A Synbiotic of Bifidobacterium animalis subsp. lactis BB-12 and 2′-FL Alleviate Infant Diarrhea and Anxiety-like Behaviors via Gut Microbiota Modulation in an EPEC O127 Infection Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Probiotic Screening Based on 2′-FL
2.3. Animal Experimental Design
2.4. Bacterial Cultivation
2.5. Animal Behavioral Assessment
2.5.1. Open Field Test
2.5.2. Elevated Cross Maze Test
2.5.3. Gastrointestinal Transit Time (GITT)
2.5.4. Diarrhea Score
2.6. Collection of Mouse Feces, Blood Samples, and Specimens
2.7. Hematoxylin and Eosin (H&E) Staining
2.8. Alcian Blue Staining
2.9. Immunofluorescence Staining
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. qRT-PCR Analysis
2.12. 16S rDNA Amplicon Sequencing
2.13. Fecal SCFAs Level Analyses
2.14. Data Processing and Analysis
3. Results
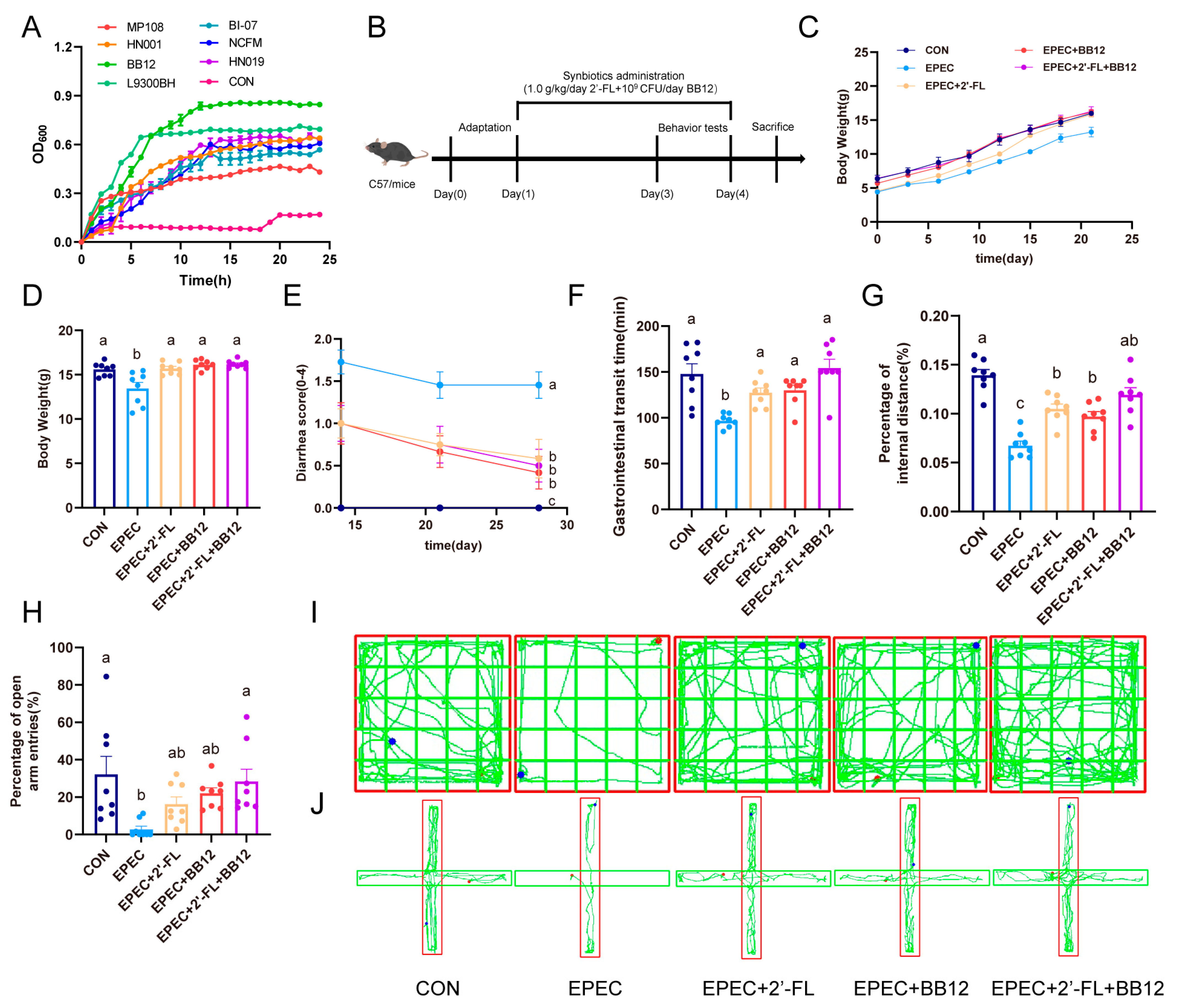
3.1. Selection of Optimal Probiotics with 2′-FL Serving as the Sole Carbon Source
3.2. Impact of Synbiotic Supplementation on EPEC-Induced Infant Diarrhea and Anxiety-like Behaviors
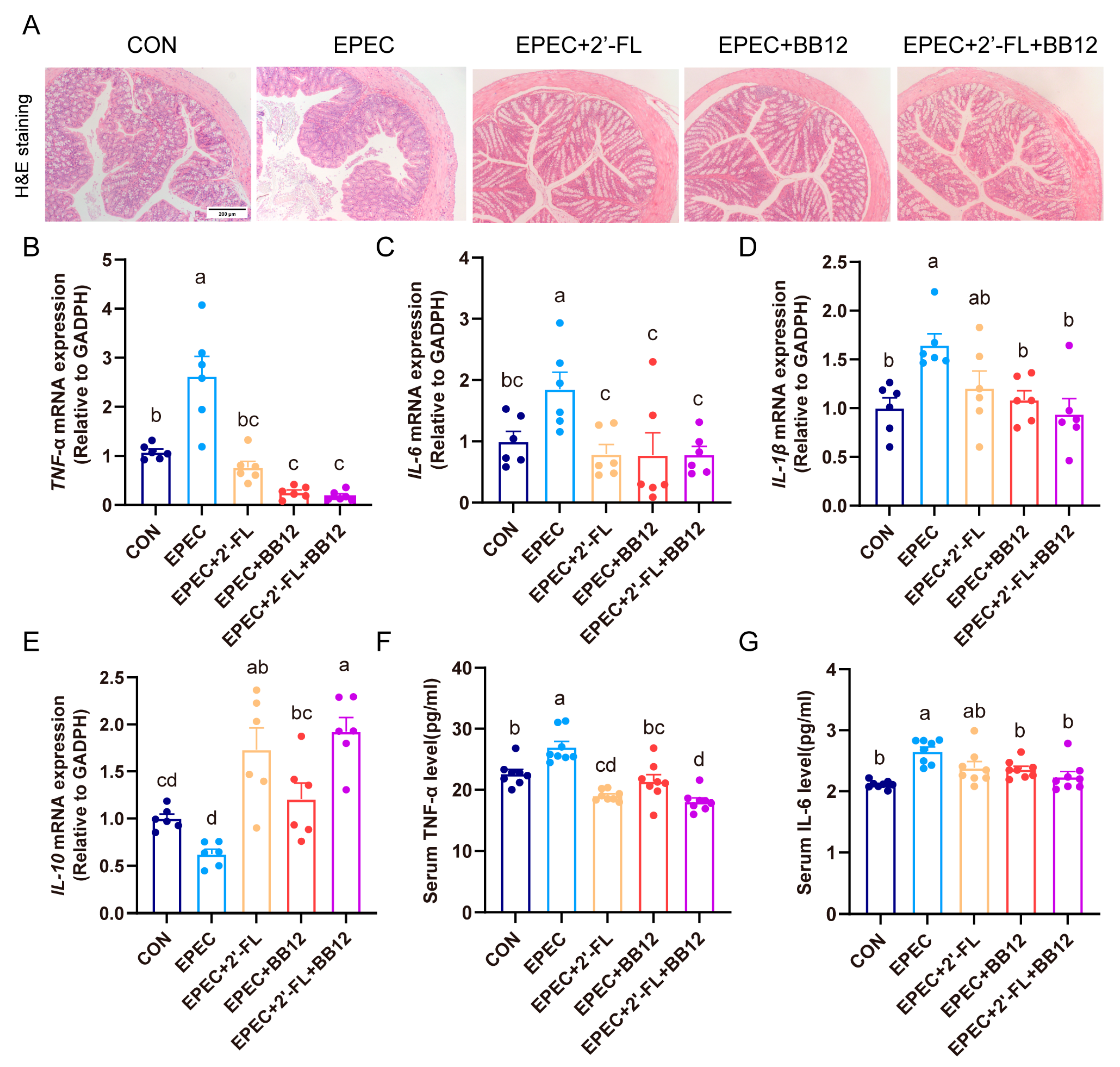
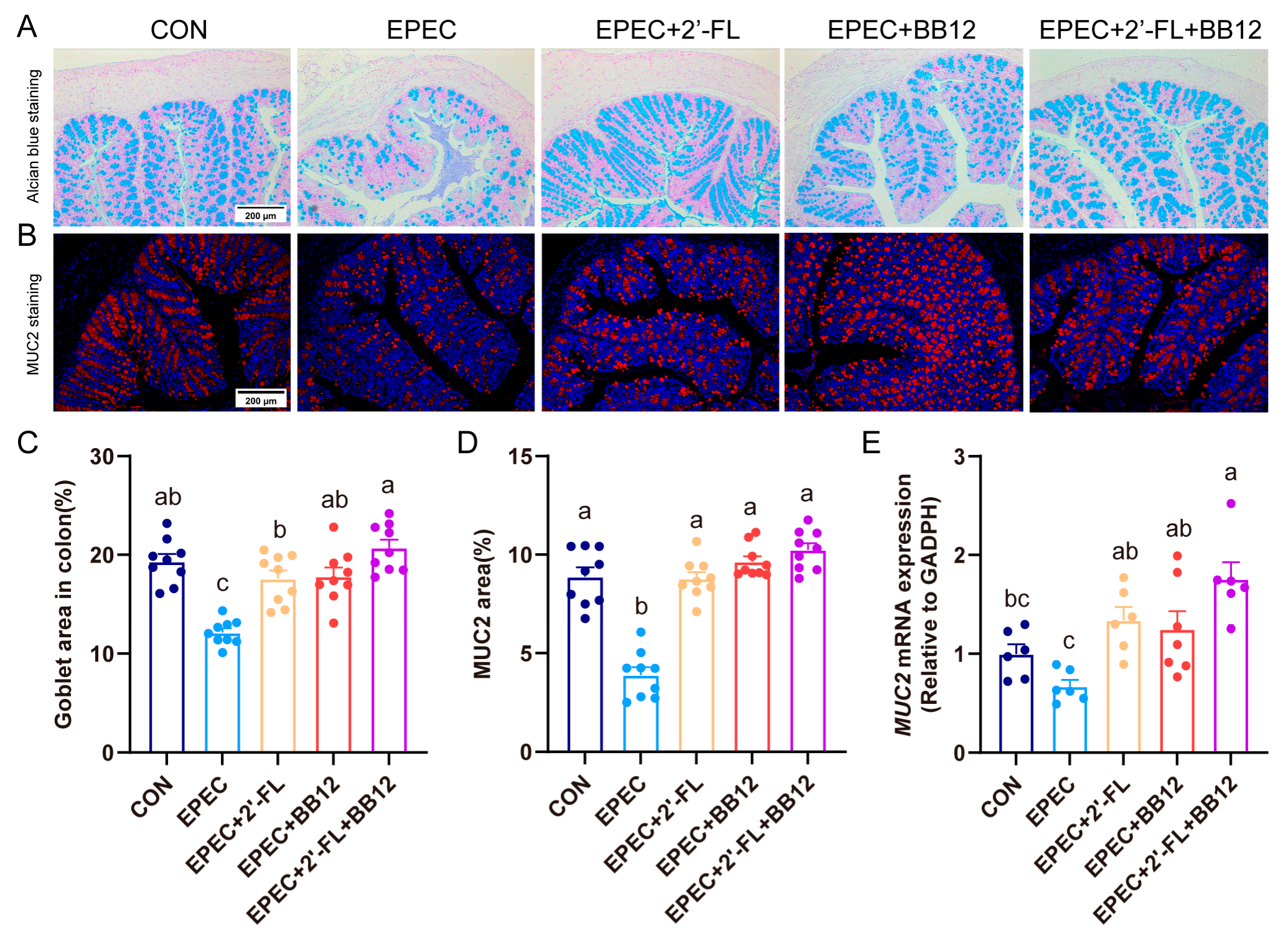
3.3. Impact of Synbiotic Supplementation on Colonic Histopathological Changes and Inflammation in EPEC-Induced Infant Diarrhea
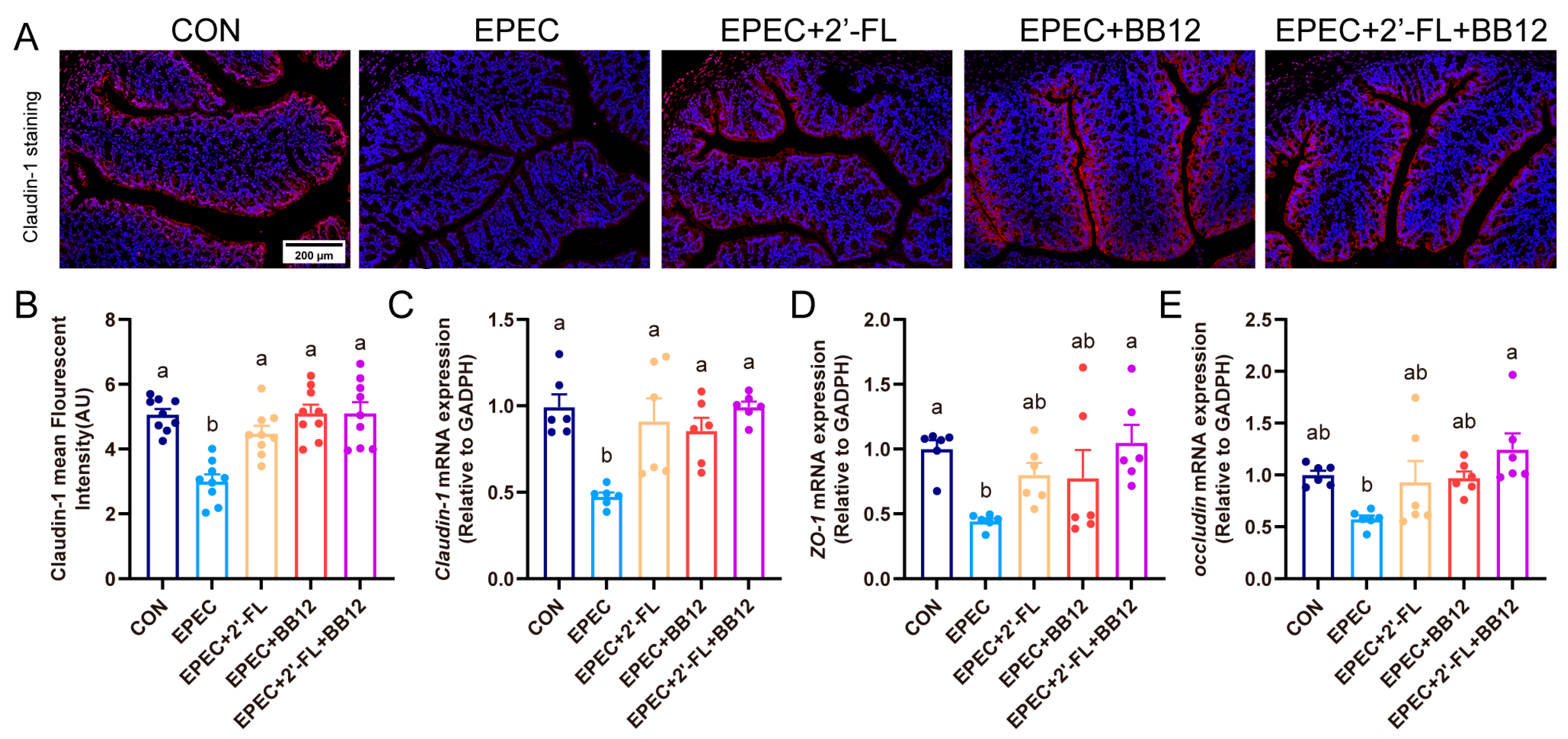
3.4. Impact of Synbiotic Supplementation on Gut Barrier Integrity Damage in EPEC-Induced Infant Diarrhea
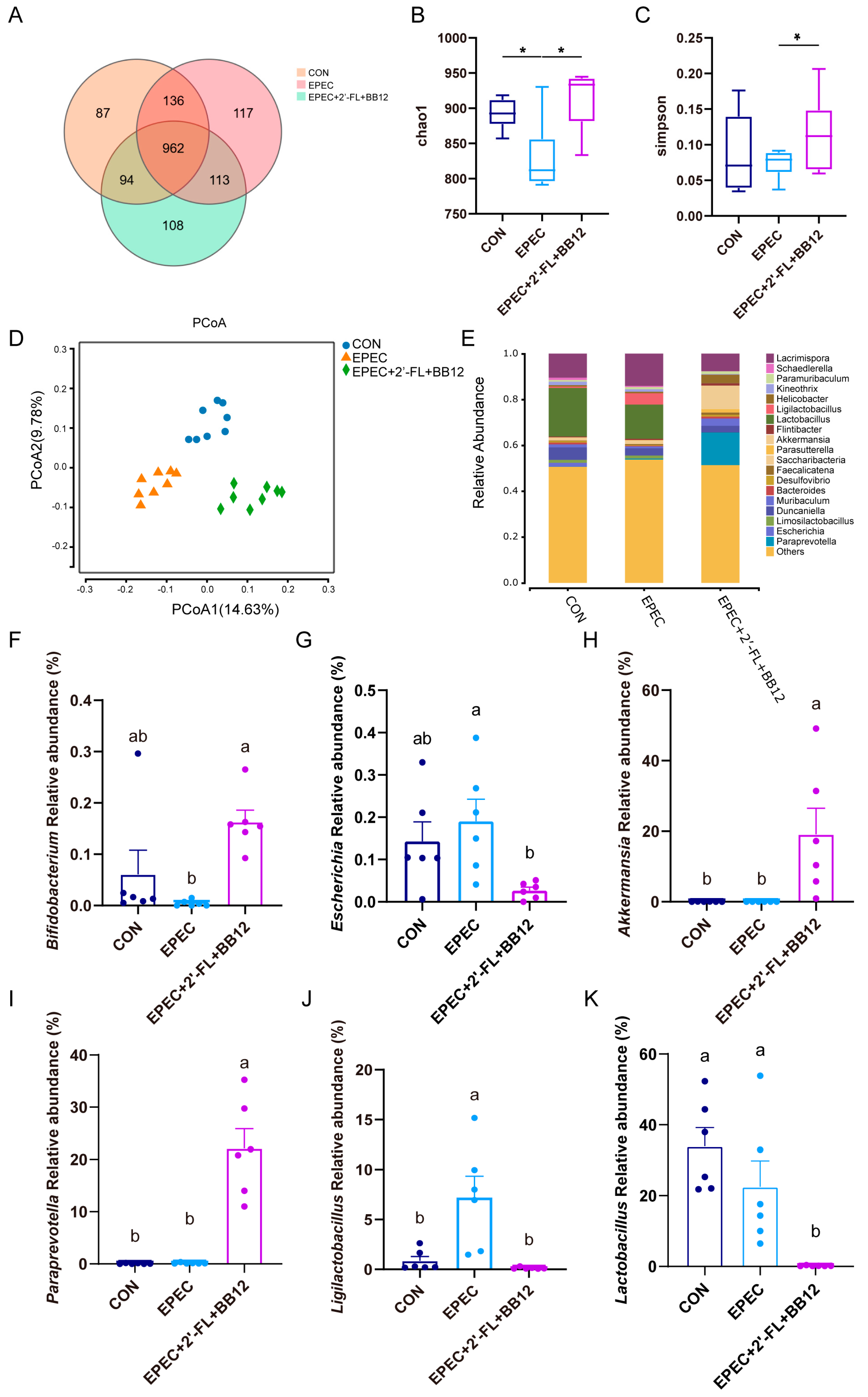
3.5. Impact of Synbiotic Supplementation on Gut Microbiota Structure in Epec-Induced Infant Diarrhea
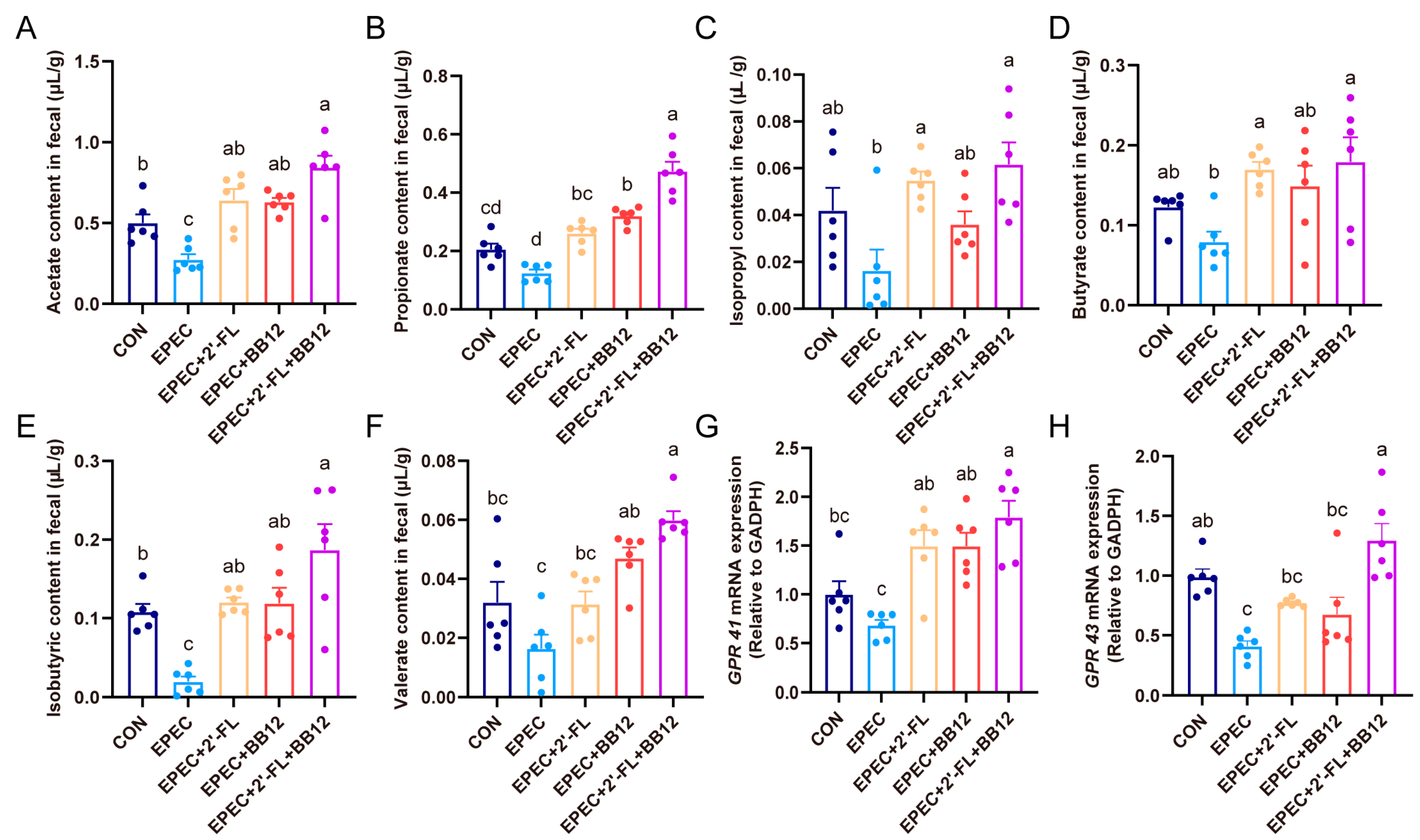
3.6. Impact of Synbiotic Supplementation on Scfas Production and Their Colonic Receptors Expressions in Epec-Induced Infant Diarrhea
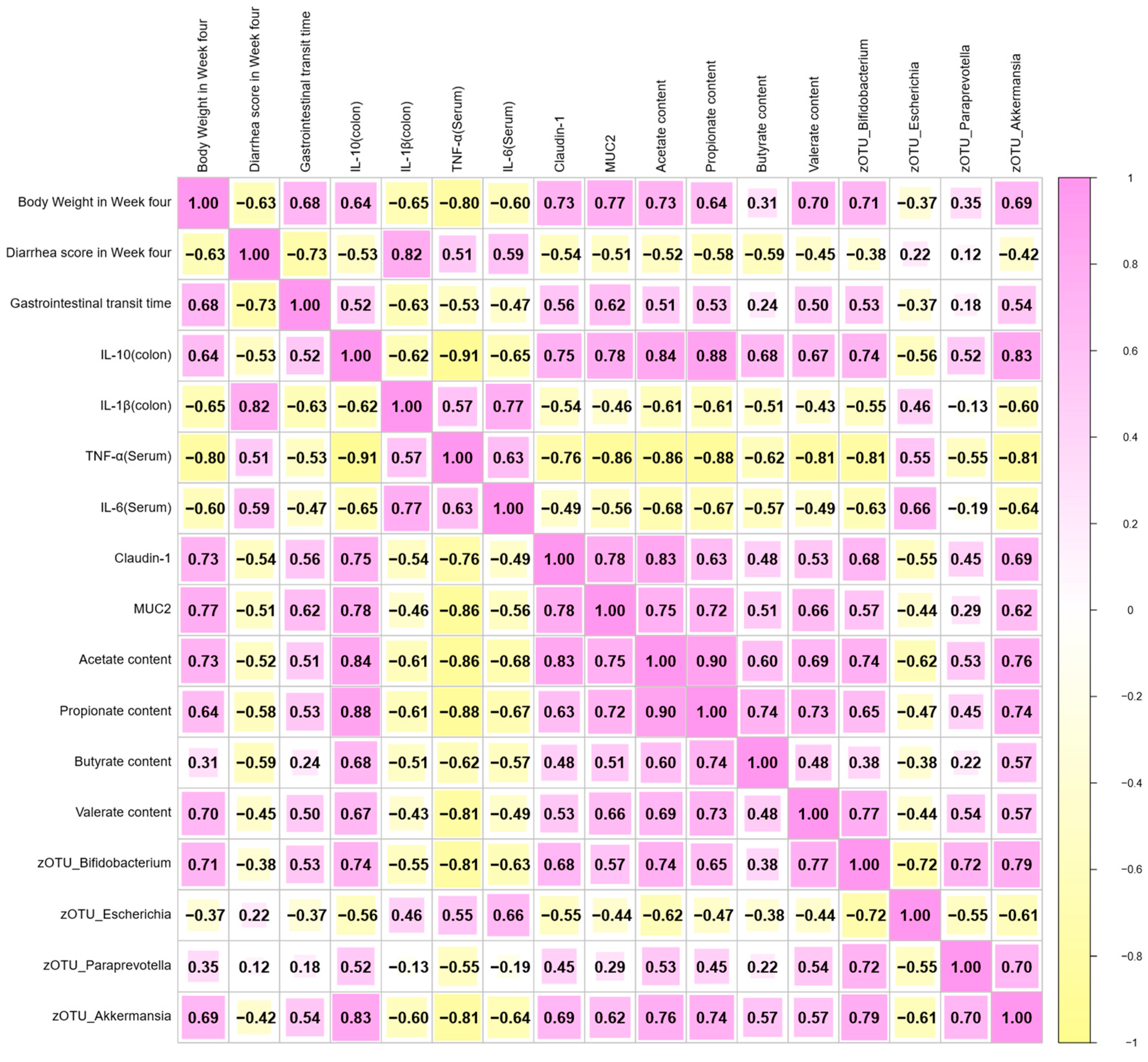
3.7. Correlation Analysis Between Diarrhea Index, Inflammation, Gut Microbiota, and Other Biochemical Indexes in EPEC-Induced Infant Diarrhea
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HMOs | human milk oligosaccharides |
| EPEC | enteropathogenic Escherichia coli |
| E. coli | Escherichia coli |
| 2′-FL | 2′-fucosyllactose |
| SCFAs | short-chain fatty acids |
| GITT | Gastrointestinal transit time |
| PBS | phosphate-buffered saline |
| LB | Luria–Bertani |
| H&E | Hematoxylin and eosin |
| PCA | principal component analysis |
| OTU | operational taxonomic units |
Appendix A
| Primer | Forward 5′-3′ | Reverse 5′-3′ |
|---|---|---|
| GAPDH | TGGAGAAACCTGCCAAGTATGA | TGGAAGAATGGGAGTTGCTGT |
| TNF-α | CTCATGCACCACCATCAAGG | ACCTGACCACTCTCCCTTTG |
| IL-1β | AGCTGGAGAGTGTGGATCCC | CCTGTCTTGGCCGAGGACTA |
| IL-6 | CCACTTCACAAGTCGGAGGC | GGAGAGCATTGGAAATTGGGGT |
| IL-10 | GCTCCAAGACCAAGGTGTCTACAA | CCGTTAGCTAAGATCCCTGGATCA |
| ZO-1 | TGGGAACAGCACACAGTGAC | GCTGGCCCTCCTTTTAACAC |
| Claudin-1 | AGCTGCCTGTTCCATGTACT | CTCCCATTTGTCTGCTGCTC |
| MUC2 | GCTGACGAGTGGTTGGTGAATG | GATGAGGTGGCAGACAGGAGAC |
| occludin | TGGCTATGGAGGCGGCTATGG | AAGGAAGCGATGAAGCAGAAGGC |
| GPR41 | GTGACCATGGGGACAAGCTTC | CCCTGGCTGTAGGTTGCATT |
| GPR43 | GGCTCCCTGCCAACCTGCTG | GTGCACAGGGGCAGGCTGAG |
References
- Liu, L.; Oza, S.; Hogan, D.; Perin, J.; Rudan, I.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: An updated systematic analysis. Lancet 2015, 385, 430–440. [Google Scholar] [CrossRef]
- Thiagarajah, J.R.; Kamin, D.S.; Acra, S.; Goldsmith, J.D.; Roland, J.T.; Lencer, W.I.; Muise, A.M.; Goldenring, J.R.; Avitzur, Y.; Martín, M.G. Advances in Evaluation of Chronic Diarrhea in Infants. Gastroenterology 2018, 154, 2045–2059.e6. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.R.; Pearson, J.S.; Bright, M.D.; Munera, D.; Robinson, K.S.; Lee, S.F.; Frankel, G.; Hartland, E.L. Enteropathogenic and enterohaemorrhagic Escherichia coli: Even more subversive elements. Mol. Microbiol. 2011, 80, 1420–1438. [Google Scholar] [CrossRef]
- Prata, M.D.M.G.; Havt, A.; Bolick, D.T.; Pinkerton, R.; Lima, A.A.M.; Guerrant, R.L. Comparisons between myeloperoxidase, lactoferrin, calprotectin and lipocalin-2, as fecal biomarkers of intestinal inflammation in malnourished children. J. Transl. Sci. 2016, 2, 134. [Google Scholar] [CrossRef]
- Ledwaba, S.E.; Costa, D.V.S.; Bolick, D.T.; Giallourou, N.; Medeiros, P.; Swann, J.R.; Traore, A.N.; Potgieter, N.; Nataro, J.P.; Guerrant, R.L. Enteropathogenic Escherichia coli Infection Induces Diarrhea, Intestinal Damage, Metabolic Alterations, and Increased Intestinal Permeability in a Murine Model. Front. Cell. Infect. Microbiol. 2020, 10, 595266. [Google Scholar] [CrossRef]
- Luis, A.S.; Hansson, G.C. Intestinal mucus and their glycans: A habitat for thriving microbiota. Cell Host Microbe 2023, 31, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zou, Y.; Wang, J.; Ma, H.; Zhang, B.; Wang, S. The Protective Effects of 2′-Fucosyllactose against E. coli O157 Infection Are Mediated by the Regulation of Gut Microbiota and the Inhibition of Pathogen Adhesion. Nutrients 2020, 12, 1284. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, J.; Marchix, J.; Aymeric, L.; Le Berre-Scoul, C.; Zoppi, J.; Bordron, P.; Burel, M.; Davidovic, L.; Richard, J.R.; Gaman, A.; et al. Fecal Supernatant from Adult with Autism Spectrum Disorder Alters Digestive Functions, Intestinal Epithelial Barrier, and Enteric Nervous System. Microorganisms 2021, 9, 1723. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Guarino, A.; Guandalini, S.; Lo Vecchio, A. Probiotics for Prevention and Treatment of Diarrhea. J. Clin. Gastroenterol. 2015, 49 (Suppl. S1), S37–S45. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Hojsak, I. Health benefits of Lactobacillus rhamnosus GG and Bifidobacterium animalis subspecies lactis BB-12 in children. Postgrad. Med. 2020, 132, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Jungersen, M.; Wind, A.; Johansen, E.; Christensen, J.E.; Stuer-Lauridsen, B.; Eskesen, D. The Science behind the Probiotic Strain Bifidobacterium animalis subsp. lactis BB-12(®). Microorganisms 2014, 2, 92–110. [Google Scholar] [CrossRef] [PubMed]
- Commane, D.M.; Shortt, C.T.; Silvi, S.; Cresci, A.; Hughes, R.M.; Rowland, I.R. Effects of fermentation products of pro- and prebiotics on trans-epithelial electrical resistance in an in vitro model of the colon. Nutr. Cancer 2005, 51, 102–109. [Google Scholar] [CrossRef]
- Du, B.; Yan, R.; Hu, X.; Lou, J.; Zhu, Y.; Shao, Y.; Jiang, H.; Hao, Y.; Lv, L. Role of Bifidobacterium animalis subsp. lactis BB-12 in mice with acute pancreatitis. AMB Express 2025, 15, 62. [Google Scholar] [CrossRef]
- Vlieger, A.M.; Robroch, A.; van Buuren, S.; Kiers, J.; Rijkers, G.; Benninga, M.A.; te Biesebeke, R. Tolerance and safety of Lactobacillus paracasei ssp. paracasei in combination with Bifidobacterium animalis ssp. lactis in a prebiotic-containing infant formula: A randomised controlled trial. Br. J. Nutr. 2009, 102, 869–875. [Google Scholar] [CrossRef]
- Merenstein, D.; Fraser, C.M.; Roberts, R.F.; Liu, T.; Grant-Beurmann, S.; Tan, T.P.; Smith, K.H.; Cronin, T.; Martin, O.A.; Sanders, M.E.; et al. Bifidobacterium animalis subsp. lactis BB-12 Protects against Antibiotic-Induced Functional and Compositional Changes in Human Fecal Microbiome. Nutrients 2021, 13, 2814. [Google Scholar] [CrossRef]
- Asakuma, S.; Hatakeyama, E.; Urashima, T.; Yoshida, E.; Katayama, T.; Yamamoto, K.; Kumagai, H.; Ashida, H.; Hirose, J.; Kitaoka, M. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J. Biol. Chem. 2011, 286, 34583–34592. [Google Scholar] [CrossRef]
- Quinn, E.M.; Joshi, L.; Hickey, R.M. Symposium review: Dairy-derived oligosaccharides—Their influence on host-microbe interactions in the gastrointestinal tract of infants. J. Dairy Sci. 2020, 103, 3816–3827. [Google Scholar] [CrossRef]
- Manthey, C.F.; Autran, C.A.; Eckmann, L.; Bode, L. Human milk oligosaccharides protect against enteropathogenic Escherichia coli attachment in vitro and EPEC colonization in suckling mice. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 165–168. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Z.; He, Y.; Wang, X.; Ma, J.; Ma, J.; Chang, P.; Huang, H.; Yuan, T.; Guo, R.; et al. Human milk oligosaccharides mitigate infant diarrhea and anxiety-like behaviors via gut microbiota modulation in an EPEC O127 infection model. Food Funct. 2025, 16, 5757–5770. [Google Scholar] [CrossRef]
- Zou, Q.; Han, S.; Liang, J.; Yan, G.; Wang, Q.; Wang, Y.; Zhang, Z.; Hu, J.; Li, J.; Yuan, T.; et al. Alleviating effect of vagus nerve cutting in Salmonella-induced gut infections and anxiety-like behavior via enhancing microbiota-derived GABA. Brain Behav. Immun. 2024, 119, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liang, J.; Zou, Q.; Wang, W.; Yan, G.; Guo, R.; Yuan, T.; Wang, Y.; Liu, X.; Liu, Z. Tryptophan Metabolism-Regulating Probiotics Alleviate Hyperuricemia by Protecting the Gut Barrier Integrity and Enhancing Colonic Uric Acid Excretion. J. Agric. Food Chem. 2024, 72, 26746–26761. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jia, M.; Ding, C.; Bao, B.; Li, H.; Ma, J.; Dong, W.; Gao, R.; Chen, X.; Chen, J.; et al. Time-restricted feeding mitigates Alzheimer’s disease-associated cognitive impairments via a B. pseudolongum-propionic acid-FFAR3 axis. iMeta 2025, 4, e70006. [Google Scholar] [CrossRef]
- Wang, C.; Chen, W.; Jiang, Y.; Xiao, X.; Zou, Q.; Liang, J.; Zhao, Y.; Wang, Q.; Yuan, T.; Guo, R.; et al. A synbiotic formulation of Lactobacillus reuteri and inulin alleviates ASD-like behaviors in a mouse model: The mediating role of the gut-brain axis. Food Funct. 2024, 15, 387–400. [Google Scholar] [CrossRef]
- Jia, M.; Ning, F.; Wen, J.; Wang, X.; Chen, J.; Hu, J.; Chen, X.; Liu, Z. Secoisolariciresinol diglucoside attenuates neuroinflammation and cognitive impairment in female Alzheimer’s disease mice via modulating gut microbiota metabolism and GPER/CREB/BDNF pathway. J. Neuroinflamm. 2024, 21, 201. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, B.; Xin, Y.; Wang, W.; Wang, X.; Liu, Z.; She, Y.; Guo, R.; Jia, G.; Wu, S.; et al. Synbiotic combination of 2′-fucosyllactose and Bifidobacterium mitigates neurodevelopmental disorders and ASD-like behaviors induced by valproic acid. Food Funct. 2025, 16, 2703–2717. [Google Scholar] [CrossRef]
- Nogacka, A.M.; Cuesta, I.; Gueimonde, M.; de Los Reyes-Gavilán, C.G. 2-Fucosyllactose Metabolism by Bifidobacteria Promotes Lactobacilli Growth in Co-Culture. Microorganisms 2023, 11, 2659. [Google Scholar] [CrossRef]
- Rondepierre, F.; Meynier, M.; Gagniere, J.; Deneuvy, V.; Deneuvy, A.; Roche, G.; Baudu, E.; Pereira, B.; Bonnet, R.; Barnich, N.; et al. Preclinical and clinical evidence of the association of colibactin-producing Escherichia coli with anxiety and depression in colon cancer. World J. Gastroenterol. 2024, 30, 2817–2826. [Google Scholar] [CrossRef]
- Dupont, A.; Sommer, F.; Zhang, K.; Repnik, U.; Basic, M.; Bleich, A.; Kühnel, M.; Bäckhed, F.; Litvak, Y.; Fulde, M.; et al. Age-Dependent Susceptibility to Enteropathogenic Escherichia coli (EPEC) Infection in Mice. PLoS Pathog. 2016, 12, e1005616. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, S.; Lv, H.; Peng, L.; Yang, W.; Chen, J.; Wu, Z.; Wan, C. Effect of Bifidobacterium animalis subsp. lactis SF on enhancing the tumor suppression of irinotecan by regulating the intestinal flora. Pharmacol. Res. 2022, 184, 106406. [Google Scholar] [CrossRef]
- Martín, R.; Laval, L.; Chain, F.; Miquel, S.; Natividad, J.; Cherbuy, C.; Sokol, H.; Verdu, E.F.; van Hylckama Vlieg, J.; Bermudez-Humaran, L.G.; et al. Bifidobacterium animalis ssp. lactis CNCM-I2494 Restores Gut Barrier Permeability in Chronically Low-Grade Inflamed Mice. Front. Microbiol. 2016, 7, 608. [Google Scholar] [CrossRef]
- Yao, Q.; Li, H.; Gao, Y.; Zheng, N.; Delcenserie, V.; Wang, J. The Milk Active Ingredient, 2′-Fucosyllactose, Inhibits Inflammation and Promotes MUC2 Secretion in LS174T Goblet Cells In Vitro. Foods 2023, 12, 186. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.H.; Shin, C.S.; Yoon, J.W.; Jeon, S.M.; Song, Y.H.; Kim, K.Y.; Kim, K. 2′-Fucosyllactose and 3-Fucosyllactose Alleviates Interleukin-6-Induced Barrier Dysfunction and Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Modulating the Intestinal Microbiome. Nutrients 2023, 15, 1845. [Google Scholar] [CrossRef]
- Devriese, S.; Eeckhaut, V.; Geirnaert, A.; Van den Bossche, L.; Hindryckx, P.; Van de Wiele, T.; Van Immerseel, F.; Ducatelle, R.; De Vos, M.; Laukens, D. Reduced Mucosa-associated Butyricicoccus Activity in Patients with Ulcerative Colitis Correlates with Aberrant Claudin-1 Expression. J. Crohns Colitis 2017, 11, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Alcazar, C.G.; Paes, V.M.; Shao, Y.; Oesser, C.; Miltz, A.; Lawley, T.D.; Brocklehurst, P.; Rodger, A.; Field, N. The association between early-life gut microbiota and childhood respiratory diseases: A systematic review. Lancet Microbe 2022, 3, e867–e880. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yin, X.; Ling, L.; Mao, H.; Dong, X.; Chang, X.; Chen, M.; Fang, S. Adding glucose delays the conversion of ethanol and acetic acid to caproic acid in Lacrimispora celerecrescens JSJ-1. Appl. Microbiol. Biotechnol. 2023, 107, 1453–1463. [Google Scholar] [CrossRef]
- Zheng, M.; Han, R.; Yuan, Y.; Xing, Y.; Zhang, W.; Sun, Z.; Liu, Y.; Li, J.; Mao, T. The role of Akkermansia muciniphila in inflammatory bowel disease: Current knowledge and perspectives. Front. Immunol. 2022, 13, 1089600. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Luo, T.; Deng, Z.; Li, J.; Zheng, L.; Zhang, B. Human Milk Oligosaccharides: A Critical Review on Structure, Preparation, Their Potential as a Food Bioactive Component, and Future Perspectives. J. Agric. Food Chem. 2023, 71, 15908–15925. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Khan, W.I. Goblet cells and mucins: Role in innate defense in enteric infections. Pathogens 2013, 2, 55–70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wang, W.; Li, N.; Chen, J.; Wang, Q.; Jia, M.; Wang, X.; Zhang, B.; Sheng, N.; Liu, Z. A Synbiotic of Bifidobacterium animalis subsp. lactis BB-12 and 2′-FL Alleviate Infant Diarrhea and Anxiety-like Behaviors via Gut Microbiota Modulation in an EPEC O127 Infection Model. Nutrients 2025, 17, 3099. https://doi.org/10.3390/nu17193099
Liu Z, Wang W, Li N, Chen J, Wang Q, Jia M, Wang X, Zhang B, Sheng N, Liu Z. A Synbiotic of Bifidobacterium animalis subsp. lactis BB-12 and 2′-FL Alleviate Infant Diarrhea and Anxiety-like Behaviors via Gut Microbiota Modulation in an EPEC O127 Infection Model. Nutrients. 2025; 17(19):3099. https://doi.org/10.3390/nu17193099
Chicago/Turabian StyleLiu, Zhuo, Wenxiu Wang, Ning Li, Jinkuan Chen, Qianxu Wang, Mengzhen Jia, Xiaorui Wang, Bo Zhang, Nan Sheng, and Zhigang Liu. 2025. "A Synbiotic of Bifidobacterium animalis subsp. lactis BB-12 and 2′-FL Alleviate Infant Diarrhea and Anxiety-like Behaviors via Gut Microbiota Modulation in an EPEC O127 Infection Model" Nutrients 17, no. 19: 3099. https://doi.org/10.3390/nu17193099
APA StyleLiu, Z., Wang, W., Li, N., Chen, J., Wang, Q., Jia, M., Wang, X., Zhang, B., Sheng, N., & Liu, Z. (2025). A Synbiotic of Bifidobacterium animalis subsp. lactis BB-12 and 2′-FL Alleviate Infant Diarrhea and Anxiety-like Behaviors via Gut Microbiota Modulation in an EPEC O127 Infection Model. Nutrients, 17(19), 3099. https://doi.org/10.3390/nu17193099








