Polygonatum sibiricum Polysaccharides Alleviate Simulated Weightlessness-Induced Cognitive Impairment by Gut Microbiota Modulation and Suppression of NLRP3/NF-κB Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Animals and Treatments
2.3. Hindlimb Unloading (HU) Procedures
2.4. Morris Water Maze Test
2.5. Passive Avoidance Test
2.6. Blood and Tissue Sample Collection
2.7. 16s rRNA Gene Sequencing
2.8. Enzyme-Linked Immunosorbent Assay
2.9. Real-Time Quantitative PCR
2.10. Neurotransmitter Detection
2.11. Western Blot Assay
2.12. Statistical Analysis
3. Results
3.1. Effects of PSP on Morris Water Maze Test in Mice with HU
3.2. Effects of PSP on Passive Avoidance Test in Mice with HU
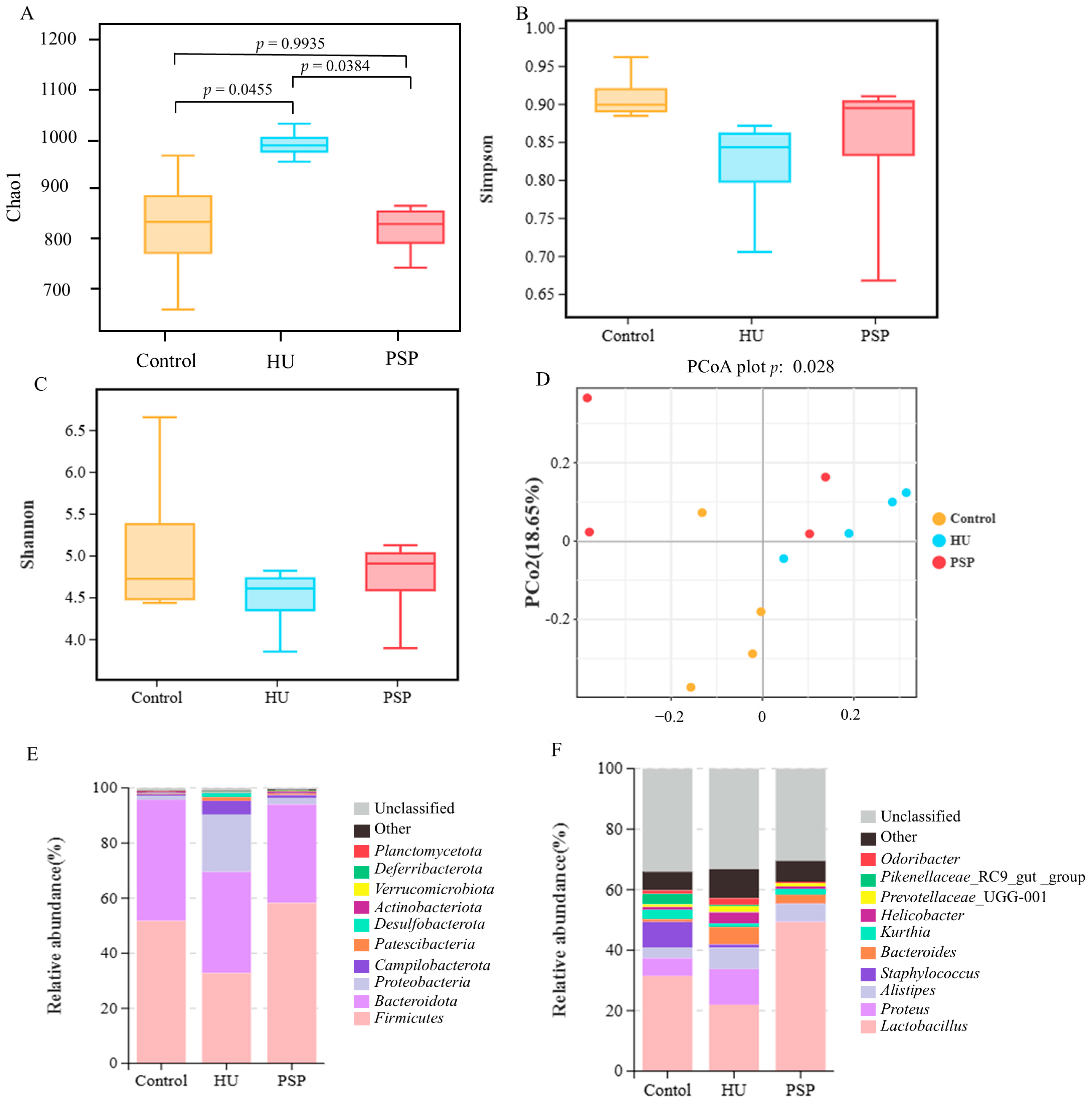
3.3. Shift in Gut Microbiome in Mice Treated with PSP
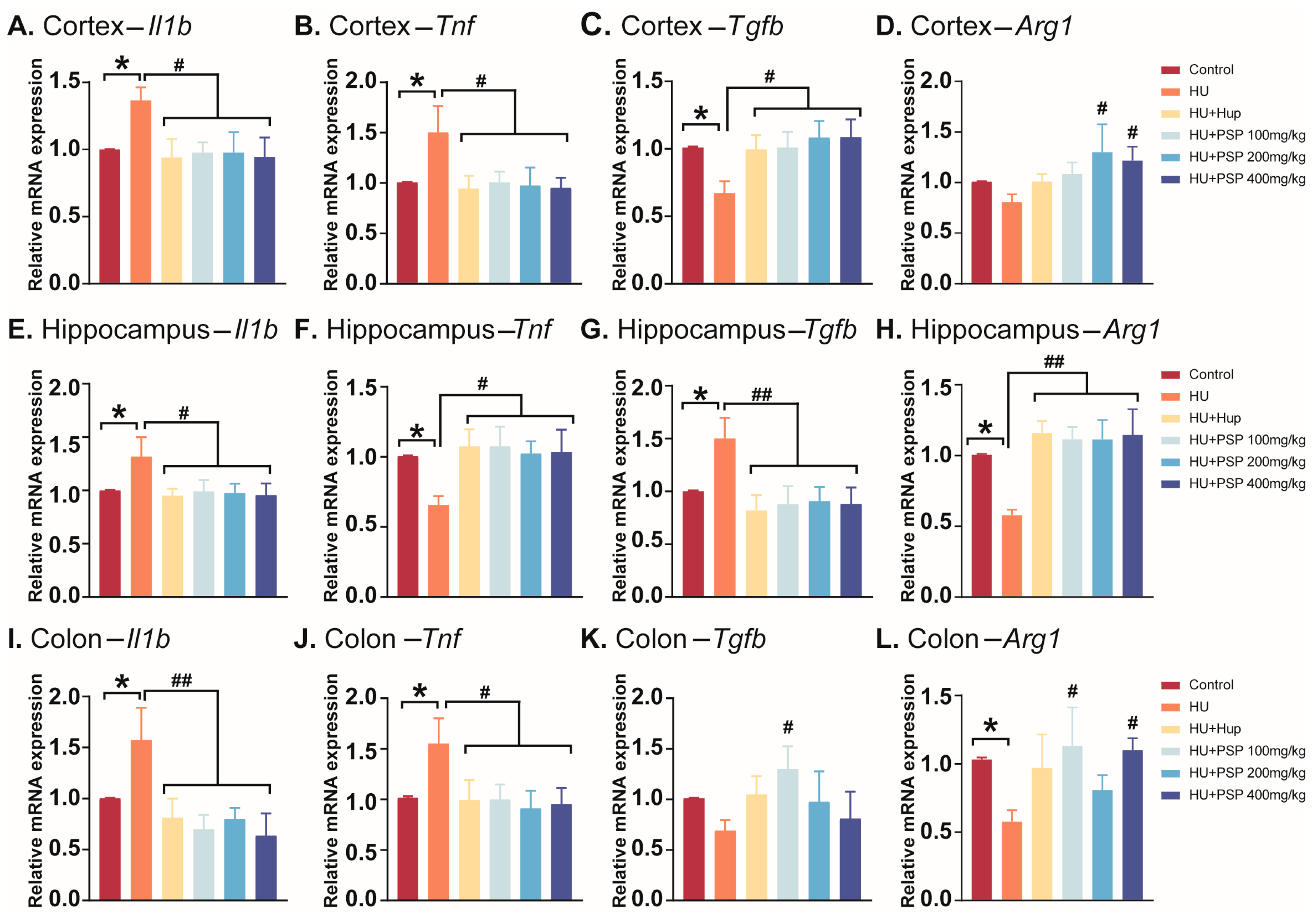
3.4. Effect of PSP on mRNA Expression of Inflammatory Cytokines in Mice with HU
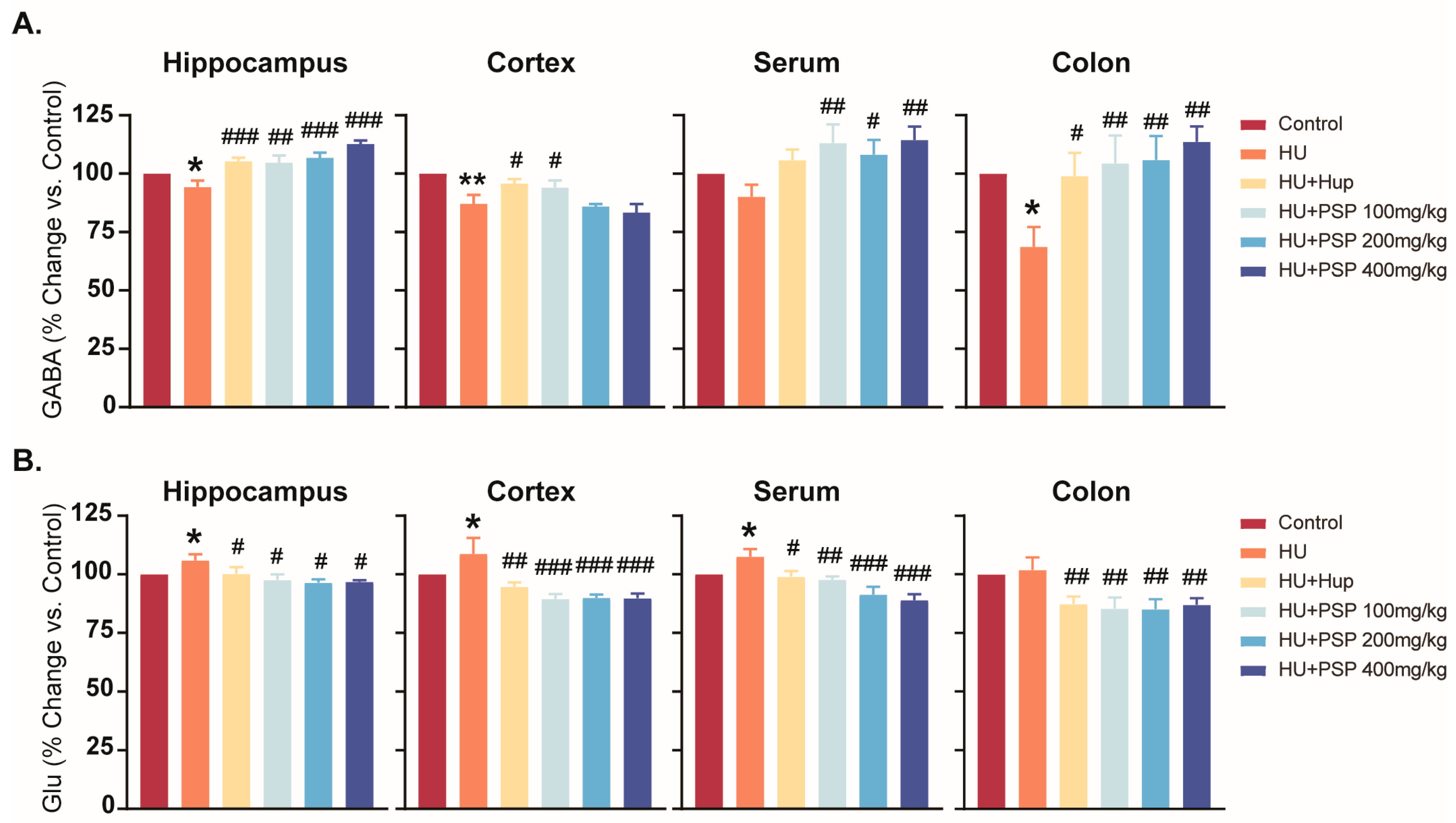
3.5. Effect of PSP on Neurotransmitter Levels in Mice with HU
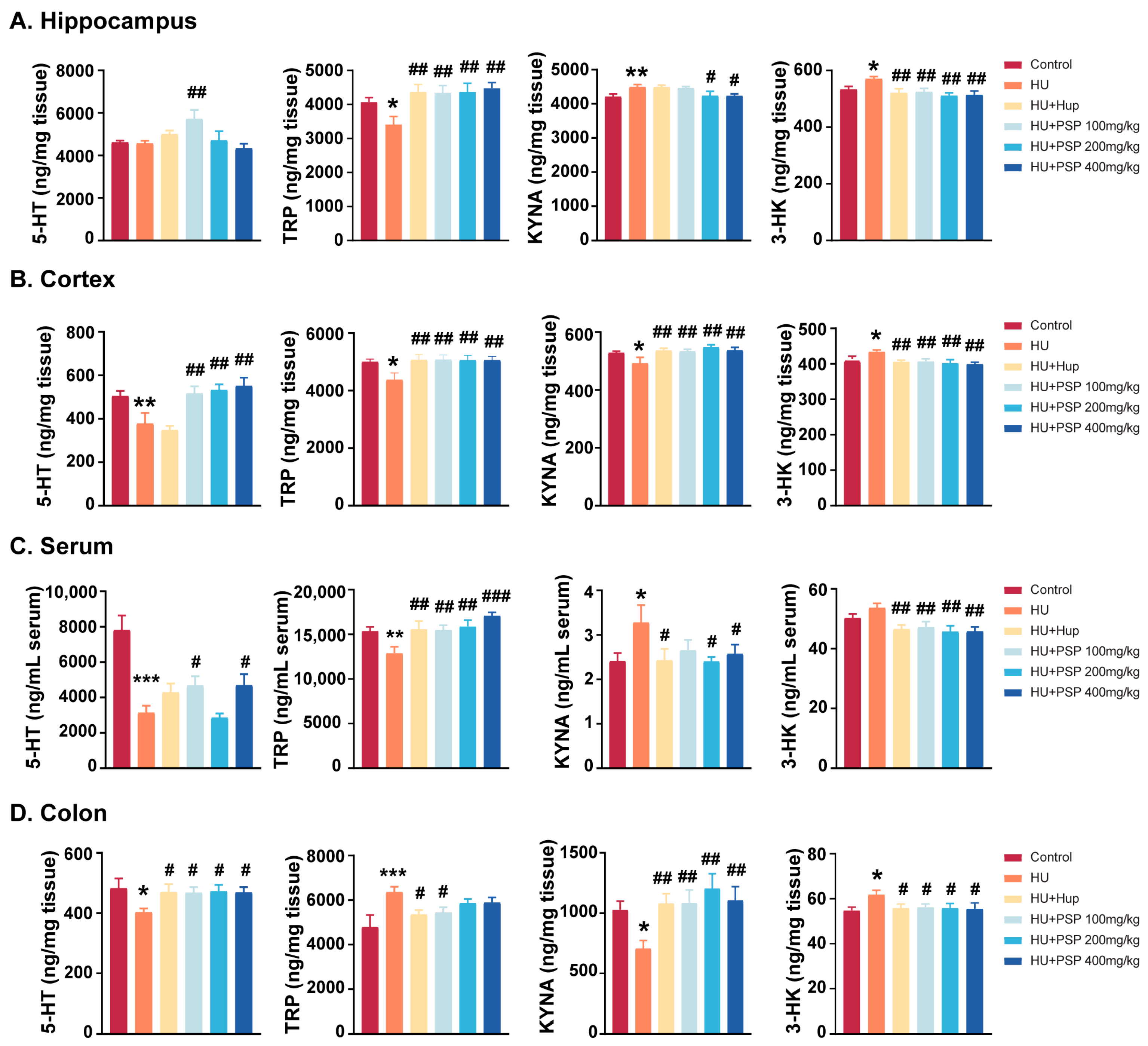
3.6. Effect of PSP on TRP Metabolites in Mice with HU
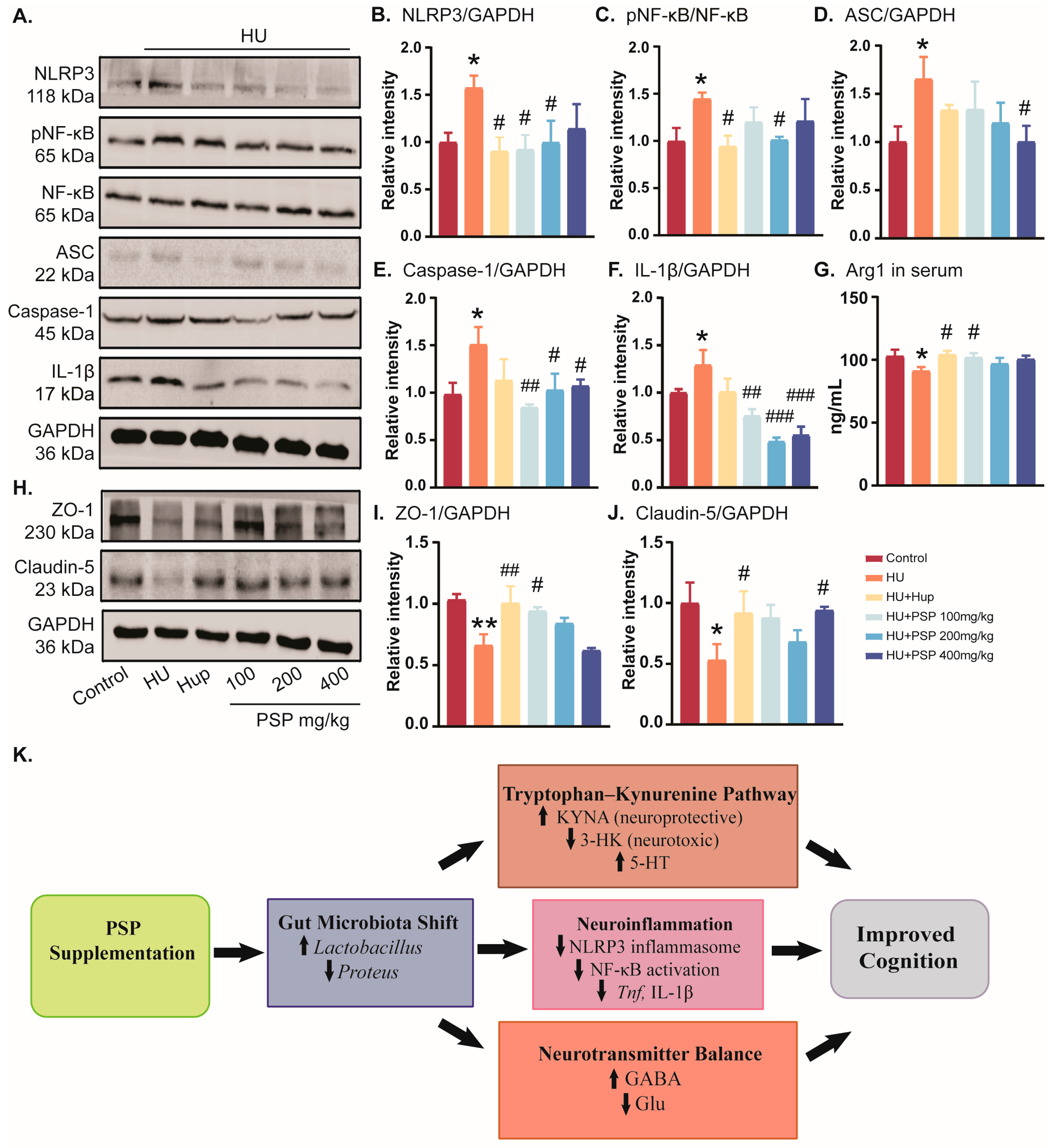
3.7. Effects of PSP on Intestinal Barrier Function of Mice with HU
3.8. Effects of PSP on Serum Inflammatory Response and NLRP3/NF-κB Pathways in Hippocampus of Mice with HU
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HU | Hindlimb unloading |
| MWMT | Morris water maze test |
| PAT | Passive avoidance test |
| NLRP3 | Nod-like receptor pyrin domain 3 |
| NF-κB | Nuclear factor kappa-B |
| ZO-1 | Zonula occludens-1 |
| MGB | Microbiota–gut–brain |
| GABA | Gamma-aminobutyric acid |
| Glu | Glutamate |
| 3-HK | 3-hydroxykynurenine |
| 5-HT | 5-hydroxy tryptamine |
| TRP | Tryptophan |
| KYNA | Kynurenic acid |
| Hup | Huperzine A |
| ASC | Apoptosis-associated speck-like protein |
| E | Epinephrine |
| NE | Norepinephrine |
References
- Koppelmans, V.; Erdeniz, B.; De Dios, Y.E.; Wood, S.J.; Reuter-Lorenz, P.A.; Kofman, I.; Bloomberg, J.J.; Mulavara, A.P.; Seidler, R.D. Study protocol to examine the effects of spaceflight and a spaceflight analog on neurocognitive performance: Extent, longevity, and neural bases. BMC Neurol. 2013, 13, 205. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, J.; Fan, Q.; Zhao, S.; Wu, X.; Wang, J.; Liu, Y.; Li, Y.; Lu, W. Long-term spaceflight composite stress induces depression and cognitive impairment in astronauts-insights from neuroplasticity. Transl. Psychiatry 2023, 13, 342. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Abrams, S.A.; Davis-Street, J.E.; Heer, M.; O’Brien, K.O.; Wastney, M.E.; Zwart, S.R. Fifty years of human space travel: Implications for bone and calcium research. Annu. Rev. Nutr. 2014, 34, 377–400. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Shi, J.; Zhang, P.; Wang, K.; Li, J.; Liu, H.; Zhou, Y.; Xu, X.; Hao, J.; Sun, X.; et al. Simulated microgravity disrupts intestinal homeostasis and increases colitis susceptibility. FASEB J. 2015, 29, 3263–3273. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; He, J.; Li, P.; Jin, R.; Wang, K.; Xu, X.; Hao, J.; Zhang, Y.; Liu, H.; et al. Intestinal microbiota contributes to colonic epithelial changes in simulated microgravity mouse model. FASEB J. 2017, 31, 3695–3709. [Google Scholar] [CrossRef]
- Hediyal, T.A.; Vichitra, C.; Anand, N.; Bhaskaran, M.; Essa, S.M.; Kumar, P.; Qoronfleh, M.W.; Akbar, M.; Kaul-Ghanekar, R.; Mahalakshmi, A.M.; et al. Protective effects of fecal microbiota transplantation against ischemic stroke and other neurological disorders: An update. Front. Immunol. 2024, 15, 1324018. [Google Scholar] [CrossRef]
- Xie, P.; Chen, L.; Wang, J.; Wang, X.; Yang, S.; Zhu, G. Polysaccharides from Polygonatum cyrtonema Hua prevent post-traumatic stress disorder behaviors in mice: Mechanisms from the perspective of synaptic injury, oxidative stress, and neuroinflammation. J. Ethnopharmacol. 2024, 319, 117165. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Gao, F.; Yang, S.; Zhen, Y.; Wang, X.; Zhu, G. Polysaccharides from Polygonatum cyrtonema Hua prevent depression-like behaviors in mice with chronic unpredictable mild stress through refining gut microbiota-lipopolysaccharide-paraventricular nucleus signal axis. Heliyon 2024, 10, e38554. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Cai, Y.; Xu, S.; Xu, Q.; Ling, C.; Li, X.; Li, W.; Liu, P.; Liu, W. Research progress on medicinal components and pharmacological activities of polygonatum sibiricum. J. Ethnopharmacol. 2024, 328, 118024. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Liu, Y.; Sun, J.; Fan, B.; Wang, F.; Lu, C. The protective effects and mechanisms of Polygonatum sibiricum polysaccharides in chronic stress-induced neural damage. J. Ethnopharmacol. 2025, 352, 120219. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, X.; Huang, S.; Feng, X.; Zhang, X.; Xiang, D. A monomeric polysaccharide from Polygonatum sibiricum improves cognitive functions in a model of Alzheimer’s disease by reshaping the gut microbiota. Int. J. Biol. Macromol. 2022, 213, 404–415. [Google Scholar] [CrossRef]
- Wu, S.; Liu, W.; Zhang, C. Polygonatum sibiricum polysaccharide ameliorates neurotoxicity in Alzheimer disease mice by inhibiting endoplasmic reticulum stress and lipid raft formation through AMPK/GSK3β/Nrf2 pathway. Neurol. Res. 2025, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Wu, M.; Diao, J.L.; Li, J.B.; Sun, Y.X.; Xiao, X.Q. Huperzine A ameliorates obesity-related cognitive performance impairments involving neuronal insulin signaling pathway in mice. Acta Pharmacol. Sin. 2020, 41, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Jiang, N.; Wang, H.; Huang, H.; Bao, Y.; Chen, Y.; Liu, X. Simulated weightlessness induces cognitive changes in rats illustrated by performance in operant conditioning tasks. Life Sci. Space Res. 2021, 29, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Jia, X.Y.; Zhang, L.; Li, Y.L.; Zhang, Z.J.; Li, L.; Zhang, L. Shenqi Xingnao Granules ameliorates cognitive impairments and Alzheimer’s disease-like pathologies in APP/PS1 mouse model. Chin. Herb. Med. 2020, 12, 421–429. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, S.; Zhang, Y.; Deng, Y.; He, Y.; Chen, Y.; Liu, C.; Lin, C.; Yang, Q. Oral Administration of Compound Probiotics Ameliorates HFD-Induced Gut Microbe Dysbiosis and Chronic Metabolic Inflammation via the G Protein-Coupled Receptor 43 in Non-alcoholic Fatty Liver Disease Rats. Probiot. Antimicrob. Proteins 2019, 11, 175–185. [Google Scholar] [CrossRef]
- Li, C.C.; Gan, L.; Tan, Y.; Yan, M.Z.; Liu, X.M.; Chang, Q.; Pan, R.L. Chronic restraint stress induced changes in colonic homeostasis-related indexes and tryptophan-kynurenine metabolism in rats. J. Proteom. 2021, 240, 104190. [Google Scholar] [CrossRef]
- Wang, L.S.; Zhang, M.D.; Tao, X.; Zhou, Y.F.; Liu, X.M.; Pan, R.L.; Liao, Y.H.; Chang, Q. LC-MS/MS-based quantification of tryptophan metabolites and neurotransmitters in the serum and brain of mice. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1112, 24–32. [Google Scholar] [CrossRef]
- Yang, C.; Wu, X.; Jiang, Z.; Ru, Y.; Shen, B.; Li, F.; Cui, J.; Zhang, C.; Wang, X.; Yu, W.; et al. Evodiamine rescues lipopolysaccharide-induced cognitive impairment via C/EBP-β-COX2 axis-regulated neuroinflammation. Int. J. Biol. Macromol. 2025, 300, 139597. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, Y.; Li, N.; Chen, Z.; Chang, J.; Deng, Y. Microgravity-Induced Cognitive Decline: Investigating the Pathogenic Mechanisms of RyR2 Hyperphosphorylation and S107 Intervention. FASEB J. 2025, 39, e70743. [Google Scholar] [CrossRef]
- Mao, X.W.; Sandberg, L.B.; Gridley, D.S.; Herrmann, E.C.; Zhang, G.; Raghavan, R.; Zubarev, R.A.; Zhang, B.; Stodieck, L.S.; Ferguson, V.L.; et al. Proteomic Analysis of Mouse Brain Subjected to Spaceflight. Int. J. Mol. Sci. 2018, 20, 7. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, N.; Zhang, Y.W.; Xie, M.Z.; Liu, X.M. Protective effect of Gastrodia elata blume ameliorates simulated weightlessness-induced cognitive impairment in mice. Life Sci. Space Res. 2023, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.H.; Zhang, J.L.; Li, D.H.; Wang, Z.Q.; Liu, Y.K.; Fan, J.X.; Jiang, S.R.; Li, X.R.; He, X.Y. Processed Polygonatum cyrtonema Hua attenuates postpartum depression in rat model by regulating monoamines and hormones. Heliyon 2024, 10, e26895. [Google Scholar] [CrossRef]
- Zhang, X.; Ni, L.; Hu, S.; Yue, B.; Chen, X.; Yuan, D.; Wang, T.; Zhou, Z. Polygonatum sibiricum ameliorated cognitive impairment of naturally aging rats through BDNF-TrkB signaling pathway. J. Food Biochem. 2022, 46, e14510. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.; Wongchitrat, P.; Govitrapong, P. A Synopsis of Multitarget Potential Therapeutic Effects of Huperzine A in Diverse Pathologies-Emphasis on Alzheimer’s Disease Pathogenesis. Neurochem. Res. 2022, 47, 1166–1182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Wang, J.; Zhang, H.Y.; Tang, X.C. Huperzine A exhibits anti-inflammatory and neuroprotective effects in a rat model of transient focal cerebral ischemia. J. Neurochem. 2008, 106, 1594–1603. [Google Scholar] [CrossRef]
- Mei, Z.; Zheng, P.; Tan, X.; Wang, Y.; Situ, B. Huperzine A alleviates neuroinflammation, oxidative stress and improves cognitive function after repetitive traumatic brain injury. Metab. Brain Dis. 2017, 32, 1861–1869. [Google Scholar] [CrossRef]
- Yang, J.; Kim, H.D.; Barrila, J.; Lee, S.H.; Nickerson, C.A.; Ott, C.M.; Israel, S.A.; Choukér, A.; Yang, J.Y. Navigating mental health in space: Gut-brain axis and microbiome dynamics. Exp. Mol. Med. 2025, 57, 1152–1163. [Google Scholar] [CrossRef]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Siddiqui, R.; Qaisar, R.; Khan, N.A.; Alharbi, A.M.; Alfahemi, H.; Elmoselhi, A. Effect of Microgravity on the Gut Microbiota Bacterial Composition in a Hindlimb Unloading Model. Life 2022, 12, 1865. [Google Scholar] [CrossRef]
- Haase, S.; Wilck, N.; Haghikia, A.; Gold, R.; Mueller, D.N.; Linker, R.A. The role of the gut microbiota and microbial metabolites in neuroinflammation. Eur. J. Immunol. 2020, 50, 1863–1870. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, T.; Shi, Z.; Dong, L.; Li, M.; Peng, B.; Li, Q.; Pan, R.; Xiao, S.; Yang, Q.; et al. Ginsenoside Reshapes Intestinal Microecology to Alleviate Microgravity Stress. Drug Des. Devel. Ther. 2025, 19, 1289–1303. [Google Scholar] [CrossRef]
- Sarubbo, F.; Cavallucci, V.; Pani, G. The Influence of Gut Microbiota on Neurogenesis: Evidence and Hopes. Cells 2022, 11, 382. [Google Scholar] [CrossRef]
- Tian, H.; Wang, J.; Feng, R.; Zhang, R.; Liu, H.; Qin, C.; Meng, L.; Chen, Y.; Fu, Y.; Liang, D.; et al. Efficacy of faecal microbiota transplantation in patients with progressive supranuclear palsy-Richardson’s syndrome: A phase 2, single centre, randomised clinical trial. eClinicalMedicine 2023, 58, 101888. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- Heneka, M.T.; McManus, R.M.; Latz, E. Author Correction: Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2019, 20, 187. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Huang, S.Y.; Zhong, K.Y.; Huang, W.G.; Huang, Z.H.; He, T.T.; Yang, M.T.; Wusiman, M.; Zhou, D.D.; Chen, S.; et al. Betaine alleviates cognitive impairment induced by homocysteine through attenuating NLRP3-mediated microglial pyroptosis in an m(6)A-YTHDF2-dependent manner. Redox Biol. 2024, 69, 103026. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Zhang, X.; Yu, Z.Z.; Wang, X.Y.; Zeng, Z.H.; Wei, M.X.; Qiu, M.T.; Wang, J.; Cheng, J.; Yi, L.T. Polygonatum sibiricum Polysaccharides Alleviate Depressive-like Symptoms in Chronic Restraint Stress-Induced Mice via Microglial Regulation in Prefrontal Cortex. Polymers 2024, 16, 2358. [Google Scholar] [CrossRef]
- Mattson, M.P.; Meffert, M.K. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006, 13, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zeng, L.; He, Q.; Wang, S.; Xu, H.; Ge, J. Advancements in research on the immune-inflammatory mechanisms mediated by NLRP3 inflammasome in ischemic stroke and the regulatory role of natural plant products. Front. Pharmacol. 2024, 15, 1250918. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Brubaker, M.; Selzler, K.J.; Gonzalez, S.T.; Patel, M.; Stahl, S.M. An overview of the pathophysiology of agitation in Alzheimer’s dementia with a focus on neurotransmitters and circuits. CNS Spectr. 2024, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.A.; Vissel, B. Excess cerebral TNF causing glutamate excitotoxicity rationalizes treatment of neurodegenerative diseases and neurogenic pain by anti-TNF agents. J. Neuroinflamm. 2016, 13, 236. [Google Scholar] [CrossRef]
- Sakurai, M.; Yamamoto, Y.; Kanayama, N.; Hasegawa, M.; Mouri, A.; Takemura, M.; Matsunami, H.; Miyauchi, T.; Tokura, T.; Kimura, H.; et al. Serum Metabolic Profiles of the Tryptophan-Kynurenine Pathway in the high risk subjects of major depressive disorder. Sci. Rep. 2020, 10, 1961. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, N.; Khan, M.N.; Zhang, Y.; Zhou, G.; Xia, C.; Xu, Y.; Lv, G.; Xie, M.; Liu, X. Protective effects of Compound Gastrodia elata Formula on the cognitive impairment induced by simulated weightlessness in mice. Life Sci. Space Res. 2025; in press. [Google Scholar] [CrossRef]
- Sun, T.; Xie, R.; He, H.; Xie, Q.; Zhao, X.; Kang, G.; Cheng, C.; Yin, W.; Cong, J.; Li, J.; et al. Kynurenic acid ameliorates NLRP3 inflammasome activation by blocking calcium mobilization via GPR35. Front. Immunol. 2022, 13, 1019365. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Lv, J.; Zhang, Y.; Sun, X.; Yao, C.; Wang, Q.; He, Q.; Liu, X. Protective effects of ginsenosides Rg1 and Rb1 against cognitive impairment induced by simulated microgravity in rats. Front. Pharmacol. 2023, 14, 1167398. [Google Scholar] [CrossRef]
- Wang, T.; Gao, C.; Dong, X.; Li, L. Therapeutic potential of traditional chinese medicine polysaccharides in Alzheimer’s disease via modulation of gut microbiota: A review. Int. J. Biol. Macromol. 2025, 321, 146160. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Khan, M.N.; Xie, M.; Zhang, Y.; Li, L.; Dar Farooq, A.; Liu, J.; He, Q.; Liu, X.; Jiang, N. Polygonatum sibiricum Polysaccharides Alleviate Simulated Weightlessness-Induced Cognitive Impairment by Gut Microbiota Modulation and Suppression of NLRP3/NF-κB Pathways. Nutrients 2025, 17, 3157. https://doi.org/10.3390/nu17193157
Chen F, Khan MN, Xie M, Zhang Y, Li L, Dar Farooq A, Liu J, He Q, Liu X, Jiang N. Polygonatum sibiricum Polysaccharides Alleviate Simulated Weightlessness-Induced Cognitive Impairment by Gut Microbiota Modulation and Suppression of NLRP3/NF-κB Pathways. Nutrients. 2025; 17(19):3157. https://doi.org/10.3390/nu17193157
Chicago/Turabian StyleChen, Fang, Muhammad Noman Khan, Mengzhou Xie, Yiwen Zhang, Liang Li, Ahsana Dar Farooq, Jixian Liu, Qinghu He, Xinmin Liu, and Ning Jiang. 2025. "Polygonatum sibiricum Polysaccharides Alleviate Simulated Weightlessness-Induced Cognitive Impairment by Gut Microbiota Modulation and Suppression of NLRP3/NF-κB Pathways" Nutrients 17, no. 19: 3157. https://doi.org/10.3390/nu17193157
APA StyleChen, F., Khan, M. N., Xie, M., Zhang, Y., Li, L., Dar Farooq, A., Liu, J., He, Q., Liu, X., & Jiang, N. (2025). Polygonatum sibiricum Polysaccharides Alleviate Simulated Weightlessness-Induced Cognitive Impairment by Gut Microbiota Modulation and Suppression of NLRP3/NF-κB Pathways. Nutrients, 17(19), 3157. https://doi.org/10.3390/nu17193157





