Abstract
Background: Physical activity, sedentary behaviour, and sleep are interdependent components of the 24 h movement profile that may influence appetite control. While acute exercise can alter appetite perceptions and food reward, less is known about how reallocating time between daily behaviours affects appetite outcomes under free-living conditions. Methods: We applied isotemporal-substitution modelling in a cross-sectional study of 130 young, healthy, active adults. Accelerometer-derived estimates of sedentary time, light physical activity (LPA), moderate-to-vigorous physical activity (MVPA), and sleep were analysed in relation to energy intake (food diaries, laboratory meals), subjective appetite perceptions, appetite-related hormones (acylated ghrelin, PYY, leptin), and psychological traits, including food reward (Leeds Food Preference Questionnaire, LFPQ), food cravings (Control of Eating Questionnaire, CoEQ), and eating behaviour traits (Three-Factor Eating Questionnaire, TFEQ). Results: Reallocating 30 min/day of sedentary time to MVPA was associated with higher energy intake in free-living (+113 kcal/day, 95% CI: 34–192) and laboratory settings (+120 kcal/day, 95% CI: 55–185), along with greater postprandial hunger and prospective food consumption, reduced fullness, elevated fasting acylated ghrelin, and lower postprandial PYY. No associations were observed for reallocations to LPA or sleep. Furthermore, sedentary time reallocations were unrelated to leptin or psychological eating traits assessed by the LFPQ, CoEQ, or TFEQ. Conclusions: In this population, reallocating sedentary time to MVPA was linked to physiological and behavioural compensation consistent with elevated energy demands, whereas reallocating to LPA or sleep showed no associations. Trait-level eating behaviours were unaffected, suggesting MVPA influences appetite primarily through acute physiological rather than enduring cognitive or hedonic pathways.
Keywords:
appetite; energy balance; eating behaviour; physical activity; sedentary time; food reward 1. Introduction
Appetite control remains a key focus of scientific enquiry given the persistent global burden of overweight and obesity [1]. Recent advances in the neurobiology of appetite have underpinned the development of new pharmacotherapies for obesity, many of which target central appetite-regulatory pathways [2]. The clinical success of these agents [3,4] underscores the pivotal role of appetite in eating behaviour and body weight regulation. However, pharmacological treatments alone cannot fully address the scale of the obesity crisis. Non-pharmacological strategies that modulate appetite remain essential, both as standalone approaches for individuals not using medication and as complementary tools alongside pharmacotherapy [5,6]. A deeper understanding of how such interventions influence appetite—across both its homeostatic and hedonic dimensions—is therefore a priority.
One promising avenue is the interaction between movement behaviours and appetite control. Sedentary behaviour has long been associated with dysregulated appetite and weight gain [7], a relationship confirmed with modern device-based assessment methods [8]. In contrast, regular physical activity appears to enhance appetite sensitivity, enabling more accurate compensation for prior energy intake [9,10]. This “fine-tuning” effect is characterised by greater fasting hunger coupled with stronger postprandial satiety [11], aligning intake more closely with energy demands. Mechanistic studies suggest that exercise-induced changes in appetite-related peptides (e.g., ghrelin, glucagon-like peptide-1 [GLP-1], peptide YY [PYY]) may contribute to this response [12,13], while behavioural evidence indicates that physical activity can also modulate hedonic aspects of eating—reducing the reward value of high-fat foods and improving control over food cravings [14]. Sleep also plays a critical role in appetite regulation, with insufficient or disrupted sleep linked to increased hunger, altered satiety signalling, and greater energy intake [15,16,17,18]. When considered alongside physical activity and sedentary behaviour, sleep represents an additional behaviour that may shape appetite regulation within the context of the full 24 h day. Together, these findings position daily movement behaviours—including physical activity, sedentary time, and sleep—as potentially powerful non-pharmacological regulators of appetite.
Yet much of the existing evidence derives from tightly controlled laboratory studies or small-scale trials that may not capture the complexity of appetite regulation in free-living populations [19,20], often focusing on one behaviour alone. Addressing this gap requires methods that account for how daily movement behaviours interact within the constraints of a 24 h day. Isotemporal substitution analysis offers one such approach [21]. By modelling the effects of reallocating time between sedentary behaviour, physical activity, and sleep, it provides ecologically valid estimates of how these behaviours influence health outcomes [22,23,24]. When applied to appetite regulation, isotemporal substitution allows for a more integrated understanding of how movement behaviours collectively shape both homeostatic and hedonic components of appetite.
Building on this rationale, the present study used a deeply-phenotyped adult cohort to examine how replacing device-measured sedentary time with different physical activity intensities and sleep influences energy intake and appetite control. Appetite and related hormonal responses were assessed dynamically during a mixed-meal tolerance test, complemented by validated measures of food craving and reward. These analyses provide novel evidence on the relationship between movement behaviours and appetite regulation in a real-world context, with implications for the design of non-pharmacological strategies to support weight management.
2. Materials and Methods
2.1. Ethical Approval and Participants
A convenience sample of 130 participants were recruited (including men and women) from the local community. Participants were aged 18–55 years with a Body Mass Index (BMI) between 16.5 and 35.0 kg/m2. All participants were healthy, weight stable (<3 kg change in the 12 weeks prior), not dieting (or using medications that affect body weight), and had no history of metabolic or cardiovascular disorders. Female participants self-reported not being pregnant. Smokers were included (smoking status was included as a co-variate in statistical models) but we excluded people who vape or use e-cigarettes as the impact on study outcomes is less clear. Written informed consent was provided by all, and the study procedures adhered to the principles of the Declaration of Helsinki [25]. This study was approved by the Loughborough University Ethics Review Sub-Committee (project ID: 10980; 30 September 2022).
2.2. Study Design
This cross-sectional study involved two laboratory visits: one for familiarisation and a second for primary assessments. Visits were separated by at least one week, during which free-living physical activity, sedentary behaviour, sleep, and free-living energy intake were monitored. Participants were instructed to fast overnight (12 h) and abstain from caffeine, alcohol, and structured exercise for 24 h before each visit. With the acquired cross-sectional data, isotemporal substitution modelling was employed to examine the associations between reallocating time from sedentary behaviour to light physical activity (LPA), moderate-to-vigorous physical activity (MVPA), or sleep and multiple appetite and energy intake-related outcomes, while accounting for the finite nature of the 24 h day.
2.2.1. Visit 1: Eligibility and Familiarisation
Participants attended the laboratory to confirm eligibility and undergo familiarisation with all study procedures. Demographic information was collected and assessments for anthropometry and resting metabolic rate (RMR) were completed. Food cravings during the previous week were assessed using the Control of Eating Questionnaire (CoEQ) [26].
Height and body mass were measured using a wireless measuring station (Seca Ltd., Hamburg, Germany) to the nearest 0.1 cm and 0.1 kg, respectively, and BMI (body mass/height2) was calculated.
RMR was measured using breath-by-breath indirect calorimetry (Cortex Metalyzer 3B, Leipzig, Germany), following standard protocols [27]. Participants rested in a quiet, supine position for 30 min, during which expired air was continuously sampled. RMR was calculated using the Weir equation from the final 20 min of data, excluding the initial 10 min to eliminate non-steady-state data [28].
2.2.2. 24 h Movement Behaviour Assessment
After Visit 1, participants wore a triaxial accelerometer (GENEActiv, ActivInsights Ltd., Kimbolton, UK) on their non-dominant wrist for 24 h per day over seven days to monitor levels of physical activity, sedentary time and sleep. Participants followed their usual routines and logged sleep/wake times and any device removal. Data, recorded at 100 Hz, were downloaded and processed using the GGIR R-package (http://cran.r-project.org. accessed on 1 August 2025) [29,30]. Dynamic acceleration (Euclidean Norm minus 1 g [ENMO]) was averaged over 5 s epochs and reported in milligravitational units (mg), alongside a sleep detection algorithm to estimate sleep duration [30,31]. ENMO reflects the average dynamic acceleration throughout the day (24 h), calculated as the vector magnitude of the three acceleration axes with 1 g subtracted and any negative values set to zero. This metric is automatically computed within the GGIR R-package [29,30] and provides an indicator of total physical activity over the 24 h period [22,30]. Data were excluded if post-calibration errors exceeded 0.01 g (10 mg), valid wear time was less than three days (defined as ≥16 h per day), or if data were missing for any 15 min interval across the 24 h cycle. Non-wear time was identified based on a 15 min window showing a standard deviation below 13 mg or a range less than 50 mg on at least two of the three axes, as previously described [22]. Missing data were imputed using average ENMO values from corresponding time points on other days [31,32]. This ensured that outcome variables covered the entire 24 h cycle (1440 min) for each participant. Sleep windows and sleep duration within the window were determined using the HDCZA automated algorithm, with sleep defined from initial sleep onset to the final awakening of the night [31,33]. For each exposure variable, the mean across all valid days was calculated.
The following sleep characteristics were obtained: sleep duration (total accumulated sleep duration within the sleep window discounting any wake time) and wake after sleep onset (WASO, the total number of minutes that a person is awake after having initially fallen asleep). Activity levels were categorized as sedentary (<40 mg), light (LPA, 40–<100 mg), or moderate-to-vigorous physical activity (MVPA; ≥100 mg) [34,35].
2.2.3. Free-Living Energy Intake Assessment
Participants completed a three-day weighed food dairy—two weekdays and one weekend day—to assess habitual dietary intake. To minimise reporting errors, participants were given instructions, a food scale, and encouraged to record intake in real time. Records were reviewed and analysed by a trained researcher using Nutritics software version 6.13 (Nutritics Ltd., Dublin, Ireland) to estimate daily energy and macronutrient intake.
2.2.4. Visit 2: Appetite and Energy Intake Assessment
Participants arrived at the laboratory at 08:00 after a 12 h overnight fast (Figure 1). On arrival, they completed the Leeds Food Preference Questionnaire (LFPQ). At ~08:15, participants began a mixed-meal tolerance test (MM-TT). Baseline appetite ratings (hunger, fullness, satisfaction, prospective food consumption) were recorded using visual analogue scales (VAS) [36], and a fasting venous blood sample was taken before consumption of a standardised meal (08:15–08:30). VAS ratings were then completed every 30 min for 240 min, with additional venous blood samples collected at 30, 60, and 120 min post-meal. At 13:00 (30 min after the MM-TT) and 17:00, participants consumed ad libitum lunch and dinner meals and were then provided with an evening snack bag to consume as desired until the next morning. Food intake from the snack bag was recorded the following day.
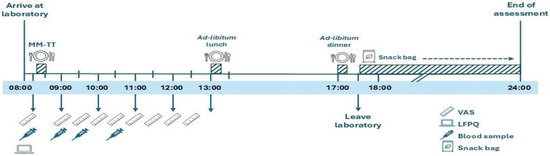
Figure 1.
Schematic illustration of study visit 2. LFPQ, Leeds Food Preference Questionnaire; MM-TT, mixed-meal tolerance test; VAS, visual analogue scale. ‘Reproduced from Alruwaili et al., 2025, Appetite, Jun 11:108194 [37], under the terms of the Creative Commons Attribution License (CC BY 4.0). https://doi.org/10.1016/j.appet.2025.108194.
2.2.5. Study Meals
In line with published guidance [38], the MM-TT meal (porridge, whole milk, honey) was calculated as 25% of estimated daily energy requirements (RMR × 1.4 × 0.25) and consisted of 55% carbohydrates, 15% protein, and 30% fat. Meal energy was determined by multiplying participants measured RMR by 1.4 (to reflect the sedentary study conditions) and subsequently by 0.25 (typical share of daily energy intake from breakfast in the UK). Ref. [39] Ad libitum meals included a standardised pasta lunch (72% CHO, 12% protein, 16% fat) and a dinner of curry, naan, and basmati rice (71% CHO, 8% protein, 21% fat). Energy intake was calculated from food consumed, with initial weights recorded and manufacturer values used for energy estimation. The snack bag contained commercially available items (e.g., cookies, cereal bars, mini rolls).
2.2.6. Leeds Food Preference Questionnaire
The LFPQ is a validated computer-based tool for assessing food preference and reward [40,41]. It measures liking and wanting for 16 foods across four categories (high-/low-fat savory/sweet). Participants select their most wanted item from paired comparisons, and reaction times (adjusted for selection frequency) are used to compute implicit wanting. Explicit liking and wanting are rated using 100 mm VAS, with fat and sweet bias scores calculated from these responses.
2.2.7. Blood Sampling and Biochemical Analyses
Venous blood samples were collected into pre-chilled EDTA monovettes (Sarstedt, Leicester, UK) and centrifuged at 2383× g for 10 min (4 °C). The resulting plasma was aliquoted into 2 mL cryovials and stored at −80 °C. Acylated ghrelin samples were processed separately to preserve the acyl group [42]. Hormone concentrations (acylated ghrelin, total PYY, leptin) were measured using enzyme-linked immunosorbent assays (ELISA) kits from commercial suppliers (Bioquote Ltd., York, UK; Merck Millipore, Darmstadt, Germany; and Bio-Techne Ltd., Abingdon, UK, respectively) with within-batch CVs < 5.14%. Acylated ghrelin and total PYY were measured at fasting and postprandial time points (0, 30, 60, and 120 min) while leptin was assessed at baseline only (fasted).
2.3. Statistical Analysis
All analyses were performed using SPSS version 28 (SPSS Inc., Chicago, IL, USA). Data distribution was assessed using Kolmogorov–Smirnov tests and histograms. Results are presented as mean ± SD (normal), median (IQR) (non-normal), or n (%) (categorical) as appropriate. Total area under the curve (AUC) for postprandial responses was calculated using the trapezoid rule.
Isotemporal substitution analysis using generalized linear models was employed to investigate the association of reallocating sedentary time to LPA, MVPA, or sleep duration (minus WASO) on appetite-related outcomes. Isotemporal substitution is a recommended tool in observational studies utilising measures based on time [21]. In this context, the “replacement” of sedentary behaviour by another behaviour represents a statistical simulation within the regression models: it estimates the expected change in outcomes if time spent sedentary were reallocated, rather than reflecting an actual experimental manipulation. To investigate these associations, all of the movement behaviours except for the one being displaced (in this case sedentary time) were concurrently entered into the regression model, as shown below:
Appetite-related outcome (y) = β0 + (β1) sleep duration + (β2) WASO + (β3) sedentary time + (β4) LPA + (β5) MVPA + (β6) covariates—with sedentary time (β3) eliminated from the model.
Importantly, incorporating sleep duration and WASO, along with other behaviours, ensures that the time reallocation is modeled and standardized to a specific time frame (such as 24 h or 1440 min). As a result, it is not necessary to include total duration as an additional covariate in the model. The regression coefficient for each behaviour in this model indicates the change in association that would occur if a unit of time spent in sedentary time is replaced with that behaviour.
We ran two regression models. Model 1 adjusted for age, sex, ethnicity, and smoking status (with postprandial AUC outcomes additionally adjusted for fasting values). Model 2 included the same covariates with the addition of BMI, given its potential role as an attenuator of associations between movement behaviours and appetite-related outcomes [43]. Although results from both models are reported, Model 2 is considered the primary model of interest because it accounts for adiposity as a key confounder. Data from Model 1 are reported in Supplementary Materials. Multicollinearity was checked using both pairwise correlations among predictors and variance inflation factors (VIFs) from Model 2. All pairwise correlations were ≤0.444 and VIFs were ≤1.577, indicating no evidence of problematic multicollinearity.
Missing data (3.4% for fasting bloods; 1.7% for appetite and field-based assessment of energy intake) were imputed across 20 datasets using regression models that included age, sex, ethnicity, BMI, and smoking status as predictors.
Given the exploratory nature of this study, formal a priori power calculations were not conducted, and no adjustments were made for multiple comparisons. Consequently, the findings should be interpreted cautiously, recognizing the risk of type I error. Results are best considered in the context of broader patterns, using beta-coefficients (β), 95% confidence intervals (CIs), and exact p-values. These outcomes are intended to guide the planning of future hypothesis-driven experimental research.
To evaluate the adequacy of the final sample (n = 119), we conducted a post hoc power analysis aligned with our primary isotemporal substitution models (Model 2) where laboratory energy intake (outcome) was regressed on the movement behaviours (total predictors = 9, error df = 109). At a two-sided alpha of 0.05 and 80% power, this design could detect partial effects of approximately partial R2 ≈ 0.07 for a single movement behaviour. Using the observed standard error of the MVPA coefficient (derived from its 95% CIs), this corresponded to a minimal detectable difference of ~94 kcal·day−1 per 30 min reallocation of sedentary time to MVPA. The observed association (+120 kcal·day−1; 95% CIs: 55, 185) therefore exceeded this threshold and corresponded to a partial R2 ≈ 0.11, indicating an adequate sample size.
3. Results
3.1. Participant Characteristics
Out of the total sample (n = 130), 11 individuals were excluded due to missing 24 h movement behaviour data related to technical issues—leaving a final sample of 119 participants. Key characteristics of these individuals are described in Table 1. Participants’ appetite, appetite-related hormone, food reward and energy intake data are shown in Supplementary Table S1. Overall, men and women were fairly equally represented, with most participants identifying as White, Indian or Asian. Generally, the sample was young, physically active and exhibiting healthy body weight.

Table 1.
Participant characteristics (n = 119).
3.2. Energy Intake
Based on food diary records, reallocating 30 min of sedentary time to MVPA was associated with a 113 (34, 192) kcal·d−1 higher daily energy intake (p = 0.005) (Figure 2, Model 2). Reallocating time from sedentary time to LPA or sleep was unrelated to daily energy intake measured via food diaries. Based on laboratory measured energy intake, reallocating 30 min of sedentary time to MVPA was associated with a 120 (55, 185) kcal·d−1 higher daily energy intake (p < 0.001) (Figure 2, Model 2). Reallocating time from sedentary time to LPA or sleep was not associated with energy intake measured in the laboratory.
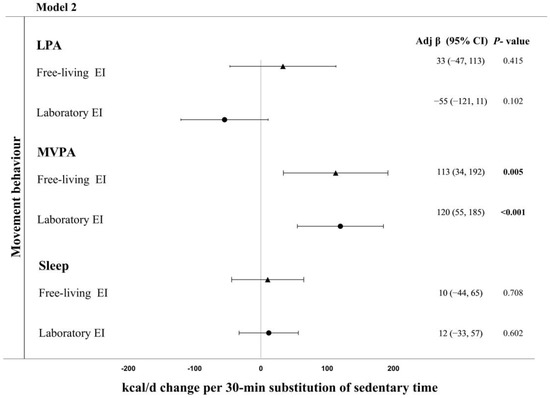
Figure 2.
Adjusted change in energy intake (measured in the laboratory and field) associated with replacing 30 min of sedentary time with sleep or physical activity in Model 2 (adjusted for age, sex, ethnicity, smoking status, and BMI). Bold indicates significant.
3.3. Appetite-Related Hormones
Reallocating 30 min of sedentary time to MVPA was associated with a 639 (−1143, −135) pg·mL·2 h−1 lower postprandial PYY AUC (p = 0.013) (Model 2, Table 2). Reallocating 30 min of sedentary time to MVPA was also associated with higher fasting acylated ghrelin concentrations (0.01 (0.00, 0.19) pg·mL−1) (p = 0.045) (Model 2, Figure 2). Reallocation of 30 min of sedentary time for LPA was associated with higher postprandial PYY concentrations (550 (35, 1065) pg·mL·2 h−1) (p = 0.036) (Model 2, Table 2).

Table 2.
Adjusted change in appetite-related hormones, and perceived ratings of appetite associated with replacing 30 min of sedentary time with sleep or physical activity in Model 2 (adjusted for age, sex, ethnicity, smoking status, and BMI).
3.4. Perceived Ratings of Appetite
Reallocating 30 min of sedentary time to MVPA was associated with higher postprandial PFC (4 h AUC: +545 mm; 95% CI: 93–996; p = 0.018; Model 2, Table 2). There was also a trend toward greater postprandial hunger (+481 mm; 95% CI: –49 to 1011; p = 0.075) and lower postprandial fullness (–454 mm; 95% CI: –937 to 30; p = 0.066) when reallocating sedentary time for MVPA. No other associations were observed for reallocations of sedentary time to physical activity (LPA or MVPA) or sleep, whether appetite perceptions measurements were fasted or postprandial.
3.5. Food Reward, Cravings and Dietary Eating Traits
Reallocation of 30 min of sedentary time to LPA, MVPA and sleep was unrelated to all measured outcomes regarding food reward (LFPQ), food cravings (CoEQ) and dietary eating traits (TFEQ) (Table 3).

Table 3.
Adjusted change in food reward (LFPQ), food cravings (CoEQ) and eating traits (TFEQ) associated with replacing 30 min of sedentary time with sleep or physical activity in Model 2 (adjusted for age, sex, ethnicity, smoking status, and BMI).
4. Discussion
The present study used isotemporal substitution modelling to examine the impact of reallocating sedentary time to sleep, LPA, or MVPA on multiple aspects of appetite control in a predominantly young, healthy, and active cohort. We investigated outcomes spanning energy intake, subjective appetite perceptions, appetite-related hormones, and psychological eating behaviour traits. Reallocation of sedentary time to MVPA was consistently associated with higher energy intake, greater postprandial appetite, and hormonal changes indicative of increased energy demands (higher fasting acylated ghrelin, lower postprandial PYY). No associations were observed for reallocations of sedentary time to LPA or sleep, and there were no links with trait-level eating behaviours, such as dietary restraint, disinhibition, food cravings, or food reward. Taken together, these findings suggest that in young, healthy, and active adults, reallocating time to MVPA under free-living conditions may elicit physiological and behavioural compensation to maintain energy balance, potentially through enhanced sensitivity of short-term appetite signals. In contrast, reallocations to LPA and sleep do not appear to meaningfully influence appetite regulation or energy intake in this population.
A key finding was that reallocating sedentary time to MVPA was associated with higher daily energy intake, with each 30 min reallocation corresponding to an additional 113 kcal (free-living measured) to 120 kcal (laboratory measured) consumed. No such associations were detected for reallocations to LPA or sleep. This likely reflects the characteristics of our cohort, who were weight-stable, highly active, and therefore required greater energy intake to meet the increased demands of higher physical activity. Such a pattern is consistent with the “activity-stat” hypothesis, which proposes that active individuals finely adjust energy intake to meet expenditure. For example, previous studies have shown that active individuals consume more energy and protein than their less active peers [44], report greater hunger and reduced satiety following meals [45], and demonstrate enhanced appetite sensitivity by downregulating intake after high-energy preloads [46]. These findings collectively suggest that active individuals may possess a more responsive appetite control system that helps align energy intake with expenditure.
This interpretation is supported by our subjective appetite data. Reallocating sedentary time to MVPA was associated with greater postprandial hunger and prospective food consumption, and with lower fullness during the MM-TT. No associations were observed for fasting appetite ratings, likely reflecting the variability and limited sensitivity of single time-point VAS measures, whereas repeated postprandial ratings more reliably capture satiety dynamics [38]. That the associations emerged only in the postprandial period could suggest that MVPA may sharpen meal-related signalling and appetite cues when energy is most needed. The absence of associations for reallocations to sleep or LPA is plausibly explained by their relatively modest energetic contribution in this cohort, alongside the homogeneity and generally healthy sleep and LPA pattern of participants.
Further insight comes from the appetite-related hormone data. Few studies have explored the interaction between habitual physical activity and appetite peptides [47], though some evidence suggests that active individuals have higher fasting acylated ghrelin concentrations than inactive controls [48]. In line with this, reallocating sedentary time to MVPA in our cohort was associated with higher fasting acylated ghrelin concentrations, consistent with an adaptive signal to stimulate intake in response to elevated energy demands [49]. Lower postprandial PYY with greater MVPA extends this picture, suggesting that satiety signalling may be downregulated in active individuals to facilitate energy replenishment [50]. In contrast, acylated ghrelin responses to meals did not differ, which may be expected given the marked postprandial decline observed across individuals (limiting variability in data). Furthermore, leptin, which primarily reflects longer-term energy reserves and adiposity [51], was not influenced by movement behaviour reallocations, likely because participants were weight stable.
The consistency of MVPA–energy intake associations across both dietary records and laboratory-based assessments strengthens confidence in these findings. While self-reported diaries are prone to underestimation [52], this bias is broadly systematic, whereas laboratory-based assessments provide greater precision but lower ecological validity. The convergence of both methods therefore provides robust evidence that reallocating sedentary time to MVPA is associated with higher energy intake in this population. Notably, the confidence intervals for associations with energy intake were wide, particularly when intake was assessed via dietary records compared with laboratory measures. This likely reflects both the limited variability in movement behaviours within our cohort and the substantial inter-individual variability in dietary intake responses, highlighting the importance of replication in larger and more diverse samples.
We also examined hedonic and cognitive influences on eating behaviour. Using the LFPQ [42], we assessed food reward (implicit and explicit liking and wanting), alongside the CoEQ (craving control and food cravings) [26] and the TFEQ (restraint, disinhibition, hunger) [53]. In contrast to our findings for energy intake and appetite physiology, reallocating sedentary time to MVPA, LPA, or sleep was unrelated to any of these outcomes. Prior research has suggested that habitual MVPA is associated with lower liking and wanting for high-fat foods [54,55] and attenuated neural reward responses to food cues [56,57], though such effects are most evident in less active or higher-adiposity populations, and the evidence from intervention studies is mixed [58]. Our null findings may therefore reflect the characteristics of our sample, namely young, active, and healthy adults with a well-regulated appetitive system and limited variability in eating behaviour traits. Furthermore, the questionnaires employed primarily capture trait-like or longer-term dispositions, which may be relatively insensitive to the subtle habitual reallocations in movement and sleep assessed here. This pattern suggests that the influence of physical activity on appetite in this population is more likely mediated through acute physiological pathways as opposed to enduring cognitive or hedonic mechanisms.
Our overall findings have both practical and clinical relevance. In our young, active cohort, reallocating sedentary time to MVPA was linked to higher intake and a hunger-promoting profile, indicating compensatory eating rather than appetite suppression. Accordingly, physical activity guidance (particularly for MVPA) should be paired with targeted nutritional guidance to align intake with goals. In a weight-management context, this means coupling MVPA with strategies to manage overall energy intake to avoid unintended surplus, whereas in a performance context, it means proactive fueling to meet higher demands while maintaining nutrient quality. These implications are population-specific and non-causal, and should be confirmed in other populations.
This study has several strengths, including the precise accelerometer-based assessment of 24 h movement behaviours, the use of isotemporal substitution modelling to capture real-world behavioural trade-offs, and the integration of comprehensive outcome measures spanning energy intake, appetite perceptions, hormone responses, and psychological traits. To our knowledge, this is the first application of isotemporal substitution in this research area. The following limitations should also be noted: the cross-sectional design prevents causal inference and raises the possibility of reverse causality, the homogeneous sample restricts applicability to older, less active, or populations living with obesity, and the limited variability in sleep and LPA may have reduced power to detect associations. Moreover, participants were recruited through convenience sampling from the local community, which may introduce selection bias and limit the extent to which these findings can be extrapolated to broader populations. Additionally, given the exploratory nature of this work we did not adjust for multiple comparisons, and therefore individual associations should be interpreted with caution, with emphasis placed on the overall pattern of findings rather than isolated results. Outcomes were measured on a single occasion, which may not fully capture longer-term patterns or account for the considerable inter-individual variability in appetite regulation. Finally, despite some inherent correlation among 24 h movement behaviours, collinearity diagnostics showed no problematic multicollinearity (r ≤ 0.444, VIFs ≤ 1.577), though some imprecision is still possible.
5. Conclusions
In conclusion, we hypothesized that reallocating sedentary time to different 24 h movement behaviours would be linked to altered appetite regulation. Our findings support this for MVPA, but not LPA or sleep; reallocating sedentary time to MVPA was associated with higher energy intake, greater postprandial appetite, and hormonal changes consistent with compensatory responses to increased energy expenditure in young, healthy, and active adults. By contrast, trait-level eating behaviours were not associated with these reallocations, reinforcing that physical activity may influence appetite primarily through acute physiological rather than cognitive mechanisms. These findings provide new insight into the interplay between daily movement behaviours and appetite regulation, and highlight the importance of considering compensatory eating in the context of MVPA promotion. Future work should employ longitudinal and interventional designs and examine subgroups (e.g., by sex or BMI category) to test causality and assess generalizability across diverse populations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17193163/s1, Figure S1: Adjusted change in energy intake (measured in the laboratory and field) associated with replacing 30 min of sedentary time with sleep or physical activity in Model 1 (adjusted for age, sex, ethnicity, and smoking status); Table S1: Participant characteristics relating to appetite perceptions, appetite related hormone and food reward outcomes (n = 119); Table S2: Adjusted change in perceived ratings of appetite and appetite related hormones associated with replacing 30 min of sedentary time with sleep or physical activity in Model 1 (adjusted for age, sex, ethnicity, and smoking); Table S3: Adjusted change in food reward (LFPQ), food cravings (CoEQ) and eating traits (TFEQ) associated with replacing 30 min of sedentary time with sleep or physical activity in Model 1 (adjusted for age, sex, ethnicity, and smoking status).
Author Contributions
Conceptualization, J.A.K., S.A.W., S.M. and A.A.; methodology, S.A.W., J.P.S., A.V.R. and J.H.; software, A.V.R., J.H., S.M. and A.A.; formal analysis, S.M., A.A., S.A.W. and A.E.T.; investigation, A.A., S.M., J.A.K. and S.A.W.; resources, D.J.S., D.T. and J.A.K.; data curation, A.A., S.M. and S.A.W.; writing—original draft preparation, J.A.K., S.M. and A.A.; writing—review and editing, S.M., A.A., J.P.S., A.E.T., D.J.S., D.T., J.H., A.V.R., S.A.W. and J.A.K.; supervision, J.A.K., S.A.W. and D.J.S.; project administration, A.A.; funding acquisition, J.A.K. and D.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the National Institute for Health and Care Research (NIHR) Leicester Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. Mrs Arwa Alruwaili received a PhD scholarship from King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Ethics Review Sub-Committee of Loughborough University (project ID: 10980; 30 September 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study (anonymized) are available on request from the corresponding author.
Acknowledgments
We would like to thank all participants that took part in this research study.
Conflicts of Interest
None of the authors have any conflicts of interest to declare.
Abbreviations
The following abbreviations are used in this manuscript:
| AU | Arbitrary units |
| AUC | Area under the curve |
| BMI | Body mass index |
| CI | Confidence interval |
| CoEQ | Control of Eating Questionnaire |
| ES | Effect size |
| LFPQ | Leeds Food Preference Questionnaire |
| PFC | Prospective food consumption |
| PYY | Peptide-YY |
| TFEQ | Three-Factor Eating Questionnaire |
| WASO | Wake after sleep onset |
References
- GBD 2021 Adult BMI Collaborators. Global, Regional, and National Prevalence of Adult Overweight and Obesity, 1990–2021, with Forecasts to 2050: A Forecasting Study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef]
- Johansen, V.B.I.; Petersen, J.; Lund, J.; Mathiesen, C.V.; Fenselau, H.; Clemmensen, C. Brain Control of Energy Homeostasis: Implications for Anti-Obesity Pharmacotherapy. Cell 2025, 188, 4178–4212. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Wadden, T.A.; Chao, A.M.; Moore, M.; Tronieri, J.S.; Gilden, A.; Amaro, A.; Leonard, S.; Jakicic, J.M. The Role of Lifestyle Modification with Second-Generation Anti-Obesity Medications: Comparisons, Questions, and Clinical Opportunities. Curr. Obes. Rep. 2023, 12, 453–473. [Google Scholar] [CrossRef]
- Pinkney, J.; Tarrant, M. Time for a New Agenda for Behavioural Treatment of Overweight and Obesity. Clin. Obes. 2024, 14, e12628. [Google Scholar] [CrossRef]
- Mayer, J.; Roy, P.; Mitra, K.P. Relation between Caloric Intake, Body Weight, and Physical Work. Am. J. Clin. Nutr. 1956, 4, 169–175. [Google Scholar] [CrossRef]
- Myers, A.; Gibbons, C.; Finlayson, G.; Blundell, J. Associations among Sedentary and Active Behaviours, Body Fat and Appetite Dysregulation: Investigating the Myth of Physical Inactivity and Obesity. Br. J. Sports Med. 2017, 51, 1540–1544. [Google Scholar] [CrossRef]
- Beaulieu, K.; Hopkins, M.; Blundell, J.; Finlayson, G. Does Habitual Physical Activity Increase the Sensitivity of the Appetite Control System? A Systematic Review. Sports Med. 2016, 46, 1897–1919. [Google Scholar] [CrossRef]
- Long, S.J.; Hart, K.; Morgan, L.M. The Ability of Habitual Exercise to Influence Appetite and Food Intake in Response to High- and Low-Energy Preloads in Man. Br. J. Nutr. 2002, 87, 517–523. [Google Scholar] [CrossRef]
- King, N.A.; Caudwell, P.P.; Hopkins, M.; Stubbs, J.R.; Naslund, E.; Blundell, J.E. Dual-Process Action of Exercise on Appetite Control: Increase in Orexigenic Drive but Improvement in Meal-Induced Satiety. Am. J. Clin. Nutr. 2009, 90, 921–927. [Google Scholar] [CrossRef]
- Rosenkilde, M.; Reichkendler, M.H.; Auerbach, P.; Toräng, S.; Gram, A.S.; Ploug, T.; Holst, J.J.; Sjödin, A.; Stallknecht, B. Appetite Regulation in Overweight, Sedentary Men after Different Amounts of Endurance Exercise: A Randomized Controlled Trial. J. Appl. Physiol. 2013, 115, 1599–1609. [Google Scholar] [CrossRef]
- Martins, C.; Kulseng, B.; Rehfeld, J.F.; King, N.A.; Blundell, J.E. Effect of Chronic Exercise on Appetite Control in Overweight and Obese Individuals. Med. Sci. Sports Exerc. 2013, 45, 805–812. [Google Scholar] [CrossRef]
- Dera, A.M.; Shen, T.; Thackray, A.E.; Hinton, E.C.; King, J.A.; James, L.; Morgan, P.S.; Rush, N.; Miyashita, M.; Batterham, R.L.; et al. The Influence of Physical Activity on Neural Responses to Visual Food Cues in Humans: A Systematic Review of Functional Magnetic Resonance Imaging Studies. Neurosci. Biobehav. Rev. 2023, 152, 105247. [Google Scholar] [CrossRef]
- Al Khatib, H.K.; Harding, S.V.; Darzi, J.; Pot, G.K. The Effects of Partial Sleep Deprivation on Energy Balance: A Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2017, 71, 614–624. [Google Scholar] [CrossRef]
- Zhu, B.; Shi, C.; Park, C.G.; Zhao, X.; Reutrakul, S. Effects of Sleep Restriction on Metabolism-Related Parameters in Healthy Adults: A Comprehensive Review and Meta-Analysis of Randomized Controlled Trials. Sleep Med. Rev. 2019, 45, 18–30. [Google Scholar] [CrossRef]
- Chaput, J.P.; McHill, A.W.; Cox, R.C.; Broussard, J.L.; Dutil, C.; da Costa, B.G.G.; Sampasa-Kanyinga, H.; Wright, K.P. The Role of Insufficient Sleep and Circadian Misalignment in Obesity. Nat. Rev. Endocrinol. 2022, 19, 82–97. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief Communication: Sleep Curtailment in Healthy Young Men Is Associ-ated with Decreased Leptin Levels, Elevated Ghrelin Levels, and Increased Hunger and Appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- Thackray, A.E.; Stensel, D.J. The Impact of Acute Exercise on Appetite Control: Current Insights and Future Perspectives. Appetite 2023, 186, 106557. [Google Scholar] [CrossRef]
- Blundell, J.E.; Beaulieu, K. The Complex Pattern of the Effects of Prolonged Frequent Exercise on Appetite Control, and Implications for Obesity. Appetite 2023, 183, 106482. [Google Scholar] [CrossRef]
- Biddle, G.J.H.; Henson, J.; Biddle, S.J.H.; Davies, M.J.; Khunti, K.; Rowlands, A.V.; Sutton, S.; Yates, T.; Edwardson, C.L. Modelling the Reallocation of Time Spent Sitting into Physical Activity: Isotemporal Substitution vs. Compositional Isotemporal Substitution. Int. J. Environ. Res. Public. Health 2021, 18, 6210. [Google Scholar] [CrossRef]
- Covenant, A.; Yates, T.; Rowlands, A.V.; Dempsey, P.C.; Edwardson, C.L.; Hall, A.P.; Davies, M.J.; Henson, J. Replacing Sedentary Time with Sleep and Physical Activity: Associations with Physical Function and Wellbeing in Type 2 Diabetes. Diabetes Res. Clin. Pract. 2024, 217, 111886. [Google Scholar] [CrossRef]
- Nagai, K.; Tamaki, K.; Kusunoki, H.; Wada, Y.; Tsuji, S.; Ito, M.; Sano, K.; Amano, M.; Shimomura, S.; Shinmura, K. Isotemporal Substitution of Sedentary Time with Physical Activity and Its Associations with Frailty Status. Clin. Interv. Aging 2018, 13, 1831–1836. [Google Scholar] [CrossRef]
- Park, J.; Nam, H.K.; Cho, S. Il Association between Accelerometer-Derived Physical Activity and Depression: A Cross-Sectional Study Using Isotemporal Substitution Analysis. BMJ Open 2024, 14, e078199. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Participants. JAMA 2024, 333, 71–74. [Google Scholar] [CrossRef]
- Dalton, M.; Finlayson, G.; Hill, A.; Blundell, J. Preliminary Validation and Principal Components Analysis of the Control of Eating Questionnaire (CoEQ) for the Experience of Food Craving. Eur. J. Clin. Nutr. 2015, 69, 1313–1317. [Google Scholar] [CrossRef]
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L. Best Practice Methods to Apply to Measurement of Resting Metabolic Rate in Adults: A Systematic Review. J. Am. Diet. Assoc. 2006, 106, 881–903. [Google Scholar] [CrossRef]
- Weir, J.B.d.V. New Methods for Calculating Metabolic Rate with Special Reference to Protein Metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef]
- van Hees, V.; Migueles, J.H. GGIR. 2025. Available online: https://zenodo.org/records/15594302 (accessed on 1 August 2025).
- Migueles, J.H.; Rowlands, A.V.; Huber, F.; Sabia, S.; Van Hees, V.T. GGIR: A Research Community–Driven Open Source R Package for Generating Physical Activity and Sleep Outcomes from Multi-Day Raw Accelerometer Data. J. Meas. Phys. Behav. 2019, 2, 188–196. [Google Scholar] [CrossRef]
- Van Hees, V.T.; Sabia, S.; Anderson, K.N.; Denton, S.J.; Oliver, J.; Catt, M.; Abell, J.G.; Kivimäki, M.; Trenell, M.I.; Singh-Manoux, A. A Novel, Open Access Method to Assess Sleep Duration Using a Wrist-Worn Accelerometer. PLoS ONE 2015, 10, e0142533. [Google Scholar] [CrossRef]
- Van Hees, V.T.; Fang, Z.; Langford, J.; Assah, F.; Mohammad, A.; Da Silva, I.C.M.; Trenell, M.I.; White, T.; Wareham, N.J.; Brage, S. Autocalibration of Accelerometer Data for Free-Living Physical Activity Assessment Using Local Gravity and Temperature: An Evaluation on Four Continents. J. Appl. Physiol. 2014, 117, 738–744. [Google Scholar] [CrossRef] [PubMed]
- van Hees, V.T.; Sabia, S.; Jones, S.E.; Wood, A.R.; Anderson, K.N.; Kivimäki, M.; Frayling, T.M.; Pack, A.I.; Bucan, M.; Trenell, M.I.; et al. Estimating Sleep Parameters Using an Accelerometer without Sleep Diary. Sci. Rep. 2018, 8, 12975. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M.; Van Hees, V.T.; Hansen, B.H.; Ekelund, U. Age Group Comparability of Raw Accelerometer Output from Wrist- and Hip-Worn Monitors. Med. Sci. Sports Exerc. 2014, 46, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M.; Hansen, B.H.; van Hees, V.T.; Ekelund, U. Evaluation of Raw Acceleration Sedentary Thresholds in Children and Adults. Scand. J. Med. Sci. Sports 2017, 27, 1814–1823. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, Power and Validity of Visual Analogue Scales in Assessment of Appetite Sensations in Single Test Meal Studies. Int. J. Obes. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Alruwaili, A.; Nayeemullah, R.; Engin, B.; Malaikah, S.; James, L.; Sanders, J.P.; Thivel, D.; Thackray, A.E.; Stensel, D.J.; King, J.A.; et al. The Association of Cigarette Smoking with Appetite, Appetite-Related Hormones and Food Reward: A Matched-Pair Cohort Study. Appetite 2025, 214, 108194. [Google Scholar] [CrossRef]
- King, J.A.; Thackray, A.E.; Gibbons, C.; Martins, C.; Broom, D.R.; Stensel, D.J.; Papamargaritis, D.; Arsenyadis, F.; Finlayson, G.; Whelehan, G.; et al. The Mixed-Meal Tolerance Test as an Appetite Assay: Methodological and Practical Considerations. Int. J. Obes. 2025, 1–16. [Google Scholar] [CrossRef]
- Jetté, M.; Sidney, K.; Blümchen, G. Metabolic Equivalents (METS) in Exercise Testing, Exercise Prescription, and Evaluation of Functional Capacity. Clin. Cardiol. 1990, 13, 555–565. [Google Scholar] [CrossRef]
- Oustric, P.; Thivel, D.; Dalton, M.; Beaulieu, K.; Gibbons, C.; Hopkins, M.; Blundell, J.; Finlayson, G. Measuring Food Preference and Reward: Application and Cross-Cultural Adaptation of the Leeds Food Preference Questionnaire in Human Experimental Research. Food Qual. Prefer. 2020, 80, 103824. [Google Scholar] [CrossRef]
- Finlayson, G.; King, N.; Blundell, J. The Role of Implicit Wanting in Relation to Explicit Liking and Wanting for Food: Implications for Appetite Control. Appetite 2008, 50, 120–127. [Google Scholar] [CrossRef]
- Thackray, A.E.; Willis, S.A.; Clayton, D.J.; Broom, D.R.; Finlayson, G.; Goltz, F.R.; Sargeant, J.A.; Woods, R.M.; Stensel, D.J.; King, J.A. Influence of Short-Term Hyperenergetic, High-Fat Feeding on Appetite, Appetite-Related Hormones, and Food Reward in Healthy Men. Nutrients 2020, 12, 2635. [Google Scholar] [CrossRef] [PubMed]
- Stuart, E.A. Matching Methods for Causal Inference: A Review and a Look Forward. Stat. Sci. 2010, 25, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Van Walleghen, E.L.; Orr, J.S.; Gentile, C.L.; Davy, K.P.; Davy, B.M. Habitual Physical Activity Differentially Affects Acute and Short-Term Energy Intake Regulation in Young and Older Adults. Int. J. Obes. 2007, 31, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, N.T.; Møller, B.K.; Raben, A.; Kristensen, S.T.; Holm, L.; Flint, A.; Astrup, A. Determinants of Appetite Ratings: The Role of Age, Gender, BMI, Physical Activity, Smoking Habits, and Diet/Weight Concern. Food Nutr. Res. 2011, 55, 7028. [Google Scholar] [CrossRef]
- Beaulieu, K.; Hopkins, M.; Long, C.; Blundell, J.; Finlayson, G. High Habitual Physical Activity Improves Acute Energy Compensation in Nonobese Adults. Med. Sci. Sports Exerc. 2017, 49, 2268–2275. [Google Scholar] [CrossRef]
- Dorling, J.; Broom, D.R.; Burns, S.F.; Clayton, D.J.; Deighton, K.; James, L.J.; King, J.A.; Miyashita, M.; Thackray, A.E.; Bat-terham, R.L.; et al. Acute and Chronic Effects of Exercise on Appetite, Energy Intake, and Appetite-Related Hormones: The Modulating Effect of Adiposity, Sex, and Habitual Physical Activity. Nutrients 2018, 10, 1140. [Google Scholar] [CrossRef]
- Lund, M.T.; Taudorf, L.; Hartmann, B.; Helge, J.W.; Holst, J.J.; Dela, F. Meal Induced Gut Hormone Secretion Is Altered in Aerobically Trained Compared to Sedentary Young Healthy Males. Eur. J. Appl. Physiol. 2013, 113, 2737–2747. [Google Scholar] [CrossRef]
- Lv, Y.; Liang, T.; Wang, G.; Li, Z. Ghrelin, a Gastrointestinal Hormone, Regulates Energy Balance and Lipid Metabolism. Biosci. Rep. 2018, 38, BSR20181061. [Google Scholar] [CrossRef]
- Le Roux, C.W.; Batterham, R.L.; Aylwin, S.J.B.; Patterson, M.; Borg, C.M.; Wynne, K.J.; Kent, A.; Vincent, R.P.; Gardiner, J.; Ghatei, M.A.; et al. Attenuated Peptide YY Release in Obese Subjects Is Associated with Reduced Satiety. Endocrinology 2006, 147, 3–8. [Google Scholar] [CrossRef]
- Friedman, J.M. Leptin and the Endocrine Control of Energy Balance. Nat. Metab. 2019, 1, 754–764. [Google Scholar] [CrossRef]
- Livingstone, M.B.E.; Black, A.E. Markers of the Validity of Reported Energy Intake. J. Nutr. 2003, 133, 895S–920S. [Google Scholar] [CrossRef]
- Stunkard, A.J.; Messick, S. The Three-Factor Eating Questionnaire to Measure Dietary Restraint, Disinhibition and Hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef]
- Oustric, P.; Myers, A.; Gibbons, C.; Buckland, N.; Dalton, M.; Long, C.; Beaulieu, K.; Sophie Hollingworth, S.; Finlayson, G. Are Objectively Measured Free-Living Physical Activity and Sedentary Behaviour Associated with Control over Eating and Food Preferences in Women? Appetite 2018, 123, 465. [Google Scholar] [CrossRef]
- Horner, K.M.; Finlayson, G.; Byrne, N.M.; King, N.A. Food Reward in Active Compared to Inactive Men: Roles for Gastric Emptying and Body Fat. Physiol. Behav. 2016, 160, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Killgore, W.D.S.; Kipman, M.; Schwab, Z.J.; Tkachenko, O.; Preer, L.; Gogel, H.; Bark, J.S.; Mundy, E.A.; Olson, E.A.; Weber, M. Physical Exercise and Brain Responses to Images of High-Calorie Food. Neuroreport 2013, 24, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; O’Connor, S.G.; Belcher, B.R.; Page, K.A. Effects of Physical Activity and Sedentary Behavior on Brain Response to High-Calorie Food Cues in Young Adults. Obesity 2018, 26, 540–546. [Google Scholar] [CrossRef]
- Beaulieu, K.; Oustric, P.; Finlayson, G. The Impact of Physical Activity on Food Reward: Review and Conceptual Synthesis of Evidence from Observational, Acute, and Chronic Exercise Training Studies. Curr. Obes. Rep. 2020, 9, 63–80. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).