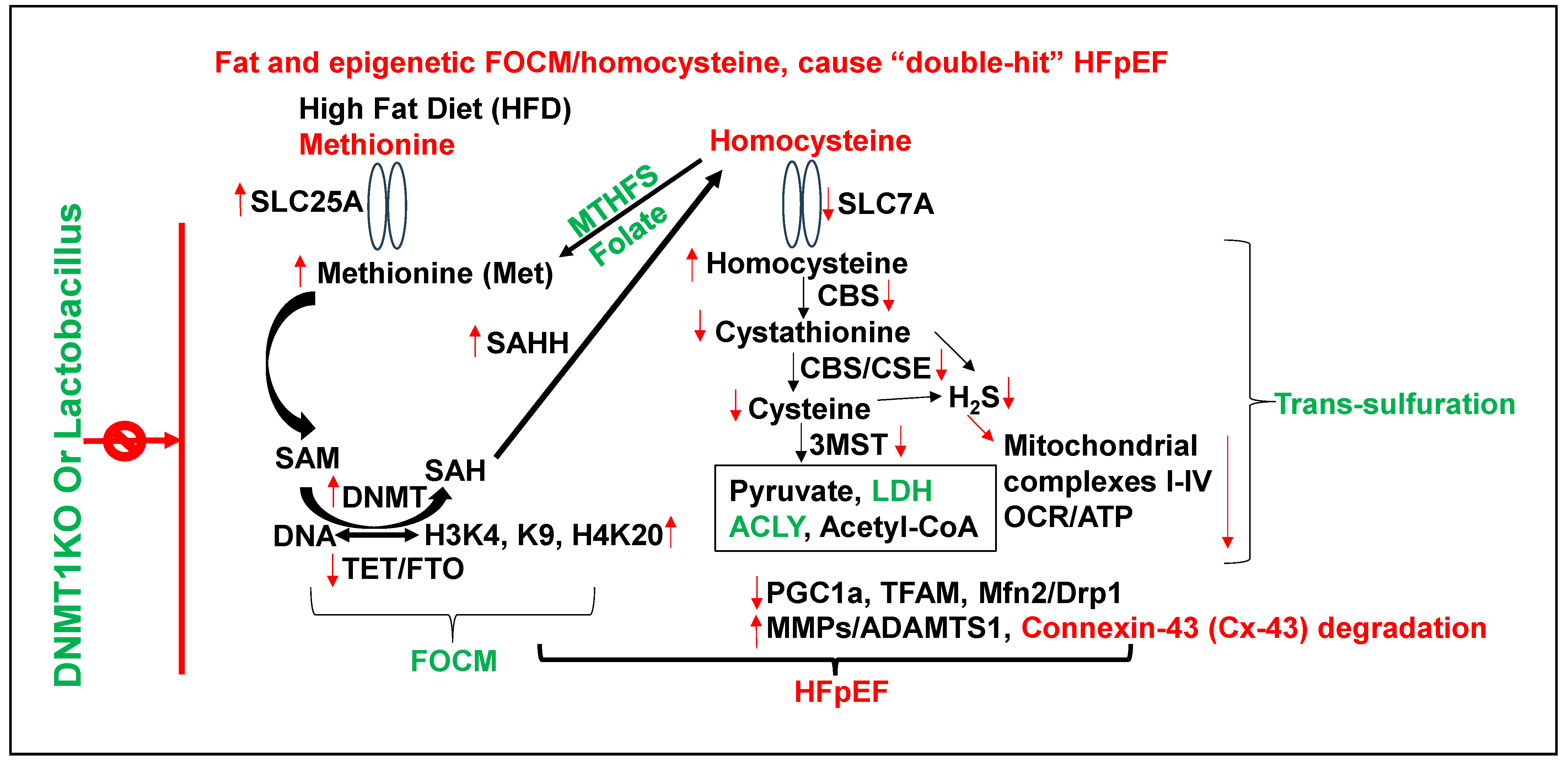
3. Epigenetics of HFpEF
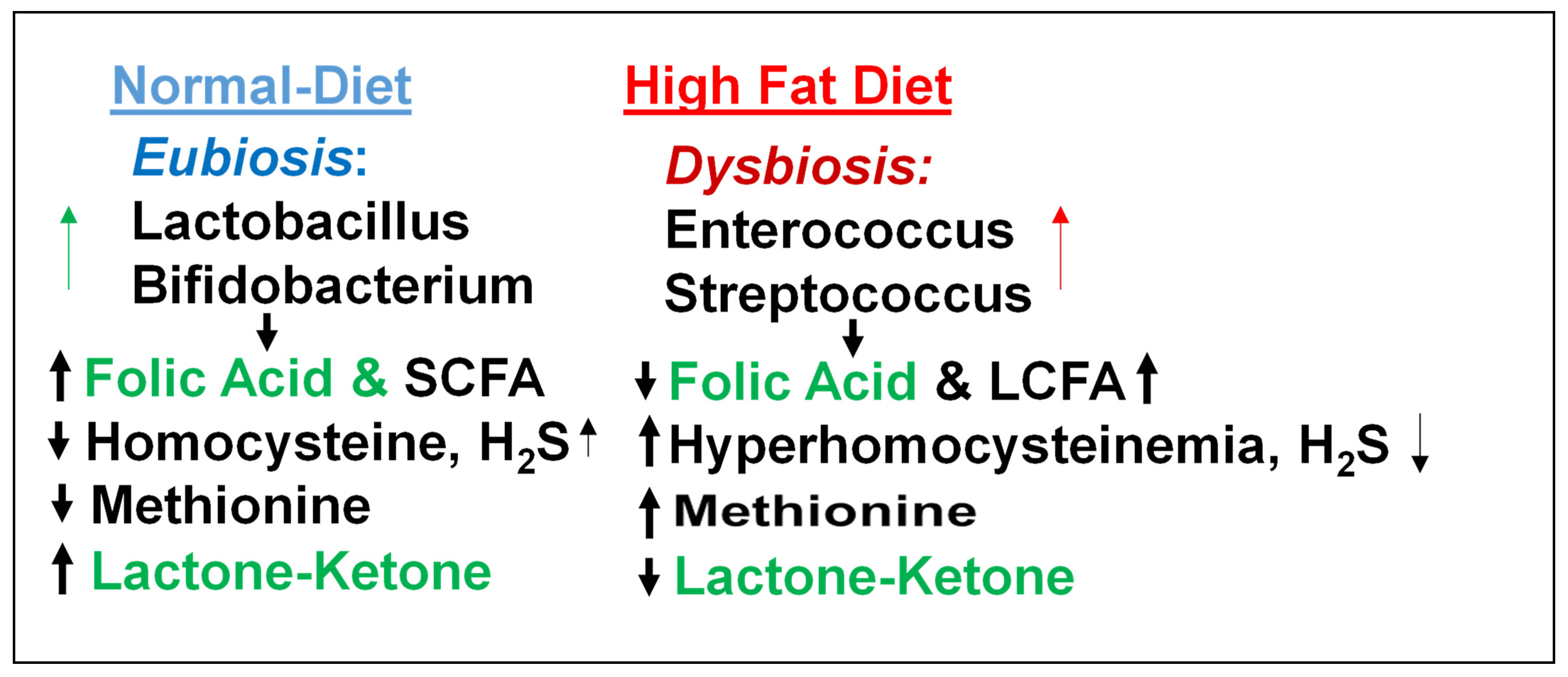
4. Homocysteine and Lipid Connection
5. Viewpoint on the Role of Epigenetics and Future Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veeranki, S.; Tyagi, S.C. Dysbiosis and Disease: Many Unknown Ends, Is It Time to Formulate Guidelines for Dysbiosis Research? J. Cell. Physiol. 2017, 232, 2929–2930. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Hardin, S.J.; George, A.K.; Eyob, W.; Stanisic, D.; Pushpakumar, S.; Tyagi, S.C. Epigenetics, 1-Carbon Metabolism, and Homocysteine During Dysbiosis. Front. Physiol. 2020, 11, 617953. [Google Scholar] [CrossRef] [PubMed]
- George, A.K.; Singh, M.; Pushpakumar, S.; Homme, R.P.; Hardin, S.J.; Tyagi, S.C. Dysbiotic 1-carbon metabolism in cardiac muscle remodeling. J. Cell. Physiol. 2020, 235, 2590–2598. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Pushpakumar, S.; Zheng, Y.; Homme, R.P.; Smolenkova, I.; Mokshagundam, S.P.L.; Tyagi, S.C. Hydrogen sulfide mitigates skeletal muscle mitophagy-led tissue remodeling via epigenetic regulation of the gene writer and eraser function. Physiol. Rep. 2022, 10, e15422. [Google Scholar] [CrossRef] [PubMed]
- Meddeb, M.; Koleini, N.; Jun, S.; Keykhaei, M.; Farshidfar, F.; Zhao, L.; Kwon, S.; Lin, B.; Keceli, G.; Paolocci, N.; et al. ATP citrate lyase supports cardiac function and NAD+/NADH balance and os depressed in human heart failure. bioRxiv 2024. [Google Scholar] [CrossRef]
- Liu, S.; Gammon, S.T.; Tan, L.; Karlstaedt, A. ATP-dependent citrate lyase drives LV dysfunction by metabolic remodeling of the heart. bioRxiv 2024. [Google Scholar] [CrossRef]
- Arnold, P.K.; Finley, L.W. Regulation and function of the mammalian tricarboxylic acid cycle. J. Biol. Chem. 2023, 299, 102838. [Google Scholar] [CrossRef]
- Greco, C.M.; Cervantes, M.; Fustin, J.-M.; Ito, K.; Ceglia, N.; Samad, M.; Shi, J.; Koronowski, K.B.; Forne, I.; Ranjit, S.; et al. S-adenosyl-l-homocysteine hydrolase links methionine metabolism to the circadian clock and chromatin remodeling. Sci. Adv. 2020, 6, eabc5629. [Google Scholar] [CrossRef]
- da Silva, L.D.; de Oliveira, Y.; de Souza, E.L.; de Luna Freire, M.O.; de Andrade Braga, V.; Magnani, M.; de Brito Alves, J.L. Effects of probiotic therapy on cardio-metabolic parameters and autonomic modulation in hypertensive women: A randomized, triple-blind, placebo-controlled trial. Food Funct. 2020, 11, 7152–7163. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Stražar, M.; Mohamed, A.M.; Pacheco, J.A.; Walker, R.L.; Lebar, T.; Zhao, S.; Lockart, J.; Dame, A.; Thurimella, K.; et al. Gut microbiome and metabolome profiling in Framingham heart study reveals cholesterol-metabolizing bacteria. Cell 2024, 187, 1834–1852.e19. [Google Scholar] [CrossRef]
- Pushpakumar, S.; Singh, M.; Zheng, Y.; Akinterinwa, O.E.; Mokshagundam, S.P.L.; Sen, U.; Kalra, D.K.; Tyagi, S.C. Renal Denervation Helps Preserve the Ejection Fraction by Preserving Endocardial-Endothelial Function during Heart Failure. Int. J. Mol. Sci. 2023, 24, 7302. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Sehly, A.; Lan, N.S.R.; Abraham, A.; Dwivedi, G. Arteriovenous fistula is associated with diastolic dysfunction in end-stage renal failure patients. Clin. Exp. Nephrol. 2023, 27, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R.; Tyagi, S.C. Impaired Folate-Mediated One-Carbon Metabolism in Type 2 Diabetes, Late-Onset Alzheimer’s Disease and Long COVID. Medicina 2021, 58, 16. [Google Scholar] [CrossRef] [PubMed]
- Katsouda, A.; Szabo, C.; Papapetropoulos, A. Reduced adipose tissue H2S in obesity. Pharmacol. Res. 2018, 128, 190–199. [Google Scholar] [CrossRef]
- Han, W.; Li, M.; Yang, M.; Chen, S.; Lu, Y.; Tang, T.; Wang, R.; Zhang, C.; Qi, K. Dietary Folic Acid Supplementation Inhibits High Fat Diet Induced Body Weight Gain through Gut Microbiota-Associated Branched-Chain Amino Acids and Mitochondria in Mice. J. Nutr. Sci. Vitaminol. 2023, 69, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Rajabian, N.; Ikhapoh, I.; Shahini, S.; Choudhury, D.; Thiyagarajan, R.; Shahini, A.; Kulczyk, J.; Breed, K.; Saha, S.; Mohamed, M.A.; et al. Methionine adenosyltransferase2A inhibition restores metabolism to improve regenerative capacity and strength of aged skeletal muscle. Nat. Commun. 2023, 14, 886. [Google Scholar] [CrossRef]
- Sendžikaitė, G.; Hanna, C.W.; Stewart-Morgan, K.R.; Ivanova, E.; Kelsey, G. A DNMT3A PWWP mutation leads to methylation of bivalent chromatin and growth retardation in mice. Nat. Commun. 2019, 10, 1884. [Google Scholar] [CrossRef]
- Doehner, W.; Anker, S.D.; Butler, J.; Zannad, F.; Filippatos, G.; Ferreira, J.P.; Salsali, A.; Kaempfer, C.; Brueckmann, M.; Pocock, S.J.; et al. Uric acid and sodium-glucose cotransporter-2 inhibition with empagliflozin in heart failure with reduced ejection fraction (HFrEF): The EMPEROR-reduced trial. Eur. Heart J. 2022, 43, 3435–3446. [Google Scholar] [CrossRef]
- Ijiri, K.; Zerbini, L.F.; Peng, H.; Correa, R.G.; Lu, B.; Walsh, N.; Zhao, Y.; Taniguchi, N.; Huang, X.-L.; Otu, H.; et al. A novel role for growth arrest DNA damage protein 45 (GADD45beta) as a mediator of MMP-13 gene expression. JBC 2005, 280, 38544–38555. [Google Scholar] [CrossRef]
- Saleem, T.H.; Algowhary, M.; Kamel, F.E.M.; I El-Mahdy, R. Plasma amino acid metabolomic pattern in heart failure patients with either preserved or reduced ejection fraction: The relation to established risk variables and prognosis. Biomed. Chromatogr. 2021, 35, e5012. [Google Scholar] [CrossRef]
- Liu, B.; Ma, S.; Wang, T.; Zhao, C.; Li, Y.; Yin, J.; Liu, C.; Gao, C.; Sun, L.; Yue, W.; et al. A novel rat model of heart failure induced by high methionine diet showing evidence of association between hyperhomocysteinemia and activation of NF-kappaB. Am. J. Transl. Res. 2016, 8, 117–124. [Google Scholar] [PubMed]
- Joseph, J.; Giczewska, A.; Alhanti, B.; Cheema, A.K.; Handy, D.E.; Mann, D.L.; Loscalzo, J.; Givertz, M.M. Associations of methyl donor and methylation inhibitor levels during anti-oxidant therapy in heart failure. J. Physiol. Biochem. 2021, 77, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Service, N.H. Meat in your diet, (2021) & Tyagi SC. Clin. Exper Hypertens. 1999, 21, 181–198. [Google Scholar]
- Albano, C.; Silvetti, T.; Brasca, M. Screening of lactic acid bacteria producing folate and their potential use as adjunct cultures for cheese bio-enrichment. FEMS Microbiol. Lett. 2020, 367, fnaa059. [Google Scholar] [CrossRef]
- Guerrero-Encinas, I.; González-González, J.N.; Santiago-López, L.; Muhlia-Almazán, A.; Garcia, H.S.; Mazorra-Manzano, M.A.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Protective Effect of Lacticaseibacillus casei CRL 431 Postbiotics on Mitochondrial Function and Oxidative Status in Rats with Aflatoxin B(1)-Induced Oxidative Stress. Probiotics Antimicrob. Proteins 2021, 13, 1033–1043. [Google Scholar] [CrossRef]
- Gu, Y.; Xiao, X.; Pan, R.; Zhang, J.; Zhao, Y.; Dong, Y.; Cui, H. Lactobacillus plantarum dy-1 fermented barley extraction activates white adipocyte browning in high-fat diet-induced obese rats. J. Food Biochem. 2021, 45, e13680. [Google Scholar] [CrossRef]
- Hong, T.; Li, J.; Guo, L.; Cavalier, M.; Wang, T.; Dou, Y.; DeLaFuente, A.; Fang, S.; Guzman, A.; Wohlan, K.; et al. TET2 modulates spatial relocalization of heterochromatin in aged hematopoietic stem and progenitor cells. Nat. Aging 2023, 3, 1387–1400. [Google Scholar] [CrossRef]
- Singh, M.; George, A.K.; Homme, R.P.; Majumder, A.; Laha, A.; Sandhu, H.S.; Tyagi, S.C. Circular RNAs profiling in the cystathionine-β-synthase mutant mouse reveals novel gene targets for hyperhomocysteinemia induced ocular disorders. Exp. Eye Res. 2018, 174, 80–92. [Google Scholar] [CrossRef]
- Aubert, G.; Martin, O.J.; Horton, J.L.; Lai, L.; Vega, R.B.; Leone, T.C.; Koves, T.; Gardell, S.J.; Krüger, M.; Hoppel, C.L.; et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation 2016, 133, 698–705. [Google Scholar] [CrossRef]
- Panigrahi, P.; Parida, S.; Nanda, N.C.; Satpathy, R.; Pradhan, L.; Chandel, D.S.; Baccaglini, L.; Mohapatra, A.; Mohapatra, S.S.; Misra, P.R.; et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 2017, 548, 407–412. [Google Scholar] [CrossRef]
- Brooks, G.A.; Osmond, A.D.; Arevalo, J.A.; Duong, J.J.; Curl, C.C.; Moreno-Santillan, D.D.; Leija, R.G. Lactate as a myokine and exerkine: Drivers and signals of physiology and metabolism. J. Appl. Physiol. 2023, 134, 529–548. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.R.; Puchalska, P.; Crawford, P.A.; Kelly, D.P. Ketones and the Heart: Metabolic Principles and Therapeutic Implications. Circ. Res. 2023, 132, 882–898. [Google Scholar] [CrossRef] [PubMed]
- Balikcioglu, P.G.; Trub, C.J.; Balikcioglu, M.; Ilkayeva, O.; White, P.J.; Muehlbauer, M.; Bain, J.R.; Armstrong, S.; Freemark, M. Branched-chain α-keto acids and glutamate/glutamine: Biomarkers of insulin resistance in childhood obesity. Endocrinol. Diabetes Metab. 2023, 6, e388. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.-N.; Kim, H.-R.; Jang, K.-A.; Hwang, Y.-J.; Kim, J.-S. Anti-obesity effects of yuja (Citrus junos Sieb ex Tanaka) pomace extract fermented with lactic acid bacteria on the hepatocytes and epididymal fat tissue of rats. Appl. Microsc. 2023, 53, 7. [Google Scholar] [CrossRef]
- Schaevitz, L.; Berger-Sweeney, J.; Ricceri, L. One-carbon metabolism in neurodevelopmental disorders: Using broad-based nutraceutics to treat cognitive deficits in complex spectrum disorders. Neurosci. Biobehav. Rev. 2014, 46 Pt 2, 270–284. [Google Scholar] [CrossRef]
- Tóth, M.E.; Sárközy, M.; Szűcs, G.; Dukay, B.; Hajdu, P.; Zvara, Á.; Puskás, L.G.; Szebeni, G.J.; Ruppert, Z.; Csonka, C.; et al. Exercise training worsens cardiac performance in males but does not change ejection fraction and improves hypertrophy in females in a mouse model of metabolic syndrome. Biol. Sex Differ. 2022, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Entcheva, E.; Kay, M.W. Cardiac optogenetics: A decade of enlightenment. Nat. Rev. Cardiol. 2021, 18, 349–367. [Google Scholar] [CrossRef]
- Zasadny, F.M.; Dyavanapalli, J.; Dowling, N.M.; Mendelowitz, D.; Kay, M.W. Cholinergic stimulation improves electrophysiological rate adaptation during pressure overload-induced heart failure in rats. Am. J. Physiol. Circ. Physiol. 2020, 319, H1358–H1368. [Google Scholar] [CrossRef]
- Whitehead, A.J.; Engler, A.J. Regenerative cross talk between cardiac cells and macrophages. Am. J. Physiol. Circ. Physiol. 2021, 320, H2211–H2221. [Google Scholar] [CrossRef]
- Yeoman, B.; Shatkin, G.; Beri, P.; Banisadr, A.; Katira, P.; Engler, A.J. Adhesion strength and contractility enable metastatic cells to become adurotactic. Cell Rep. 2021, 34, 108816. [Google Scholar] [CrossRef]
- Banisadr, A.; Eick, M.; Beri, P.; Parisian, A.D.; Yeoman, B.; Placone, J.K.; Engler, A.J.; Furnari, F. EGFRvIII uses intrinsic and extrinsic mechanisms to reduce glioma adhesion and increase migration. J. Cell Sci. 2020, 133, jcs247189. [Google Scholar] [CrossRef] [PubMed]
- Dees, C.; Pötter, S.; Zhang, Y.; Bergmann, C.; Zhou, X.; Luber, M.; Wohlfahrt, T.; Karouzakis, E.; Ramming, A.; Gelse, K.; et al. TGF-β–induced epigenetic deregulation of SOCS3 facilitates STAT3 signaling to promote fibrosis. J. Clin. Investig. 2020, 130, 2347–2363. [Google Scholar] [CrossRef]
- Kakoki, M.; Ramanathan, P.V.; Hagaman, J.R.; Grant, R.; Wilder, J.C.; Taylor, J.M.; Jennette, J.C.; Smithies, O.; Maeda-Smithies, N. Cyanocobalamin prevents cardiomyopathy in type 1 diabetes by modulating oxidative stress and DNMT-SOCS1/3-IGF-1 signaling. Commun. Biol. 2021, 4, 775. [Google Scholar] [CrossRef]
- Kanai, S.M.; Edwards, A.J.; Rurik, J.G.; Osei-Owusu, P.; Blumer, K.J. Proteolytic degradation of regulator of G protein signaling 2 facilitates temporal regulation of G(q/11) signaling and vascular contraction. J. Biol. Chem. 2017, 292, 19266–19278. [Google Scholar] [CrossRef]
- Felisbino, M.B.; McKinsey, T.A. Epigenetics in Cardiac Fibrosis. JACC Basic Transl. Sci. 2018, 3, 704–715. [Google Scholar] [CrossRef]
- Trial, J.; Cieslik, K.A. Changes in cardiac resident fibroblast physiology and phenotype in aging. Am. J. Physiol. Circ. Physiol. 2018, 315, H745–H755. [Google Scholar] [CrossRef]
- Stratton, M.S.; Bagchi, R.A.; Felisbino, M.B.; Hirsch, R.A.; Smith, H.E.; Riching, A.S.; Enyart, B.Y.; Koch, K.A.; Cavasin, M.A.; Alexanian, M.; et al. Dynamic Chromatin Targeting of BRD4 Stimulates Cardiac Fibroblast Activation. Circ. Res. 2019, 125, 662–677. [Google Scholar] [CrossRef]
- Tyagi, S.C.; Sood, H.S.; Hunt, M.J.; Mujumdar, V.S.; Tummalapalli, C.M.; Aru, G.M. Homocysteine redox receptor and regulation of extracellular matrix components in vascular cells. Am. J. Physiol. Physiol. 1998, 274, C396–C405. [Google Scholar] [CrossRef]
- Sen, U.; Moshal, K.S.; Tyagi, N.; Kartha, G.K.; Tyagi, S.C. Homocysteine-induced myofibroblast differentiation in mouse aortic endothelial cells. J. Cell. Physiol. 2006, 209, 767–774. [Google Scholar] [CrossRef]
- Hunt, M.J.; Aru, G.M.; Hayden, M.R.; Moore, C.K.; Hoit, B.D.; Tyagi, S.C. Induction of oxidative stress and disintegrin metalloproteinase in human heart end-stage failure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L239–L245. [Google Scholar] [CrossRef] [PubMed]
- Miro-Casas, E.; Ruiz-Meana, M.; Agullo, E.; Stahlhofen, S.; Rodríguez-Sinovas, A.; Cabestrero, A.; Jorge, I.; Torre, I.; Vazquez, J.; Boengler, K.; et al. Connexin43 in cardiomyocyte mitochondria contributes to mitochondrial potassium uptake. Cardiovasc. Res. 2009, 83, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Ruiz-Meana, M.; Gent, S.; Ungefug, E.; Soetkamp, D.; Miro-Casas, E.; Cabestrero, A.; Fernandez-Sanz, C.; Semenzato, M.; Di Lisa, F.; et al. Mitochondrial connexin 43 impacts on respiratory complex I activity and mitochondrial oxygen consumption. J. Cell. Mol. Med. 2012, 16, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- McCully, H. Pliny’s Pheromonic Abortifacients. Science 1969, 165, 236–237. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.S. Vascular pathology of homocysteinemia: Implications for the pathogenesis of arteriosclerosis. Am. J. Pathol. 1969, 56, 111–128. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyagi, S.C. A High-Fat Diet Induces Epigenetic 1-Carbon Metabolism, Homocystinuria, and Renal-Dependent HFpEF. Nutrients 2025, 17, 216. https://doi.org/10.3390/nu17020216
Tyagi SC. A High-Fat Diet Induces Epigenetic 1-Carbon Metabolism, Homocystinuria, and Renal-Dependent HFpEF. Nutrients. 2025; 17(2):216. https://doi.org/10.3390/nu17020216
Chicago/Turabian StyleTyagi, Suresh C. 2025. "A High-Fat Diet Induces Epigenetic 1-Carbon Metabolism, Homocystinuria, and Renal-Dependent HFpEF" Nutrients 17, no. 2: 216. https://doi.org/10.3390/nu17020216
APA StyleTyagi, S. C. (2025). A High-Fat Diet Induces Epigenetic 1-Carbon Metabolism, Homocystinuria, and Renal-Dependent HFpEF. Nutrients, 17(2), 216. https://doi.org/10.3390/nu17020216




