Abstract
Weissella, a genus of Gram-positive, facultatively anaerobic lactic acid bacteria, has emerged as a significant component of human microbiota with diverse biotechnological and therapeutic applications. This narrative review examines the current state of knowledge regarding Weissella taxonomy, physiological characteristics, and functional properties based on research spanning from 1993 to present. Weissella species demonstrate remarkable versatility, producing bioactive metabolites including exopolysaccharides (EPS), bacteriocins, and organic acids that confer antimicrobial, antioxidant, and anti-inflammatory properties. These bacteria show significant potential in food fermentation, probiotic applications, and therapeutic interventions for gut health, obesity, and inflammatory conditions. However, challenges persist regarding strain-specific pathogenicity, particularly with W. confusa as an opportunistic pathogen, and the need for comprehensive safety evaluations. Current limitations include variability in probiotic efficacy, incomplete understanding of host-microbe interactions, and gaps in metabolic pathway characterization. This review provides a foundation for advancing Weissella research and applications while highlighting critical areas requiring further investigation to fully harness their biotechnological and therapeutic potential.
1. Introduction
The human microbiome comprises a complex ecosystem of microorganisms, predominantly represented by the bacterial phyla Bacillota, Bacteroidetes, Proteobacteria, and Actinobacteria, which collectively maintain dynamic homeostasis with the host under physiological conditions [1,2]. Within this intricate microbial landscape, the genus Weissella, a significant member of the Bacillota phylum, has emerged as a microorganism of considerable interest due to its diverse physiological functions and potential therapeutic applications.
Originally classified within the genus Leuconostoc, Weissella was established as a distinct genus in 1993 following comprehensive phylogenetic analyses that revealed its unique taxonomic identity [3]. Morphologically, Weissella species are characterized as Gram-positive bacteria exhibiting pleomorphic cell shapes ranging from short rods to cocci, demonstrating remarkable environmental adaptability [4]. The genus displays ubiquitous ecological distribution, inhabiting diverse environments including soil, aquatic systems, plant surfaces, and the gastrointestinal tracts of humans and animals, where it constitutes an integral component of the gut microbiota.
The multifaceted roles of Weissella extend beyond mere colonization, encompassing significant contributions to host physiology through both direct bacterial effects and indirect metabolite-mediated mechanisms. Clinical investigations have demonstrated the immunomodulatory potential of Weissella species, exemplified by W. cibaria JW15, which significantly enhanced Natural Killer cell activity following daily administration of 1 × 1010 CFU to human subjects [5]. Recent advances have revealed novel applications for Weissella-derived products, including bacterial ghosts generated through sodium hydroxide treatment, which effectively stimulate macrophage activation and upregulate pro-inflammatory cytokine expression (IL-1β, TNF-α, and iNOS), suggesting potential as immunotherapeutic agents [6]. Furthermore, certain Weissella strains demonstrate antimicrobial properties through bacteriocin production, with W. hellenica QU 13 synthesizing broad-spectrum antibacterial compounds that lack typical N-terminal signal sequences [7].
These beneficial characteristics have positioned Weissella as a promising candidate for probiotic and biotechnological applications, particularly in food fermentation and biopharmaceutical development [8,9]. However, the clinical significance of Weissella is complicated by reports of opportunistic pathogenicity, with W. conflusa identified as a causative agent of neonatal sepsis in veterinary settings [10]. This dual nature underscores the complexity of host-microbe interactions and highlights the necessity for comprehensive strain-level characterization. Despite substantial progress in Weissella research, several critical knowledge gaps persist, including challenges in accurate strain identification, variability in probiotic efficacy across different strains, and incomplete understanding of the molecular mechanisms governing host-microbe interactions. These limitations impede the translation of research findings into practical applications and clinical interventions.
This comprehensive narrative review aims to synthesize current knowledge regarding the taxonomy, physiological characteristics, biotechnological potential, and health implications of Weissella species. Through systematic analysis of research published in the Web of Science database since the genus establishment in 1993, we provide an evidence-based foundation for advancing Weissella research and applications, while identifying future research directions essential for realizing its full potential in human health promotion and industrial innovation.
2. Taxonomy and Functional Characteristics of Weissella
Prior to 1993, Weissella species were classified within the genus Lactobacillus. The establishment of Weissella as a distinct genus was initiated following the seminal work of Collins et al., who demonstrated discernible differences between Weissella and Leuconostoc based on comprehensive phenotypic, biochemical, and 16S rRNA sequence analyses, particularly within the V6 hypervariable region (positions 1007–1022) [3]. This reclassification marked the beginning of a new era in Weissella research and taxonomy.
Building upon this foundational work, recent phylogenetic studies have significantly advanced our understanding of Weissella taxonomy through comprehensive genomic analyses. Based on Average Nucleotide Identity, Average Amino Acid Identity, 16S rRNA gene sequences, and homology analysis between 16S rRNA genes and complete genomes, several species have been confirmed as distinct taxonomic entities. These include W. beninensis LMG 25373, W. fabalis LMG 26217, W. uvarum B18NM42, W. fabaria LMG 24289, W. diestrammenae DSM 27840, and W. ghanensis DSM 19935, with each strain representing individual species within the genus [11]. Weissella comprises a genus of Gram-positive, facultatively anaerobic, lactic acid-producing bacteria that exhibit wide ecological distribution across diverse environments. These microorganisms are commonly found in host organisms, feces, soil, and fermented plant-based foods (Table 1), with the genus encompassing several well-characterized species, including W. cibaria, W. confusa, W. kimchi, W. sagaensis, and other ecologically significant strains. However, the clinical significance of this genus extends beyond its ecological distribution. While most Weissella strains are recognized for their probiotic properties, certain species exhibit opportunistic pathogenic behavior under specific conditions. W. confusa represents the most clinically significant species within this genus, having been isolated from various clinical cases including bacteremia, endocarditis, postoperative osteomyelitis, sepsis, and more recently, meningitis [12]. The pathogenicity of W. confusa is primarily attributed to the production of heat-stable exopolysaccharides (EPSs), which are considered potential virulence factors [13]. Interestingly, host-specific interactions appear to modulate pathogenic expression significantly. For instance, W. confusa strains isolated from locusts demonstrate reduced virulence potential, lacking toxin production and secretion systems, suggesting strain-specific genomic adaptations to particular host niches [14]. These findings underscore the critical importance of source-dependent safety evaluations for Weissella applications in food and therapeutic contexts.
Paradoxically, despite pathogenic concerns with certain strains, Weissella species demonstrate considerable beneficial properties with significant industrial potential. Glucans derived from W. confusa exhibit remarkable functional characteristics, including excellent hydrophilicity, thermal stability, and heavy metal chelation activity, supporting their application as functional additives in food processing and environmental remediation strategies [15]. Similarly, α-D-glucans synthesized by W. cibaria PDER21 demonstrate superior antioxidant properties, enhanced water-binding capacity, and improved solubility characteristics, thereby expanding their utility in diverse food processing applications [16]. These bioactive compounds represent promising candidates for the development of functional food ingredients and nutraceutical products. Furthermore, the probiotic potential of Weissella species is evidenced by their ability to promote the growth of beneficial bacterial populations through specific mechanisms. In vitro studies have demonstrated that Weissella strains can significantly enhance the proliferation of beneficial bacteria, including Lactobacillus spp. and Bifidobacterium spp., primarily through EPS production and associated prebiotic effects [17]. This symbiotic relationship highlights the potential of Weissella as a probiotic adjuvant in maintaining gut microbiota homeostasis and supporting digestive health.

Table 1.
Common species and sources of Weissella.
Table 1.
Common species and sources of Weissella.
| Strain | Source | References |
|---|---|---|
| W. ceti | Beaked whales (Mesoplodon bidens) | [18] |
| W. cibaria | Sorghum | [19] |
| W. cibaria GM93m3 | Raw goat milk | [20] |
| W. cibaria strain CXO-01 | Saliva | [21] |
| W. confusa | Feces | [22] |
| W. diestrammenae | Gut (Diestrammena coreana) | [23] |
| W. fabaria | Cocoa fermentation | [24] |
| W. fangxianensis | Chinese rice wine starter | [25] |
| W. fermenti | Kimchi | [26] |
| W. jogaejeotgali | Korean jogae jeotgal (Fermented clams) | [27] |
| W. kimchii | Green onion | [28] |
| W. muntiaci | Faece (Muntiacus reevesi) | [29] |
| W. oryzae | Fermented rice grains | [30] |
| W. sagaensis | Traditional Chinese yogurt | [31] |
| W. soli | Soil | [32] |
3. Growth Conditions and Physiological Characteristics
Weissella species exhibit distinctive morphological and physiological characteristics that define their growth requirements and metabolic capabilities. Colonies present as small, circular, gray-white formations with a characteristic moist appearance. Microscopically, Weissella cells are short, rod-shaped bacteria that typically occur in pairs or short chains [33]. These bacteria demonstrate species-specific growth optima, although most strains favor mildly acidic conditions with pH ranges of 5.0–6.5 and temperature ranges of 30–37 °C. Growth becomes significantly inhibited under neutral to alkaline conditions, resulting in reduced biomass accumulation and extended lag phases [34]. Weissella species are heterotrophic and facultatively anaerobic, enabling survival in diverse oxygen environments.
Weissella species utilize various carbohydrates including glucose, maltose, and sucrose as primary carbon sources. Through heterofermentative pathways, particularly the phosphoketolase pathway, these bacteria generate multiple bioactive metabolites including lactic acid, bacteriocins, hydrogen peroxide, exopolysaccharides (EPSs), and diacetyl [35,36,37]. W. viridescens demonstrates additional metabolic versatility by producing volatile organic acids (acetic, propionic, and butyric acids), esters, and free amino acids during fermentation processes. The concentrations of three sweet amino acids—threonine, serine, and glycine—increase proportionally with salt concentration, contributing to both flavor development and preservation properties in food systems [38,39].
Recent metabolomic investigations have revealed remarkable stress tolerance capabilities in Weissella species. W. confusa demonstrates exceptional salinity tolerance, surviving concentrations up to 35% NaCl in MRS medium for 24 h. This tolerance is mediated through the accumulation of osmoprotectants such as ectoine, lactose metabolism alterations, and modified amino acid and nucleotide metabolic pathways [40]. Under combined acid and bile salt stress conditions, W. confusa ZJU exhibits significant metabolic reprogramming, including upregulation of alanine, aspartate, and glutamate metabolism pathways. Enhanced glycolysis and gluconeogenesis pathways serve as central mechanisms for carbohydrate metabolism, conferring increased tolerance to bile salt stress [41].
EPS production in Weissella species has been systematically optimized using Response Surface Methodology (RSM). For W. confusa 126, optimal cultivation parameters were determined as: cultivation time of 48.50 h, sucrose concentration of 24.00 g/L, pH 7.00, and yeast extract concentration of 2.50%. Under these optimized conditions, EPS production reached 3.00 g/L [42]. Comparative analysis between wild-type and mutant strains revealed minimal variation in EPS production capacity, with yields ranging from 5490.16 to 5580.72 mg/L [43].
4. Microbial Regulation Mechanisms of Weissella
Weissella species demonstrate substantial antimicrobial potential through diverse biological mechanisms involving synergistic metabolite interactions and multiple inhibitory pathways (Figure 1). These lactic acid bacteria exhibit broad-spectrum activity against both bacterial and fungal pathogens through distinct yet complementary mechanisms of action. The antimicrobial efficacy of Weissella species has been extensively documented against various pathogenic bacteria. W. cibaria JW15 demonstrates inhibitory activity against multiple foodborne pathogens, including Listeria monocytogenes, Salmonella typhimurium, Salmonella enteritidis, and Escherichia coli. Beyond direct antimicrobial effects, this strain interferes with pathogen adhesion to intestinal epithelial cells, thereby providing protective benefits to the intestinal barrier. Additionally, W. cibaria JW15 exhibits significant antioxidant properties through the scavenging of reactive oxygen species, including DPPH, ABTS, and hydroxyl radicals, consequently preventing lipid peroxidation processes [44]. Mechanistic studies have elucidated specific pathways through which Weissella species exert antimicrobial effects. Kibar et al. investigated the impact of Weissella-derived compounds on Streptococcus mutans survival and biofilm formation through controlled in vitro experiments. Their findings demonstrated that glucan at 50 mg/mL concentration inhibited biofilm formation by 70%, with antioxidant activity exhibiting dose-dependent responses [45]. The cell-free culture supernatant of W. confusa WM36 contains multiple antimicrobial compounds, primarily organic acids (lactic and acetic acids) and 2,4-di-tert-butylphenol. These metabolites demonstrate concentration-dependent antimicrobial activity against Salmonella typhi. At 40% concentration, the supernatant achieved greater than 99.99% inhibition of biofilm formation, while 20% concentration completely suppressed bacterial motility. Furthermore, at 10% concentration, these metabolites downregulated critical virulence genes associated with type III secretion systems, effector proteins, and quorum-sensing mechanisms [46].
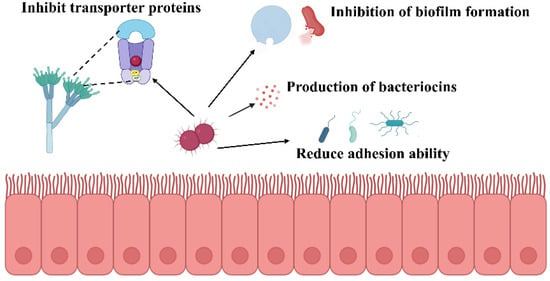
Figure 1.
Potential antibacterial mechanisms of Weissella.
Bacteriocin production represents another significant antimicrobial mechanism in Weissella species. These ribosomally synthesized antimicrobial proteins or peptides are encoded by bacterial genes and provide competitive advantages in natural microbial environments. In W. confusa MBF8-1, a 17,643 bp plasmid designated pWcMBF8-1 governs bacteriocin production. This genetic element encodes double-glycine motif peptides (Bac1, Bac2, Bac3), an ABC transporter complex (BacTE), and a putative immunity protein (BacI), enabling the strain to survive in highly competitive microbial ecosystems [47]. Weissella species also exhibit notable antifungal properties through distinct mechanisms. W. confusa AL3, isolated from human gut microbiota, demonstrates inhibitory activity against Aspergillus flavus MTCC 2798. Chemical analysis of the methanol extract identified cyclo-leucyl-proline and 1,2-benzenedicarboxylic acid as the primary antifungal compounds responsible for this activity [48]. Similarly, the cell-free supernatant of W. cibaria BYL4.2 exhibits pH-dependent antifungal activity that remains stable following protein treatment. Metabolomic analysis using liquid chromatography-mass spectrometry identified D-tartaric acid as a key antifungal metabolite that inhibits Penicillium chrysogenum growth through disruption of ABC transporter metabolic pathways [49].The antimicrobial activity of Weissella species operates through multiple coordinated mechanisms, encompassing pathogen growth inhibition, adhesion interference, reactive oxygen species scavenging, antimicrobial metabolite production, and fungal growth suppression. The synergistic interactions among these mechanisms underscore the considerable potential of Weissella species in antimicrobial and antioxidant applications, providing substantial theoretical foundations and practical frameworks for developing novel biotherapeutic agents.
5. Potential of Weissella in the Food Industry
Probiotics are defined as live microorganisms that confer health benefits to the host through multiple mechanisms, including adherence to host tissues, immune function enhancement, modulation of host metabolic activities, and performance of beneficial metabolic functions [50]. Weissella species have emerged as promising probiotic candidates due to their exceptional physiological characteristics, including superior environmental tolerance, antimicrobial activity, β-glucosidase activity, and intestinal adhesion capabilities. Among these attributes, the remarkable ability of Weissella species to produce exopolysaccharides represents a particularly valuable trait for industrial applications.
The exopolysaccharides produced by Weissella species possess distinctive adhesive, cohesive, antioxidant, and immunomodulatory properties that confer significant value in both food additive and biomedical applications. W. confusa demonstrates exceptional utility in sourdough fermentation processes, producing hydrophilic EPS that substantially enhances the rehydration properties and cooking quality of fat-free instant noodles (FFNs). This improvement is achieved through increased gelatinization and reduced relative crystallinity, which additionally enhances the in vitro starch digestibility of FFNs [51]. Furthermore, W. confusa strains W1 and W2 exhibit enhanced adhesion properties and environmental stress resistance attributable to their robust EPS production capabilities, positioning them as suitable candidates for diverse food industry applications [52].
Genomic analysis of Weissella species has provided insights into their probiotic potential and safety profiles. Rocha et al. conducted complete genome sequencing of W. paramesenteroides UFTM 2.6.1, isolated from unpasteurized milk, followed by comprehensive pangenomic analysis. This investigation identified 99 unique genes associated with probiotic functions, encompassing genes involved in stress response mechanisms, gastrointestinal persistence, and vitamin biosynthesis pathways. In vitro antimicrobial assays confirmed the strain’s activity against Listeria spp. The absence of CRISPR-Cas arrays, Cas proteins, and resistance or virulence genes demonstrated the safety profile of this strain, providing a foundation for Weissella applications in food safety and biotechnology [53].
Exopolysaccharides represent complex carbohydrate compounds synthesized during microbial growth and development, functioning as adaptive responses to environmental conditions. These polymers are classified into homopolysaccharides and heteropolysaccharides based on their compositional characteristics. Probiotic bacteria constitute a significant proportion of EPS-producing microorganisms, with Weissella species exhibiting exceptional glucan EPS production capabilities. Environmental factors influence both EPS production and bioactivity, with sucrose enhancing overall EPS yield while galactose specifically enhances the anti-inflammatory properties of the resulting polymers [54,55].
The EPSs produced by Weissella species are predominantly heteropolysaccharides composed of eight distinct monosaccharides, with glucose and galactose representing the most abundant components [43]. The complex polysaccharide architecture of Weissella-derived EPS contributes to their substantial antioxidant activity, particularly through interactions with DPPH radicals. The synergistic effects of antioxidant components and unique glycosidic configurations play crucial roles in preventing oxidative damage in biological systems [56].
The biosynthesis of EPS occurs primarily through extracellular synthesis pathways involving three sequential steps: monosaccharide and sugar nucleotide synthesis, polysaccharide assembly, and transport of synthesized polysaccharides to the extracellular environment [57]. The efficiency and purity of EPS extraction vary considerably depending on the producing strain and isolation methodology employed. Common EPS isolation techniques include precipitation from culture media, alkaline treatment, ion scavenging approaches, and ultrasonication methods [58]. Size-exclusion chromatography represents an efficient approach for separating crude EPS preparations. Purification procedures typically employ protein removal techniques, EPS precipitation methods, and membrane filtration processes. Repeated purification cycles using gel permeation chromatography can yield sufficient quantities of highly purified EPS [59,60]. However, the lack of comprehensive information regarding EPS cellular localization continues to present challenges for developing efficient extraction and purification methodologies.
6. Weissella’s Association with Health
6.1. Weissella and Gut Health
The gut microbiota represents a critical component of human health maintenance, with Weissella serving as one of the essential cellulose-degrading genera that contribute significantly to intestinal homeostasis (Figure 2). Specifically, Weissella species employ specialized enzymatic systems to degrade indigestible polysaccharides and subsequently produce glucan, which functions within the body’s antioxidant defense system to protect the intestinal barrier and mitigate intestinal damage. For instance, W. cibaria produces β-xylosidase enzyme, which hydrolyzes ammonia-pretreated rice straw (A-PRS) to generate bioactive xylooligosaccharides [61]. In addition, after the rainbow trout was fed a plant-based diet for the first time, the intestinal microorganisms changed to Firmicutes, which can metabolize carbohydrates, mainly including Streptococcus, Leuconostoc and Weissella [62,63].

Figure 2.
Mechanisms by which Weissella promotes intestinal health.
Given that the gut microbiota comprises a complex ecosystem consisting primarily of bacteria, fungi, viruses, and archaea, microbial diversity serves as the fundamental basis for maintaining intestinal health. In this context, research demonstrates that galactan exopolysaccharides produced by W. confusa KR780676 promote the growth of beneficial bacteria, specifically enhancing the proliferation of Lactobacillus plantarum MTCC9510 and Lactobacillus fermentum MTCC903 [64]. Similarly, glucans synthesized by W. cibaria RBA12, characterized by a 97% α-(1→6) backbone structure with 3% α-(1→3) branching, stimulate the in vitro growth of Bifidobacterium and Lactobacillus species [65]. Collectively, these findings indicate that Weissella species support gut health through mechanisms that increase both the abundance and diversity of beneficial bacterial populations.
Beyond promoting beneficial bacterial growth, specific polysaccharide components produced by Weissella species demonstrate distinct protective mechanisms for intestinal barrier function. Notably, Zhao et al. isolated a polysaccharide component designated EPS-2 with a molecular weight of 845 kDa from W. cibaria. In contrast to other exopolysaccharides, EPS-2 exhibits direct protective effects on the intestinal barrier by reversing the propionate level decreases associated with colitis, thereby improving colonic goblet cell function and mucin content. Furthermore, mechanistic studies reveal that propionate binds to G protein-coupled receptor 43 on intestinal epithelial cell surfaces, consequently increasing histone acetylation levels, promoting expression of tight junction proteins occludin and ZO-1, and enhancing mucin production [66,67].
In addition to barrier protection, Weissella species demonstrate substantial capacity for alleviating oxidative stress within the intestinal environment, consequently reducing oxidative damage. The exopolysaccharides produced by these bacteria exhibit exceptional antioxidant activity, which represents a key mechanism underlying their protective effects [42,68]. More specifically, during lipopolysaccharide (LPS) exposure, W. cibaria MW01 inhibits nuclear translocation of NF-κB and inactivates the myosin light chain kinase MLCK-pMLC pathway, thereby attenuating the secretion of pro-inflammatory cytokines including TNF-α and IL-6, and mitigating intestinal inflammation [69].
Moreover, the regulatory mechanisms governing NF-κB activity involve SIRT1-mediated pathways. Particularly, SIRT1 activation induces deacetylation of NF-κB-p65 at lysine 310 (K310) and histone H3 at lysine 9 (K9). Consequently, Sirtuin1 (SIRT1) activation results in reduced NF-κB-p65 DNA binding capacity and mitigates oxidative stress through decreased transcription of NADPH oxidase subunits [70]. Supporting these mechanisms, in DSS-induced mouse models of intestinal inflammation, W. paramesenteroides NRIC1542 increased SIRT1 protein expression, suppressed NF-κB activation, and reduced TNF-α and IL-1β levels [71].
The antioxidant properties of Weissella species have been further validated through studies utilizing alternative model systems. Specifically, the antioxidant effects were demonstrated in the Caenorhabditis elegans model system, where fructan produced by W. cibaria MD2 enhanced oxidative stress resistance and extended lifespan in C. elegans through promotion of nuclear translocation of the transcription factor DAF-16 [72].
Finally, Weissella species demonstrate direct adhesive interactions with intestinal tissues to exert protective functions. Remarkably, in gut barrier dysfunction models, W. cibaria MW01 exhibited superior adhesion to intestinal cells compared with Lactobacillus rhamnosus GG, while simultaneously mitigating LPS-induced inflammation, preventing tight junction protein downregulation, and restoring gut barrier integrity [69]. Similarly, in the hydrogen peroxide-induced inflammatory bowel disease model, W. confusa F213 could increase the transmembrane epithelial resistance and ZO-1 expression to maintain mucosal integrity [73]. These multifaceted protective mechanisms underscore the significant potential of Weissella species in maintaining and restoring intestinal health.
6.2. Weissella and Other Diseases
The probiotic properties of Weissella extend beyond gut health, encompassing reported benefits in obesity prevention, inflammation modulation, and detoxification processes. These diverse therapeutic applications demonstrate the multifaceted potential of Weissella species in health promotion and disease prevention (Table 2). Regarding obesity prevention, W. koreensis OK1-6 inhibits fat accumulation in 3T3-L1 adipocytes through downregulation of key lipogenic genes, including CCAAT enhancer-binding protein α (C/EBPα), sterol regulatory element binding protein 1 (SREBP1), and fatty acid synthase (FAS), thereby indicating its potential in preventing obesity development [74]. Complementing these findings, W. cibaria MG5285 suppresses lipogenic protein expression and promotes phosphorylation of adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) and acetyl-CoA carboxylase in hepatic tissue, consequently reducing lipid accumulation [75]. Collectively, these findings highlight the capacity of Weissella species to inhibit fat synthesis pathways, thereby driving further investigation into their potential as next-generation anti-obesity probiotics. In addition to metabolic regulation, Weissella species exhibit significant anti-inflammatory properties through multiple mechanisms. In vitro studies have demonstrated that W. cibaria strains alleviate lipopolysaccharide (LPS)-induced inflammatory responses by reducing the production of nitric oxide, IL-6, and IL-1β in macrophage cultures [76]. Furthermore, in dermatological inflammation models, oral administration of W. cibaria WIKIM28 effectively reduced atopic dermatitis-like skin lesions, epidermal thickening, and serum immunoglobulin E (IgE) levels [77].
To further elucidate the anti-inflammatory mechanisms of Weissella, Hong et al. isolated lipoteichoic acid from W. cibaria and reported that this compound promoted immune factor secretion in a dose-dependent manner. Additionally, lipoteichoic acid activates NF-κB, p38, and c-Jun N-terminal kinase phosphorylation in THP-1 cells while simultaneously stimulating immune factor secretion in mouse splenocytes, suggesting potential therapeutic applications in treating immunosuppressive diseases [78]. Aging could cause chronic inflammation. Surprisingly, compared with middle-aged people, the abundance of Weissella in intestinal microorganisms in the elderly was significantly increased, which suggested that the anti-inflammatory effect of Weissella might be achieved by compensatory increase in its abundance [79]. In Alzheimer’s disease, microglia and astrocytes are involved in its inflammatory response [80]. Studies have shown that Weisslla can reduce oxidative stress and reduce cognitive impairment of Alzheimer’s disease through SIRT1/PGC-1α [81]. Beyond metabolic and immunological benefits, Weissella species also demonstrate significant potential in detoxification processes, particularly for heavy metal remediation. The liver and kidneys, which serve as critical organs for heavy metal metabolism, are particularly vulnerable to heavy metal toxicity. In vivo investigations have shown that W. cibaria WD2 effectively alleviates cadmium- and lead-induced hepatic and renal damage [82]. Mechanistically, in vitro studies revealed that W. viridescens ZY-6 adsorbs cadmium ions (Cd2+) primarily through electrostatic interactions with negatively charged functional groups, including hydroxyl (-OH), amino (-NH2), carboxylate (COO−), and phosphoryl (P=O) groups present on the bacterial cell surface. Supporting these findings, scanning electron microscopy and Fourier transform infrared spectroscopy confirmed that Cd2+ adsorption induces physiological changes in bacterial cells, characterized by cellular wrinkling and elongation [83]. These observations suggest that Weissella species can function as effective biosorbents for mitigating cadmium contamination in environmental systems, human dietary sources, and animal feed applications.

Table 2.
Probiotic characteristics and applications of Weissella.
Table 2.
Probiotic characteristics and applications of Weissella.
| Strain | Probiotic Properties | Application | References |
|---|---|---|---|
| W. confusa WM36 | Produce antimicrobial substances such as antimicrobial peptides, organic acids, and 2,4-di-tert-butylphenol | Alternative therapies as non-antibiotic approaches for typhoid fever control | [47] |
| W. cibaria CMS1 | Inhibit the formation of the Streptococcus mutans biofilm | Decrease the risk of respiratory tract and intestinal infections | [84] |
| W. cibaria WIKIM28 | Inhibit local accumulation and degranulation rate of mast cells | Potential use as a dietary supplement or therapeutic agent | [77] |
| W. confusa PL9001 | Target and destroy bacterial cell wall by secreting bacteriocin with bactericidal activity | Development of a novel gastric probiotic | [85] |
| W. paramesenteroides MYPS5.1 | Product high concentrations of extracellular polysaccharides | Potential anticancer agent | [86] |
| W. confusa F213 | Enhance the resistance of the gastrointestinal environment | Adjuvant therapy for inflammatory bowel diseases | [73] |
| W. viridescens UCO-SMC3 | Produce lactic acid, hydrogen peroxide, and bacteriocins with strong bactericidal activity | Reduce inflammatory response. | [87] |
| W. cibaria CMU | Produce bactericidal substances: hydrogen peroxide and organic acids (lactic acid, acetic acid, and citric acid) | Improve oral health and prevent oral diseases | [88] |
| W. sp. SNUL2 | Produce peptidases | Regulate gut microbiota composition | [89] |
| W. viridescens Wv2365 | Produce exopolysaccharides | Improve symptoms of metabolic dysfunction-associated steatotic liver disease | [90] |
7. Challenges and Future Research Directions
Despite significant advances in Weissella research, several critical challenges and limitations continue to impede comprehensive understanding and practical application of these microorganisms. Although whole-genome sequencing, metabolomics, and isotope tracing techniques have provided valuable insights into the metabolic pathways of Weissella, their application remains constrained by methodological limitations. For instance, metagenomics approaches may not adequately distinguish functional differences between closely related strains, thereby necessitating more refined genomic and metabolomic analytical approaches to elucidate strain-specific characteristics and capabilities. Furthermore, while exopolysaccharides represent the most extensively studied metabolites of Weissella, other potentially significant metabolites, particularly short-chain fatty acids (SCFAs), have received comparatively limited research attention. Consequently, the structural and functional diversity of bioactive compounds produced by Weissella species, as well as their specific roles in different environmental contexts, require comprehensive investigation to fully understand their therapeutic and industrial potential.
Notably, a promising characteristic of Weissella species is their demonstrated tolerance to gastric acid and bile salts [64,91], a trait that significantly enhances their potential for broader industrial and therapeutic applications. However, despite the well-documented probiotic properties of most Weissella strains, their potential pathogenicity cannot be overlooked and represents a critical safety concern. Specifically, W. ceti has been reported to cause mortality rates reaching 60% in farmed rainbow trout populations in Peru, highlighting the substantial risks associated with insufficient understanding of the pathogenic potential within the Weissella genus [92]. Nevertheless, current safety evaluations of Weissella species have yielded promising results, particularly in mammalian model systems. Studies conducted in rat models have demonstrated that W. cibaria exhibits safety at doses as high as 5000 mg/kg body weight (bw)/day (1.8 × 109 CFU/kg bw/day), with no evidence of mutagenicity or chromosomal aberrations [93]. Additionally, phenotypic safety assessments of W. cibaria JW15 have not detected the presence of virulence genes, including cytolysin (cylA), aggregation substance (asa1), hyaluronidase (hyl), and gelatinase (gelE) [94]. Moreover, the exopolysaccharides produced by Weissella bacteria have been granted generally recognized as safe status, supporting their potential for food industry applications [55]. Despite these encouraging safety profiles in animal models, Weissella research still lacks adequate clinical validation in human populations, representing a significant gap in translational research. Furthermore, in view of the resistance to vancomycin of Weisseria isolated from different environments, comprehensive safety evaluations are still required, particularly regarding quorum-sensing systems and long-term health effects in human hosts [95]. Even if Weissella species receive regulatory approval as probiotics, substantial research efforts will be necessary to address remaining challenges related to encapsulation technologies, the safety and efficacy profiles of their metabolites, and their long-term impact on host health dynamics. These multifaceted research and development efforts will be critical to fully harness the therapeutic and industrial potential of Weissella species while ensuring their safe and effective application in both commercial and clinical settings. Addressing these challenges through systematic investigation will be essential for advancing Weissella from promising research candidates to validated therapeutic and industrial agents.
8. Conclusions
This comprehensive review demonstrates that Weissella represents a versatile genus of lactic acid bacteria with significant potential for human health and industrial applications. The research reveals Weissella’s multifaceted nature, encompassing probiotic properties, antimicrobial activities, and biotechnological applications, particularly in exopolysaccharide production. The genus produces diverse bioactive compounds including bacteriocins, organic acids, and EPS that contribute to antioxidant, anti-inflammatory, and prebiotic functions while promoting gut health through enhancing beneficial bacterial growth, strengthening intestinal barrier function, and modulating immune responses. However, this review highlights the dual nature of Weissella, with certain strains like W. confusa exhibiting opportunistic pathogenic behavior, underscoring the critical importance of strain-specific characterization and safety assessments before clinical applications. Future research should prioritize multi-omics approaches to understand strain-specific differences and identify genetic markers distinguishing beneficial from pathogenic strains. Critical areas include elucidating molecular mechanisms of host-microbe interactions, conducting long-term clinical trials to establish safety profiles and therapeutic efficacy, optimizing industrial cultivation methods and encapsulation technologies, developing regulatory frameworks for safety assessment and quality control, and exploring environmental applications in bioremediation and agriculture. The remarkable potential of Weissella warrants continued investment in research and development, promising innovative solutions for human health, food technology, and sustainable biotechnological applications.
Author Contributions
Conceptualization, W.M. and C.W.; methodology, X.Z.; writing—original draft preparation, X.L. and Y.J.; writing—review and editing, W.M. and M.Z.K.; visualization, M.Z. (Meixia Zhang); funding acquisition, M.Z. (Mingxia Zhu). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Start Up Funds for Doctoral Research of Liaocheng University (318052512), the Key R&D Program of Shandong Province, China (2024LZGC020 and 2024LZGC002), the National Student Innovation and Entrepreneurship Program (202410447021), and the University-level Student Innovation and Entrepreneurship Project (CXCY2024302).
Data Availability Statement
No new data were created or analyzed in this study. Data Sharing is not applicable to this article.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AMPK | adenosine 5′-monophosphate (AMP)-activated protein kinase |
| A-PRS | ammonia-pretreated rice straw |
| CCAAT | CCAAT enhancer-binding protein α |
| EPS | exopolysaccharides |
| FAS | fatty acid synthase |
| FFNs | fat-free instant noodles |
| IgE | immunoglobulin E |
| RSM | Response Surface Methodology |
| SCFAs | short-chain fatty acids |
| SIRT1 | Sirtuin1 |
| SREBP1 | sterol regulatory element binding protein 1 |
References
- Hugon, P.; Dufour, J.C.; Colson, P.; Fournier, P.E.; Sallah, K.; Raoult, D. A comprehensive repertoire of prokaryotic species identified in human beings. Lancet Infect. Dis. 2015, 15, 1211–1219. [Google Scholar] [CrossRef]
- Oren, A.; Göker, M.; Sutcliffe, I.C.; Executive, B.I.C.S. New phylum names harmonize prokaryotic nomenclature. mBio 2022, 13, 01479. [Google Scholar] [CrossRef]
- Collins, M.D.; Samelis, J.; Metaxopoulos, J.; Wallbanks, S. Taxonomic studies on some Leuconostoc-like organisms from fermented sausages: Description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J. Appl. Bacteriol. 1993, 75, 595–603. [Google Scholar] [CrossRef]
- Teixeira, C.G.; Fusieger, A.; Miliao, G.L.; Martins, E.; Drider, D.; Nero, L.A.; de Carvalho, A.F. Weissella: An emerging bacterium with promising health benefits. Probiotics Antimicrob. Proteins 2021, 13, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, A.; Yoo, H.J.; Kim, M.; Noh, G.M.; Lee, J.H. Supplementation with the probiotic strain Weissella cibaria JW15 enhances natural killer cell activity in nondiabetic subjects. J. Funct. Foods 2018, 48, 153–158. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, K.S.; Kim, W.M.; Kim, M.; Park, H.O.; Choi, C.W.; Han, J.S.; Park, S.Y.; Lee, K.S. Sodium hydroxide-induced Weissella kimchii ghosts (WKGs) as immunostimulant. Mol. Cell Toxicol. 2023, 19, 805–815. [Google Scholar] [CrossRef]
- Masuda, Y.; Zendo, T.; Sawa, N.; Perez, R.H.; Nakayama, J.; Sonomoto, K. Characterization and identification of weissellicin y and weissellicin m, novel bacteriocins produced by Weissella hellenica QU 13. J. Appl. Microbiol. 2012, 112, 99–108. [Google Scholar] [CrossRef]
- Liu, X.J.; Qu, H.Y.; Gou, M.X.; Guo, H.Y.; Wang, L.Y.; Yan, X.H. Application of Weissella cibaria x31 or Weissella confusa l2 as a starter in low nitrite dry-fermented sausages. Int. J. Food Eng. 2020, 16, 20190344. [Google Scholar] [CrossRef]
- Xiang, W.L.; Zhang, N.D.; Lu, Y.; Zhao, Q.H.; Xu, Q.; Rao, Y.; Liu, L.; Zhang, Q. Effect of Weissella cibaria co-inoculation on the quality of sichuan pickle fermented by lactobacillus plantarum. Lebensm. Wiss. Technol. 2020, 121, 108975. [Google Scholar] [CrossRef]
- Lawhon, S.D.; Lopez, F.R.; Joswig, A.; Black, H.C.; Watts, A.E.; Norman, T.E.; Porter, B.F. Weissella confusa septicemia in a foal. J. Vet. Diagn. Investig. 2014, 26, 150–153. [Google Scholar] [CrossRef]
- Fanelli, F.; Montemurro, M.; Chieffi, D.; Cho, G.S.; Franz, C.; Dell’Aquila, A.; Rizzello, C.; Fusco, V. Novel insights into the phylogeny and biotechnological potential of Weissella species. Front. Microbiol. 2022, 13, 914036. [Google Scholar] [CrossRef]
- Cheaito, R.A.; Awar, G.; Alkozah, M.; Cheaito, M.A.; El Majzoub, I. Meningitis due to Weissella confusa. Am. J. Emerg. Med. 2020, 38, 1298.e1–1298.e3. [Google Scholar] [CrossRef] [PubMed]
- Siavoshi, F.; Ebrahimi, H.; Sarrafnejad, A. Weissella confusa with thermostable β-hemolytic exopolysaccharide. Toxicon 2021, 202, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.L.; Wang, Y.D.; Zhao, F.Q.; Kang, L. Complete genome sequence of Weissella confusa LM1 and comparative genomic analysis. Front. Microbiol. 2021, 12, 749218. [Google Scholar] [CrossRef] [PubMed]
- Du, R.P.; Pei, F.Y.; Kang, J.; Zhang, W.; Wang, S.; Ping, W.X.; Ling, H.Z.; Ge, J.P. Analysis of the structure and properties of dextran produced by Weissella confusa. Int. J. Biol. Macromol. 2022, 204, 677–684. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Ispirli, H.; Taylan, O.; Alamoudi, M.; Dertli, E. Bioactive and technological properties of an α-d-glucan synthesized by Weissella cibaria PDER21. Carbohydr. Polym. 2022, 285, 119227. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, J.; Liu, L.N.; Wang, S.; Ping, W.X.; Ge, J.P. Characterization of exopolysaccharides produced by Weissella confusa XG-3 and their potential biotechnological applications. Int. J. Biol. Macromol. 2021, 178, 306–315. [Google Scholar] [CrossRef]
- Vela, A.I.; Fernández, A.; de Quirós, Y.B.; Herráez, P.; Domínguez, L.; Fernández-Garayzábal, J.F. weissella ceti sp nov., Isolated from beaked whales (Mesoplodon bidens). Int. J. Syst. Evol. Microbiol. 2011, 61, 2758–2762. [Google Scholar] [CrossRef]
- Falasconi, I.; Fontana, A.; Patrone, V.; Rebecchi, A.; Garrido, G.D.; Principato, L.; Callegari, M.L.; Spigno, G.; Morelli, L. Genome-assisted characterization of lactobacillus fermentum, Weissella cibaria, and Weissella confusa strains isolated from sorghum as starters for sourdough fermentation. Microorganisms 2020, 8, 1388. [Google Scholar] [CrossRef]
- Akinyemi, M.O.; Oyedele, O.A.; Kleyn, M.S.; Onarinde, B.A.; Adeleke, R.A.; Ezekiel, C.N. Draft genome sequences of Weissella cibaria GM93m3, a promising probiotic strain from raw goat milk. Microbiol. Resour. Announc. 2024, 13, e0027024. [Google Scholar] [CrossRef]
- Jo, H.; Lee, D.; Kim, H.; Kim, J.; Kang, S.; Mun, S.; Karm, M.; Kim, H.J. Complete genome sequence of Weissella cibaria strain CXO-01 isolated from korean saliva. Microbiol. Resour. Announc. 2024, 13, e0015124. [Google Scholar] [CrossRef]
- Wang, W.; Liu, W.; Chu, W. Isolation and preliminary screening of potentially probiotic weissella confusa strains from healthy human feces by culturomics. Microb. Pathog. 2020, 147, 104356. [Google Scholar] [CrossRef]
- Oh, S.J.; Shin, N.R.; Hyun, D.W.; Kim, P.S.; Kim, J.Y.; Kim, M.S.; Yun, J.H.; Bae, J.W. Weissella diestrammenae sp nov., Isolated from the gut of a camel cricket (Diestrammena coreana). Int. J. Syst. Evol. Microbiol. 2013, 63, 2951–2956. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, K.; Camu, N.; De Vuyst, L.; Vandamme, P. Weissella fabaria sp. Nov., From a ghanaian cocoa fermentation. Int. J. Syst. Evol. Microbiol. 2010, 60, 1999–2005. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.S.; Dong, Y.; Cai, W.C.; Zhao, H.J.; Liu, H.J.; Shan, C.H.; Guo, Z. Comparative genomic analysis of the genus Weissella and taxonomic study of Weissella fangxianensis sp. Nov., Isolated from chinese rice wine starter. Int. J. Syst. Evol. Microbiol. 2023, 73, 005870. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Baek, J.H.; Han, D.M.; Lee, S.H.; Kim, S.Y.; Jeon, C.O. Description and genomic characteristics of Weissella fermenti sp. Nov., Isolated from kimchi. J. Microbiol. Biotechnol. 2023, 33, 1448–1456. [Google Scholar] [CrossRef]
- Lee, S.H.; Ku, H.J.; Ahn, M.J.; Hong, J.S.; Lee, S.H.; Shin, H.; Lee, K.C.; Lee, J.S.; Ryu, S.; Jeon, C.O.; et al. Weissella jogaejeotgali sp nov., Isolated from jogae jeotgal, a traditional korean fermented seafood. Int. J. Syst. Evol. Microbiol. 2015, 65, 4674–4681. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, J.Y.; Song, H.S.; Park, J.H.; Ji, G.E.; Kim, H.Y. Isolation and identification of Weissella kimchii from green onion by cell protein pattern analysis. J. Microbiol. Biotechnol. 2004, 14, 105–109. [Google Scholar]
- Lin, S.T.; Wang, L.T.; Wu, Y.C.; Guu, J.; Tamura, T.; Mori, K.; Huang, L.; Watanabe, K. Weissella muntiaci sp. Nov., Isolated from faeces of formosan barking deer (Muntiacus reevesi). Int. J. Syst. Evol. Microbiol. 2020, 70, 1578–1584. [Google Scholar] [CrossRef]
- Tohno, M.; Kitahara, M.; Inoue, H.; Uegaki, R.; Irisawa, T.; Ohkuma, M.; Tajima, K. weissella oryzae sp nov., Isolated from fermented rice grains. Int. J. Syst. Evol. Microbiol. 2013, 63, 1417–1420. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.Y.; Wang, T. Cadmium biosorption by lactic acid bacteria Weissella viridescens ZY-6. Food Control 2021, 123, 107747. [Google Scholar] [CrossRef]
- Magnusson, J.; Jonsson, H.; Schnürer, J.; Roos, S. Weisselia soli sp nov., A lactic acid bacterium isolated from soil. Int. J. Syst. Evol. Microbiol. 2002, 52, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Björkroth, K.J.; Schillinger, U.; Geisen, R.; Weiss, N.; Vandamme, P. Taxonomic study of Weissella confusa and description of Weissella cibaria sp. Nov., Detected in food and clinical samples. Int. J. Syst. Evol. Microbiol. 2002, 52, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Ramana, K.V. Purification and characterization of bacteriocin from Weissella paramesenteroides DFR-8, an isolate from cucumber (Cucumis sativus). J. Food Biochem. 2010, 34, 932–948. [Google Scholar] [CrossRef]
- Fusco, V.; Quero, G.M.; Cho, G.S.; Kabisch, J.; Meske, D.; Neve, H.; Bockelmann, W.; Franz, C. The genus Weissella: Taxonomy, ecology and biotechnological potential. Front. Microbiol. 2015, 6, 155. [Google Scholar] [CrossRef]
- Dey, D.K.; Koo, B.G.; Sharma, C.; Kang, S.C. Characterization of Weissella confusa DD_a7 isolated from kimchi. Lebensm. Wiss. Technol. 2019, 111, 663–672. [Google Scholar] [CrossRef]
- Galli, V.; Venturi, M.; Coda, R.; Maina, N.H.; Granchi, L. Isolation and characterization of indigenous Weissella confusa for in situ bacterial exopolysaccharides (EPS) production in chickpea sourdough. Food Res. Int. 2020, 138, 109785. [Google Scholar] [CrossRef]
- Diez, A.M.; Björkroth, J.; Jaime, I.; Rovira, J. Microbial, sensory and volatile changes during the anaerobic cold storage of morcilla de burgos previously inoculated with Weissella viridescens and leuconostoc mesenteroides. Int. J. Food Microbiol. 2009, 131, 168–177. [Google Scholar] [CrossRef]
- Mi, T.; Wang, D.; Yao, S.; Yang, H.; Che, Y.; Wu, C. Effects of salt concentration on the quality and microbial diversity of spontaneously fermented radish paocai. Food Res. Int. 2022, 160, 111622. [Google Scholar] [CrossRef]
- Wang, A.L.; Du, Q.Q.; Li, X.M.; Cui, Y.M.; Luo, J.H.; Li, C.R.; Peng, C.; Zhong, X.F.; Huang, G.D. Intracellular and extracellular metabolic response of the lactic acid bacterium Weissella confusa under salt stress. Metabolites 2024, 14, 695. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Shi, Y.; Liu, D.; Zhang, P.; Chen, Q. A multi-omics analysis reveals the response mechanism of Weissella confusa ZJU.2 to gastric acid and bile salts. Food Biosci. 2025, 63, 105667. [Google Scholar] [CrossRef]
- Adesulu-Dahunsi, A.T.; Sanni, A.I.; Jeyaram, K. Production, characterization and in vitro antioxidant activities of exopolysaccharide from weissella cibaria GA44. Lebensm. Wiss. Technol. 2018, 87, 432–442. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.; Ishola, R.; Oyewunmi, T. Characterization, antioxidant and immunomodulatory potential on exopolysaccharide produced by wild type and mutant Weissella confusa strains. Biotechnol. Rep. 2018, 19, e00271. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.S.; Lee, N.K.; Choi, A.J.; Choe, J.S.; Bae, C.H.; Paik, H.D. Antagonistic and antioxidant effect of probiotic Weissella cibaria JW15. Food Sci. Biotechnol. 2019, 28, 851–855. [Google Scholar] [CrossRef]
- Kibar, H.; Arslan, Y.E.; Ceylan, A.; Karaca, B.; Haliscelik, O.; Kiran, F. Weissella cibaria EIR/p2-derived exopolysaccharide: A novel alternative to conventional biomaterials targeting periodontal regeneration. Int. J. Biol. Macromol. 2020, 165, 2900–2908. [Google Scholar] [CrossRef]
- Pelyuntha, W.; Chaiyasut, C.; Kantachote, D.; Sirilun, S. Organic acids and 2,4-di-tert-butylphenol: Major compounds of Weissella confusa WM36 cell-free supernatant against growth, survival and virulence of salmonella typhi. PeerJ 2020, 8, e8410. [Google Scholar] [CrossRef]
- Malik, A.; Sumayyah, S.; Yeh, C.W.; Heng, N. Identification and sequence analysis of pWcMBF8-1, a bacteriocin-encoding plasmid from the lactic acid bacterium Weissella confusa. FEMS Microbiol. Lett. 2016, 363, fnw059. [Google Scholar] [CrossRef]
- Parappilly, S.J.; Idicula, D.V.; Chandran, A.; Radhakrishnan, K.M.; George, S.M. Antifungal activity of human gut lactic acid bacteria against aflatoxigenic aspergillus flavus MTCC 2798 and their potential application as food biopreservative. J. Food Saf. 2021, 41, e12942. [Google Scholar] [CrossRef]
- Yao, D.; Wang, X.Y.; Ma, L.X.; Wu, M.N.; Xu, L.; Yu, Q.R.; Zhang, L.Y.; Zheng, X.Q. Impact of Weissella cibaria BYL4.2 and its supernatants on penicillium chrysogenum metabolism. Front. Microbiol. 2022, 13, e12942. [Google Scholar] [CrossRef]
- Caggia, C.; De Angelis, M.; Pitino, I.; Pino, A.; Randazzo, C.L. Probiotic features of lactobacillus strains isolated from ragusano and pecorino siciliano cheeses. Food Microbiol. 2015, 50, 109–117. [Google Scholar] [CrossRef]
- Ji, S.X.; Yang, Y.; Li, H.P.; Li, Z.; Suo, B.; Fan, M.H.; Ai, Z.L. Enhancement of the quality and in vitro starch digestibility of fried-free instant noodles with rapid rehydration using sourdough fermented with exopolysaccharide-producing Weissella confusa. Food Chem. 2025, 464, e12942. [Google Scholar] [CrossRef]
- Thant, E.P.; Surachat, K.; Chusri, S.; Romyasamit, C.; Pomwised, R.; Wonglapsuwan, M.; Yaikhan, T.; Suwannasin, S.; Singkhamanan, K. Exploring Weissella confusa w1 and w2 strains isolated from khao-mahk as probiotic candidates: From phenotypic traits to genomic insights. Antibiotics 2024, 13, 604. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.; Sabino, Y.; de Almeida, T.C.; Palacio, F.B.; Rotta, I.S.; Dias, V.C.; Da Silva, V.L.; Diniz, C.G.; Azevedo, V.; Brenig, B.; et al. Unlocking probiotic potential: Genomic insights into Weissella paramesenteroides UFTM 2.6.1. Probiotics Antimicrob. Proteins 2024, 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Wu, Y.J.; Hu, C.Y. Monosaccharide composition influence and immunomodulatory effects of probiotic exopolysaccharides. Int. J. Biol. Macromol. 2019, 133, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Kavitake, D.; Devi, P.B.; Shetty, P.H. Overviewof exopolysaccharides produced by Weissella genus—A review. Int. J. Biol. Macromol. 2020, 164, 2964–2973. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, C.; Cheng, Q.; Wang, Y.; Chu, C. Oral administration of exopolysaccharide from aphanothece halophytica (Chroococcales) significantly inhibits influenza virus (h1n1)-induced pneumonia in mice. Int. Immunopharmacol. 2006, 6, 1093–1099. [Google Scholar] [CrossRef]
- Liu, X.T.; Yao, T. Types, synthesis pathways, purification, characterization, and agroecological physiological functions of microbial exopolysaccharides: A review. Int. J. Biol. Macromol. 2024, 281, 136317. [Google Scholar] [CrossRef]
- Ferrari, M.; Hameleers, L.; Stuart, M.; Oerlemans, M.; de Vos, P.; Jurak, E.; Walvoort, M. Efficient isolation of membrane-associated exopolysaccharides of four commercial bifidobacterial strains. Carbohydr. Polym. 2022, 278, 118913. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; de Los Reyes-Gavilán, C.G. invited review: Methods for the screening, isolation, and characterization of exopolysaccharides produced by lactic acid bacteria. J. Dairy. Sci. 2005, 88, 843–856. [Google Scholar] [CrossRef]
- Buksa, K.; Kowalczyk, M.; Boreczek, J. Extraction, purification and characterisation of exopolysaccharides produced by newly isolated lactic acid bacteria strains and the examination of their influence on resistant starch formation. Food Chem. 2021, 362, 130221. [Google Scholar] [CrossRef]
- Le, B.; Yang, S.H. Production of prebiotic xylooligosaccharide from aqueous ammonia-pretreated rice straw by β-xylosidase of Weissella cibaria. J. Appl. Microbiol. 2019, 126, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Ingerslev, H.C.; Jorgensen, L.V.; Strube, M.L.; Larsen, N.; Dalsgaard, I.; Boye, M.; Madsen, L. The development of the gut microbiota in rainbow trout (Oncorhynchus mykiss) is affected by first feeding and diet type. Aquaculture 2014, 424, 24–34. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, J.; Wang, X.R.; Xuan, H.Z. Antibacterial and antibiofilm activities of chinese propolis essential oil microemulsion against streptococcus mutans. J. Appl. Microbiol. 2023, 134, lxad056. [Google Scholar] [CrossRef]
- Devi, P.B.; Kavitake, D.; Jayamanohar, J.; Shetty, P.H. Preferential growth stimulation of probiotic bacteria by galactan exopolysaccharide from Weissella confusa KR780676. Food Res. Int. 2021, 143, 110333. [Google Scholar] [CrossRef]
- Baruah, R.; Maina, N.H.; Katina, K.; Juvonen, R.; Goyal, A. Functional food applications of dextran from weissella cibaria RBA12 from pummelo (Citrus maxima). Int. J. Food Microbiol. 2017, 242, 124–131. [Google Scholar] [CrossRef]
- Zhao, H.; Abbas, S.; Ren, J.; Huang, H.; Song, Y.; Su, X.; Wu, Q.; Ma, Y.; Tang, H.; Gao, Y.Z. Dextran from human feces-derived Weissella cibaria facilitates intestinal mucosal barrier function by modulating gut bacteria and propionate levels. Carbohydr. Polym. 2025, 354, 123330. [Google Scholar] [CrossRef]
- He, K.Y.; Lei, X.Y.; Wu, D.H.; Zhang, L.; Li, J.Q.; Li, Q.T.; Yin, W.T.; Zhao, Z.L.; Liu, H.; Xiang, X.Y. Akkermansia muciniphila protects the intestine from irradiation-induced injury by secretion of propionic acid. Gut Microbes 2023, 15, 2293312. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Wang, C.T.; Jia, S.S.; Wang, B.Y.; Zhou, K.; Chen, S.J.; Yang, Y.; Liu, S.L. Purification, characterization and antioxidant activity of the exopolysaccharide from Weissella cibaria SJ14 isolated from sichuan paocai. Int. J. Biol. Macromol. 2018, 115, 820–828. [Google Scholar] [CrossRef]
- Huang, L.; Cui, K.; Mao, W.; Du, Y.; Yao, N.; Li, Z.; Zhao, H.; Ma, W. Weissella cibaria attenuated LPS-induced dysfunction of intestinal epithelial barrier in a caco-2 cell monolayer model. Front. Microbiol. 2020, 11, 2039. [Google Scholar] [CrossRef]
- Bagul, P.K.; Deepthi, N.; Sultana, R.; Banerjee, S.K. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J. Nutr. Biochem. 2015, 26, 1298–1307. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Zheng, Y.; Zhang, Y. Weissella paramesenteroides NRIC1542 inhibits dextran sodium sulfate-induced colitis in mice through regulating gut microbiota and SIRT1/NF-κb signaling pathway. FASEB J. 2024, 38, e23791. [Google Scholar] [CrossRef]
- Lakra, A.K.; Ramatchandirane, M.; Kumar, S.; Suchiang, K.; Arul, V. Physico-chemical characterization and aging effects of fructan exopolysaccharide produced by Weissella cibaria MD2 on caenorhabditis elegans. Lebensm. Wiss. Technol. 2021, 143, 111100. [Google Scholar] [CrossRef]
- Fatmawati, N.N.D.; Gotoh, K.; Mayura, I.P.B.; Nocianitri, K.A.; Suwardana, G.N.R.; Komalasari, N.L.G.Y.; Ramona, Y.; Sakaguchi, M.; Matsushita, O.; Sujaya, I.N. Enhancement of intestinal epithelial barrier function by Weissella confusa f213 and Lactobacillus rhamnosus FBB81 probiotic candidates in an in vitro model of hydrogen peroxide-induced inflammatory bowel disease. BMC Res. Notes 2020, 13, 489. [Google Scholar] [CrossRef]
- Moon, Y.J.; Soh, J.R.; Yu, J.J.; Sohn, H.S.; Cha, Y.S.; Oh, S.H. Intracellular lipid accumulation inhibitory effect of Weissella koreensis OK1-6 isolated from kimchi on differentiating adipocyte. J. Appl. Microbiol. 2012, 113, 652–658. [Google Scholar] [CrossRef]
- Choi, S.I.; You, S.H.; Kim, S.; Won, G.; Kang, C.H.; Kim, G.H. Weissella cibaria MG5285 and Lactobacillus reuteri MG5149 attenuated fat accumulation in adipose and hepatic steatosis in high-fat diet-induced c57BL/6j obese mice. Food Nutr. Res. 2021, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bhatia, R.; Singh, A.; Singh, P.; Kaur, R.; Khare, P.; Purama, R.K.; Boparai, R.K.; Rishi, P.; Ambalam, P.; et al. Probiotic attributes and prevention of LPS-induced pro-inflammatory stress in RAW264.7 macrophages and human intestinal epithelial cell line (caco-2) by newly isolated Weissella cibaria strains. Food Funct. 2018, 9, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Kwon, M.; Lee, J.; Oh, Y.J.; Jang, J.; Lee, J.; Park, H.W.; Nam, Y.; Seo, M.; Roh, S.W.; et al. Weissella cibaria WIKIM28 ameliorates atopic dermatitis-like skin lesions by inducing tolerogenic dendritic cells and regulatory t cells in BALB/c mice. Sci. Rep. 2017, 7, 40040. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.F.; Lee, Y.D.; Park, J.Y.; Kim, S.; Lee, Y.W.; Jeon, B.; Jagdish, D.; Kim, H.; Chung, D.K. Lipoteichoic acid isolated from Weissella cibaria increases cytokine production in human monocyte-like THP-1 cells and mouse splenocytes. J. Microbiol. Biotechnol. 2016, 26, 1198–1205. [Google Scholar] [CrossRef]
- Shimizu, Y.; Nakamura, K.; Kikuchi, M.; Ukawa, S.; Nakamura, K.; Okada, E.; Imae, A.; Nakagawa, T.; Yamamura, R.; Tamakoshi, A.; et al. Lower human defensin 5 in elderly people compared to middle-aged is associated with differences in the intestinal microbiota composition: The DOSANCO health study. Geroscience 2022, 44, 997–1009. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Zhang, N.; Zhao, Y.; Che, H.; Wang, Y.; Zhang, T.; Wen, M. DHA and EPA alleviate epileptic depression in PTZ-treated young mice model by inhibiting neuroinflammation through regulating microglial m2 polarization and improving mitochondrial metabolism. Antioxidants 2023, 12, 2079. [Google Scholar] [CrossRef]
- Lv, X.H.; Ye, T.; Yang, W.W.; Zhu, Z.C.; Xiang, K.; Zhan, L.; Sun, J.; Liu, J.M. Weissella confusa attenuates cognitive deficits in alzheimer’s disease by reducing oxidative stress via the SIRT1/PGC-1α signaling pathway. Neurochem. Res. 2025, 50, 175. [Google Scholar] [CrossRef]
- Ojekunle, O.; Banwo, K.; Sanni, A.I. In vitro and invivo evaluation of Weissella cibaria and lactobacillus plantarum for their protective effect against cadmium and lead toxicities. Lett. Appl. Microbiol. 2017, 64, 379–385. [Google Scholar] [CrossRef]
- Li, Y.Q.; Tian, W.L.; Gu, C.T. Weissella sagaensis sp. Nov., Isolated from traditiona chinese yogurt. Int. J. Syst. Evol. Microbiol. 2020, 70, 2485–2492. [Google Scholar] [CrossRef]
- Kang, M.; Park, G. In vitro inactivation of respiratory viruses and rotavirus by the oral probiotic strain Weissella cibaria CMS1. Probiotics Antimicrob. Proteins 2022, 14, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Ha, M.; Bae, O.; Lee, Y. Effect of Weissella confusa strain PL9001 on the adherence and growth of helicobacter pylori. Appl. Environ. Microbiol. 2002, 68, 4642–4645. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Sunita; Shukla, P. Probiotic potential of Weissella paramesenteroides MYPS5.1 isolated from customary dairy products and its therapeutic application. 3 Biotech 2022, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Monje, M.; Campos, J.; Alvarez Villamil, E.; Jerez, A.; Dentice Maidana, S.; Elean, M.; Salva, S.; Kitazawa, H.; Villena, J.; García-Cancino, A. Characterization of Weissella viridescens UCO-SMC3 as a potential probiotic for the skin: Its beneficial role in the pathogenesis of acne vulgaris. Microorganisms 2021, 9, 1486. [Google Scholar] [CrossRef]
- Kang, M.; Lee, D.; Lee, S.; Kim, M.; Nam, S. Effects of probiotic bacterium Weissella cibaria CMU on periodontal health and microbiota: A randomised, double-blind, placebo-controlled trial. BMC Oral Health 2020, 20, 243. [Google Scholar] [CrossRef]
- Han, J.W.; Lee, N.; Kim, H.J.; Moon, S.J.; Lee, S.C.; Kim, H.J. Weissella sp. SNUL2 as potential probiotics with broad-spectrum antimicrobial activities. Heliyon 2024, 10, e28481. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, R.; Wang, R.; Lu, Y.; Xu, M.; Lin, X.; Lan, R.; Zhang, S.; Tang, H.; Fan, Q.; et al. Weissella viridescens attenuates hepatic injury, oxidative stress, and inflammation in a rat model of high-fat diet-induced MASLD. Nutrients 2025, 17, 1585. [Google Scholar] [CrossRef]
- Anas, A.; Sukumaran, V.; Devarajan, D.N.; Maniyath, S.; Chekidhenkuzhiyil, J.; Mary, A.; Kuttan, S.P.; Tharakan, B. Probiotics inspired from natural ecosystem to inhibit the growth of vibrio spp. Causing white gut syndrome in litopenaeus vannamei. 3 Biotech 2021, 11, 66. [Google Scholar] [CrossRef]
- Medina, M.; Fernandez-Espinel, C.; Sotil, G.; Yunis-Aguinaga, J.; Flores-Dominick, V. First description of Weissella ceti associated with mortalities in farmed rainbow trout (Oncorhynchus mykiss) in peru. Aquaculture 2020, 529, 735608. [Google Scholar] [CrossRef]
- Dolan, L.C.; Arceneaux, B.G.; Do, K.H.; Lee, W.K.; Park, G.Y.; Kang, M.S.; Choi, K.C. Toxicological and safety evaluations of Weissella cibaria strain CMU in animal toxicity and genotoxicity. Toxicol. Res. 2022, 38, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Gwon, H.M.; Jeong, W.S.; Yeo, S.H.; Kim, S.Y. Safety evaluation of Weissella cibaria JW15 by phenotypic and genotypic property analysis. Microorganisms 2021, 9, 2450. [Google Scholar] [CrossRef] [PubMed]
- Fhoula, I.; Boumaiza, M.; Tayh, G.; Rehaiem, A.; Klibi, N.; Ouzari, I.H. Antimicrobial activity and safety features assessment of Weissella spp. From environmental sources. Food Sci. Nutr. 2022, 10, 2896–2910. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).