Effects of Food Preferences and Supplement Intake During Pregnancy on the Cleft Lip and Palate Incidence: The Japan Environment and Children’s Study
Abstract
1. Introduction
2. Materials and Methods
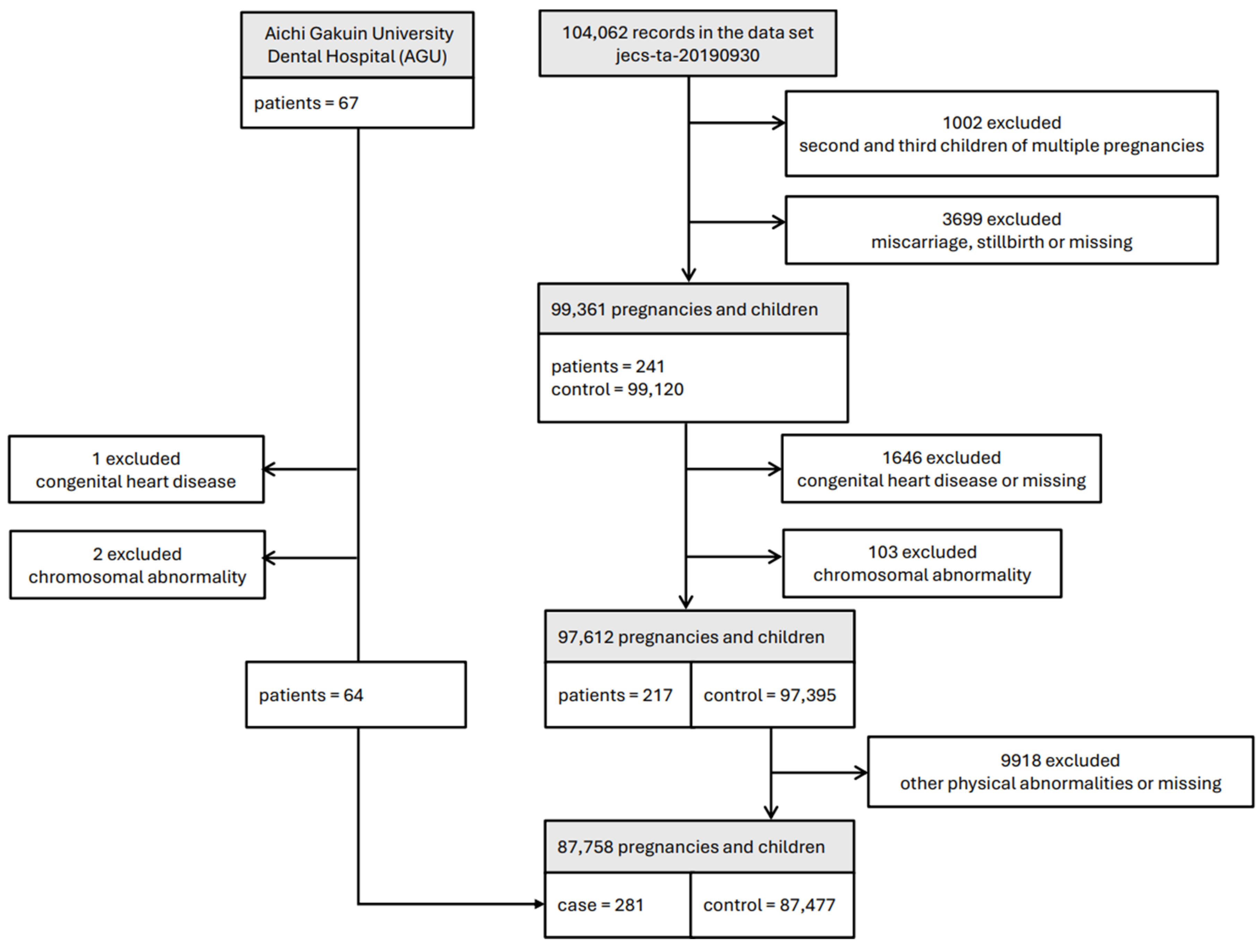
2.1. Study Design and Participants
2.2. Variables
2.3. Statistical Analysis
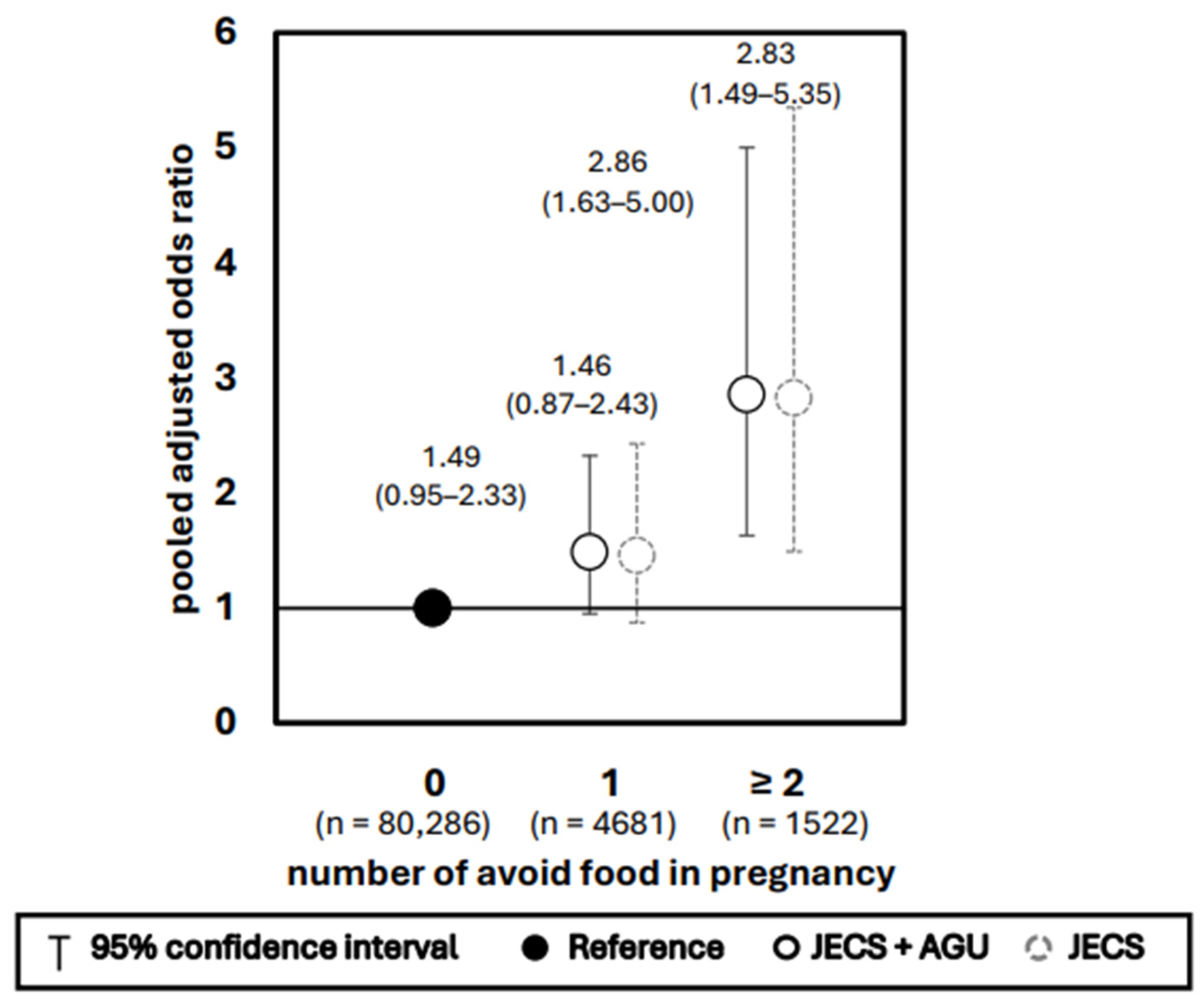
3. Results
4. Discussion
4.1. Effects of Avoiding Specific Foods
4.2. Effects of Taking Supplements
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoshida, S.; Takeuchi, M.; Kawakami, C.; Kawakami, K.; Ito, S. Maternal multivitamin intake and orofacial clefts in offspring: Japan Environment and Children’s Study (JECS) cohort study. BMJ Open 2020, 10, e035817. [Google Scholar] [CrossRef]
- Molina-Solana, R.; Yáñez-Vico, R.M.; Iglesias-Linares, A.; Mendoza-Mendoza, A.; Solano-Reina, E. Current concepts on the effect of environmental factors on cleft lip and palate. Int. J. Oral Maxillofac. Surg. 2013, 42, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Hozyasz, K.K.; Kaczmarczyk, M.; Dudzik, J.; Bulska, E.; Dudkiewicz, Z.; Szymanski, M. Relation between the concentration of zinc in maternal whole blood and the risk of an infant being born with an orofacial cleft. Br. J. Oral Maxillofac. Surg. 2009, 47, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Mossey, P.A.; Little, J.; Munger, R.G.; Dixon, M.J.; Shaw, W.C. Cleft lip and palate. Lancet 2009, 374, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.A.; Reynolds, K.; Zhou, C.J. Environmental mechanisms of orofacial clefts. Birth Defects Res. A Clin. Mol. Teratol. 2020, 112, 1660–1698. [Google Scholar] [CrossRef]
- Jamilian, A.; Sarkarat, F.; Jafari, M.; Neshandar, M.; Amini, E.; Khosravi, S.; Ghassemi, A. Family history and risk factors for cleft lip and palate patients and their associated anomalies. Stomatologija 2017, 19, 78–83. [Google Scholar]
- Li, H.; Luo, M.; Luo, J.; Zheng, J.; Zeng, R.; Du, Q.; Fang, J.; Ouyang, N. A discriminant analysis prediction model of non-syndromic cleft lip with or without cleft palate based on risk factors. BMC Pregnancy Childbirth 2016, 16, 368. [Google Scholar] [CrossRef]
- McKinney, C.M.; Pisek, A.; Chowchuen, B.; DeRouen, T.; Muktabhant, B.; Pradubwong, S.; Yeung, C.; Pitiphat, W. Case-control study of nutritional and environmental factors and the risk of oral clefts in Thailand. Birth Defects Res. A Clin. Mol. Teratol. 2016, 106, 624–632. [Google Scholar] [CrossRef]
- Interrante, J.D.; Ailes, E.C.; Lind, J.N.; Anderka, M.; Feldkamp, M.L.; Werler, M.M.; Taylor, L.G.; Trinidad, J.; Gilboa, S.M.; Broussard, C.S. Risk comparison for prenatal use of analgesics and selected birth defects, National Birth Defects Prevention Study 1997–2011. Ann. Epidemiol. 2017, 27, 645–653.e2. [Google Scholar] [CrossRef]
- Krapels, I.P.; van Rooij, I.A.; Ocké, M.C.; West, C.E.; van der Horst, C.M.; Steegers-Theunissen, R.P. Maternal nutritional status and the risk for orofacial cleft offspring in humans. J. Nutr. 2004, 134, 3106–3113. [Google Scholar] [CrossRef]
- Natsume, N.; Kawai, T.; Suzuki, T. Preference for dairy products and manifestation of cleft lip and/or palate. Plast. Reconstr. Surg. 1996, 98, 900–901. [Google Scholar] [CrossRef]
- Weyer, P.; Rhoads, A.; Suhl, J.; Luben, T.J.; Conway, K.M.; Langlois, P.H.; Shen, D.; Liang, D.; Puzhankara, S.; Anderka, M.; et al. Drinking water disinfection byproducts and risk of orofacial clefts in the National Birth Defects Prevention Study. Birth Defects Res. A Clin. Mol. Teratol. 2018, 110, 1027–1042. [Google Scholar] [CrossRef]
- Neogi, S.B.; Singh, S.; Pallepogula, D.R.; Pant, H.; Kolli, S.R.; Bharti, P.; Datta, V.; Gosla, S.R.; Bonanthaya, K.; Ness, A.; et al. Risk factors for orofacial clefts in India: A case-control study. Birth Defects Res. A Clin. Mol. Teratol. 2017, 109, 1284–1291. [Google Scholar] [CrossRef]
- Ni, W.; Yang, W.; Yu, J.; Li, Z.; Jin, L.; Liu, J.; Zhang, Y.; Wang, L.; Ren, A. Association between selected essential trace element concentrations in umbilical cord and risk for cleft lip with or without cleft palate: A case-control study. Sci. Total Environ. 2019, 661, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Wang, C.; Wei, J.; Jin, L.; Liu, J.; Wang, L.; Li, Z.; Yin, C.; Ren, A. Selected essential trace elements in maternal serum and risk for fetal orofacial clefts. Sci. Total Environ. 2020, 712, 136542. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.M.; Carmichael, S.L.; Laurent, C.; Rasmussen, S.A. Maternal nutrient intakes and risk of orofacial clefts. Epidemiology 2006, 17, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, I.A.; Ocké, M.C.; Straatman, H.; Zielhuis, G.A.; Merkus, H.M.; Steegers-Theunissen, R.P. Periconceptional folate intake by supplement and food reduces the risk of nonsyndromic cleft lip with or without cleft palate. Prev. Med. 2004, 39, 689–694. [Google Scholar] [CrossRef]
- Johansen, A.M.; Lie, R.T.; Wilcox, A.J.; Andersen, L.F.; Drevon, C.A. Maternal dietary intake of vitamin A and risk of orofacial clefts: A population-based case-control study in Norway. Am. J. Epidemiol. 2008, 167, 1164–1170. [Google Scholar] [CrossRef]
- Zhou, Y.; Sinnathamby, V.; Yu, Y.; Sikora, L.; Johnson, C.Y.; Mossey, P.; Little, J. Folate intake, markers of folate status and oral clefts: An updated set of systematic reviews and meta-analyses. Birth Defects Res. A Clin. Mol. Teratol. 2020, 112, 1699–1719. [Google Scholar] [CrossRef]
- Nishigori, H.; Obara, T.; Nishigori, T.; Ishikuro, M.; Sakurai, K.; Hoshiai, T.; Saito, M.; Fujiwara, I.; Arima, T.; Nakai, K.; et al. Preconception folic acid supplementation use and the occurrence of neural tube defects in Japan: A nationwide birth cohort study of the Japan Environment and Children’s Study. Congenit. Anom. 2019, 59, 110–117. [Google Scholar] [CrossRef]
- Eshak, E.S.; Okada, C.; Kimura, T.; Baba, S.; Ikehara, S.; Iso, H. For The JECS Group Low Periconceptional Dietary Intakes among Japanese Women: The Japan Environment and Children’s Study (JECS). J. Nutr. Sci. Vitaminol. 2022, 68, 260–269. [Google Scholar] [CrossRef]
- Hovdenak, N.; Haram, K. Influence of mineral and vitamin supplements on pregnancy outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 164, 127–132. [Google Scholar] [CrossRef]
- Ishitsuka, K.; Nakayama, S.F.; Kishi, R.; Mori, C.; Yamagata, Z.; Ohya, Y.; Kawamoto, T.; Kamijima, M. Japan Environment and Children’s Study: Backgrounds, activities, and future directions in global perspectives. Environ. Health Prev. Med. 2017, 22, 61. [Google Scholar] [CrossRef]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Yamazaki, S.; Isobe, T.; Tamura, K.; Suda, E.; Ono, M.; Yonemoto, J.; Iwai-Shimada, M.; et al. Baseline Profile of Participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2018, 28, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Eshete, M.; Butali, A.; Abate, F.; Hailu, T.; Hailu, A.; Degu, S.; Demissie, Y.; Gravem, P.E.; Derbew, M.; Mossey, P.; et al. The Role of Environmental Factors in the Etiology of Nonsyndromic Orofacial Clefts. J. Craniofac Surg. 2020, 31, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Munger, R.G.; Tamura, T.; Johnston, K.E.; Feldkamp, M.L.; Pfister, R.; Carey, J.C. Plasma zinc concentrations of mothers and the risk of oral clefts in their children in Utah. Birth Defects Res. A Clin. Mol. Teratol. 2009, 85, 151–155. [Google Scholar] [CrossRef]
- Jahanbin, A.; Shadkam, E.; Miri, H.H.; Shirazi, A.S.; Abtahi, M. Maternal Folic Acid Supplementation and the Risk of Oral Clefts in Offspring. J. Craniofac. Surg. 2018, 29, e534–e541. [Google Scholar] [CrossRef]
- Gildestad, T.; Bjørge, T.; Vollset, S.E.; Klungsøyr, K.; Nilsen, R.M.; Haaland, Ø.A.; Øyen, N. Folic acid supplements and risk for oral clefts in the newborn: A population-based study. Br. J. Nutr. 2015, 114, 1456–1463. [Google Scholar] [CrossRef]
- Gildestad, T.; Bjørge, T.; Haaland, Ø.A.; Klungsøyr, K.; Vollset, S.E.; Øyen, N. Maternal use of folic acid and multivitamin supplements and infant risk of birth defects in Norway, 1999–2013. Br. J. Nutr. 2020, 124, 316–329. [Google Scholar] [CrossRef]
- Michikawa, T.; Yamazaki, S.; Sekiyama, M.; Kuroda, T.; Nakayama, S.F.; Isobe, T.; Kobayashi, Y.; Iwai-Shimada, M.; Suda, E.; Kawamoto, T.; et al. Maternal dietary intake of vitamin A during pregnancy was inversely associated with congenital diaphragmatic hernia: The Japan Environment and Children’s Study. Br. J. Nutr. 2019, 122, 1295–1302. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Tanaka, H.; Akutsu, T.; Koide, K.; Sakuma, M.; Okazaki, M.; Ida, H.; Urashima, M. Prenatal vitamin A supplementation associated with adverse child behavior at 3 years in a prospective birth cohort in Japan. Pediatr. Int. 2016, 58, 855–861. [Google Scholar] [CrossRef]
- Sato, Y.; Yoshioka, E.; Saijo, Y.; Miyamoto, T.; Sengoku, K.; Azuma, H.; Tanahashi, Y.; Ito, Y.; Kobayashi, S.; Minatoya, M.; et al. Population Attributable Fractions of Modifiable Risk Factors for Nonsyndromic Orofacial Clefts: A Prospective Cohort Study From the Japan Environment and Children’s Study. J. Epidemiol. 2021, 31, 272–279. [Google Scholar] [CrossRef]
- Ishikawa, T.; Obara, T.; Nishigori, H.; Nishigori, T.; Metoki, H.; Ishikuro, M.; Tatsuta, N.; Mizuno, S.; Sakurai, K.; Nishijima, I.; et al. Update on the prevalence and determinants of folic acid use in Japan evaluated with 91,538 pregnant women: The Japan Environment and Children’s Study. J. Matern.-Fetal Neonatal Med. 2020, 33, 427–436. [Google Scholar] [CrossRef]
- Suzuki, T.; Nishigori, T.; Obara, T.; Masumoto, T.; Mori, M.; Murata, T.; Kyozuka, H.; Ogata, Y.; Sato, A.; Sampei, M.; et al. Maternal folic acid supplement use/dietary folate intake from preconception to early pregnancy and neurodevelopment in 2-year-old offspring: The Japan Environment and Children’s Study. Br. J. Nutr. 2022, 128, 2480–2489. [Google Scholar] [CrossRef] [PubMed]
- Simmer, K.; Thompson, R.P. Zinc in the fetus and newborn. Acta Paediatr. Scand. Suppl. 1985, 319, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, L.E.; Zavaleta, N.; Shankar, A.H.; Merialdi, M. Potential contribution of maternal zinc supplementation during pregnancy to maternal and child survival. Am. J. Clin. Nutr. 1998, 68, 499s–508s. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, E.; Martín-Grau, C.; Bedmar, C.; Serrat Orus, N.S.; Basora, J.; Arija, V. The Eclipses Study Group Maternal Factors Associated with Levels of Fatty Acids, Specifically n-3 PUFA during Pregnancy: ECLIPSES Study. Nutrients 2021, 13, 317. [Google Scholar] [CrossRef]

| Total (%) | JECS | JECS and AGU | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (%) | Patients (%) | p-Value | Patients (%) | p-Value | |||||||
| Avoid intake of milk and dairy products in pregnancy | 0.29 | 0.17 | |||||||||
| No | 84,912 | (96.8) | 84,644 | (98.2) | 206 | (97.2) | 268 | (97.1) | |||
| Yes | 1578 | (1.8) | 1570 | (1.8) | 6 | (2.8) | 8 | (2.9) | |||
| Missing | 1268 | (1.4) | |||||||||
| Avoid egg intake in pregnancy | 1.00 | 1.00 | |||||||||
| No | 84,791 | (96.6) | 84,520 | (98.0) | 208 | (98.1) | 271 | (98.2) | |||
| Yes | 1699 | (1.9) | 1694 | (2.0) | 4 | (1.9) | 5 | (1.8) | |||
| Missing | 1268 | (1.4) | |||||||||
| Avoid soy intake in pregnancy | 0.51 | 0.60 | |||||||||
| No | 86,198 | (98.2) | 85,924 | (99.7) | 211 | (99.5) | 274 | (99.6) | |||
| Yes | 291 | (0.3) | 290 | (0.3) | 1 | (0.5) | 1 | (0.4) | |||
| Missing | 1269 | (1.4) | |||||||||
| Avoid fish intake in pregnancy | 0.00 | 0.00 | |||||||||
| No | 84,525 | (96.3) | 84,266 | (97.7) | 200 | (94.3) | 259 | (93.8) | |||
| Yes | 1965 | (2.2) | 1948 | (2.3) | 12 | (5.7) | 17 | (6.2) | |||
| Missing | 1268 | (1.4) | |||||||||
| Avoid beef intake in pregnancy | 0.03 | 0.00 | |||||||||
| No | 85,536 | (97.5) | 85,269 | (98.9) | 206 | (97.2) | 267 | (96.7) | |||
| Yes | 954 | (1.1) | 945 | (1.1) | 6 | (2.8) | 9 | (3.3) | |||
| Missing | 1268 | (1.4) | |||||||||
| Avoid peanuts intake in pregnancy | 0.02 | 0.05 | |||||||||
| No | 84,815 | (96.6) | 84,549 | (98.1) | 203 | (95.8) | 266 | (96.4) | |||
| Yes | 1675 | (1.9) | 1665 | (1.9) | 9 | (4.2) | 10 | (3.6) | |||
| Missing | 1268 | (1.4) | |||||||||
| Folic acid supplements use in pregnancy | 0.23 | 0.14 | |||||||||
| No | 49,737 | (56.7) | 49,594 | (57.8) | 112 | (53.6) | 143 | (53.2) | |||
| Yes | 36,391 | (41.5) | 36,265 | (42.2) | 97 | (46.4) | 126 | (46.8) | |||
| Missing | 1630 | (1.9) | |||||||||
| Zinc supplements use in pregnancy | 0.50 | 0.14 | |||||||||
| No | 82,094 | (93.5) | 81,843 | (95.6) | 197 | (94.7) | 251 | (93.7) | |||
| Yes | 3811 | (4.3) | 3794 | (4.4) | 11 | (5.3) | 17 | (6.3) | |||
| Missing | 1853 | (2.1) | |||||||||
| EPA supplements use in pregnancy | 0.77 | 0.60 | |||||||||
| No | 84,540 | (96.3) | 84,273 | (98.6) | 206 | (99.0) | 267 | (99.3) | |||
| Yes | 1233 | (1.4) | 1231 | (1.4) | 2 | (1.0) | 2 | (0.7) | |||
| Missing | 1985 | (2.3) | |||||||||
| DHA supplements use in pregnancy | 0.67 | 0.26 | |||||||||
| No | 83,458 | (95.1) | 83,193 | (97.2) | 204 | (98.1) | 265 | (98.5) | |||
| Yes | 2366 | (2.7) | 2362 | (2.8) | 4 | (1.9) | 4 | (1.5) | |||
| Missing | 1934 | (2.2) | |||||||||
| Lactic acid drink supplements use in pregnancy | 0.63 | 0.39 | |||||||||
| No | 41,217 | (47.0) | 41,081 | (47.9) | 96 | (46.2) | 136 | (50.6) | |||
| Yes | 44,813 | (51.1) | 44,680 | (52.1) | 112 | (53.8) | 133 | (49.4) | |||
| Missing | 1728 | (2.0) | |||||||||
| Avoid foods and use supplements in pregnancy | 0.05 | 0.01 | |||||||||
| Avoid foods: No Use supplements: No | 24,442 | (27.9) | 24,370 | (28.7) | 53 | (25.6) | 72 | (27.2) | |||
| Avoid foods: Yes Use supplements: No | 1763 | (2.0) | 1753 | (2.1) | 7 | (3.4) | 10 | (3.8) | |||
| Avoid foods: No Use supplements: Yes | 54,557 | (62.2) | 54,397 | (64.1) | 129 | (62.3) | 160 | (60.4) | |||
| Avoid foods: Yes Use supplements: Yes | 4323 | (4.9) | 4300 | (5.1) | 18 | (8.7) | 23 | (8.7) | |||
| Missing | 2673 | (3.0) | |||||||||
| Maternal smoking history | 0.84 | 0.90 | |||||||||
| No | 49,421 | (56.3) | 49,263 | (57.6) | 119 | (56.9) | 158 | (58.1) | |||
| Yes | 36,332 | (41.4) | 36,218 | (42.4) | 90 | (43.1) | 114 | (41.9) | |||
| Missing | 2005 | (2.3) | |||||||||
| Paternal smoking history | 0.70 | 0.37 | |||||||||
| No | 22,998 | (26.3) | 22,918 | (27.1) | 59 | (28.4) | 80 | (29.5) | |||
| Yes | 61,852 | (70.5) | 61,661 | (72.9) | 149 | (71.6) | 191 | (70.5) | |||
| Missing | 2908 | (3.3) | |||||||||
| Maternal passive smoking | 1.00 | 0.71 | |||||||||
| No | 53,480 | (60.9) | 53,313 | (62.0) | 131 | (62.1) | 167 | (60.9) | |||
| Yes | 32,750 | (37.3) | 32,643 | (38.0) | 80 | (37.9) | 107 | (39.1) | |||
| Missing | 1528 | (1.7) | |||||||||
| Alcohol intake during second/third trimesters | 0.89 | 0.16 | |||||||||
| No | 28,753 | (32.8) | 28,650 | (33.5) | 72 | (34.0) | 103 | (37.6) | |||
| Yes | 57,031 | (65.0) | 56,860 | (66.5) | 140 | (66.0) | 171 | (62.4) | |||
| Missing | 1974 | (2.2) | |||||||||
| Maternal educational status | 0.65 | 0.85 | |||||||||
| Junior high school or high school | 31,556 | (36.0) | 31,452 | (36.7) | 85 | (40.7) | 104 | (38.1) | |||
| Higher professional school or professional school | 36,183 | (41.2) | 36,074 | (42.1) | 80 | (38.3) | 109 | (39.9) | |||
| Junior college or college | 17,021 | (19.4) | 16,964 | (19.8) | 41 | (19.6) | 57 | (20.9) | |||
| Postgraduate college | 1213 | (1.4) | 1210 | (1.4) | 3 | (1.4) | 3 | (1.1) | |||
| Missing | 1785 | (2.0) | |||||||||
| Annual income (JPY × 10,000) | 0.34 | 0.07 | |||||||||
| <400 | 32,528 | (37.1) | 32,439 | (40.6) | 76 | (39.2) | 89 | (34.6) | |||
| 400–<800 | 39,120 | (44.6) | 38,987 | (48.8) | 95 | (49.0) | 133 | (51.8) | |||
| 800–<1200 | 7077 | (8.1) | 7045 | (8.8) | 22 | (11.3) | 32 | (12.5) | |||
| ≥1200 | 1480 | (1.7) | 1477 | (1.8) | 1 | (0.5) | 3 | (1.2) | |||
| Missing | 7553 | (8.6) | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujiwara, K.; Tamada, H.; Imura, H.; Matsuki, T.; Furukawa, H.; Natsume, N.; Yamada, Y.; Ebara, T.; Kamijima, M.; The Japan Environment and Children’s Study (JECS) Group. Effects of Food Preferences and Supplement Intake During Pregnancy on the Cleft Lip and Palate Incidence: The Japan Environment and Children’s Study. Nutrients 2025, 17, 3175. https://doi.org/10.3390/nu17193175
Fujiwara K, Tamada H, Imura H, Matsuki T, Furukawa H, Natsume N, Yamada Y, Ebara T, Kamijima M, The Japan Environment and Children’s Study (JECS) Group. Effects of Food Preferences and Supplement Intake During Pregnancy on the Cleft Lip and Palate Incidence: The Japan Environment and Children’s Study. Nutrients. 2025; 17(19):3175. https://doi.org/10.3390/nu17193175
Chicago/Turabian StyleFujiwara, Kumiko, Hazuki Tamada, Hideto Imura, Taro Matsuki, Hiroo Furukawa, Nagato Natsume, Yasuyuki Yamada, Takeshi Ebara, Michihiro Kamijima, and The Japan Environment and Children’s Study (JECS) Group. 2025. "Effects of Food Preferences and Supplement Intake During Pregnancy on the Cleft Lip and Palate Incidence: The Japan Environment and Children’s Study" Nutrients 17, no. 19: 3175. https://doi.org/10.3390/nu17193175
APA StyleFujiwara, K., Tamada, H., Imura, H., Matsuki, T., Furukawa, H., Natsume, N., Yamada, Y., Ebara, T., Kamijima, M., & The Japan Environment and Children’s Study (JECS) Group. (2025). Effects of Food Preferences and Supplement Intake During Pregnancy on the Cleft Lip and Palate Incidence: The Japan Environment and Children’s Study. Nutrients, 17(19), 3175. https://doi.org/10.3390/nu17193175






