Evaluation of Antioxidant-Rich Mexican Oregano (Lippia graveolens) Infusion and Carvacrol: Impact on Metabolic Activity and Cytotoxicity in Breast Cancer Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Cell Lines and Culture Conditions
2.3. Plant Material
2.4. Bromatological Analysis of L. graveolens
2.5. Aqueous Extraction of Mexican Oregano (Lippia graveolens Kunth)
2.6. Preparation of Cv Solution
2.7. Antioxidant Activity Protocols (DPPH and ABTS) in MoI
2.8. Determination of Total Phenolic Content in MoI
2.9. Determination of Total Flavonoids in MoI
2.10. FRAP in MoI
2.11. FTIR Measurement
2.12. HPLC Analysis of MoI
2.13. MoI Time Response Curve
2.14. Evaluation of Cell Metabolism (MTT Assay)
2.15. Cytotoxicity Assay (LDH in Supernatant)
2.16. Statistical Analysis
3. Results
3.1. Nutritional Composition of L. graveolens
3.2. Antioxidant Capability of MoI
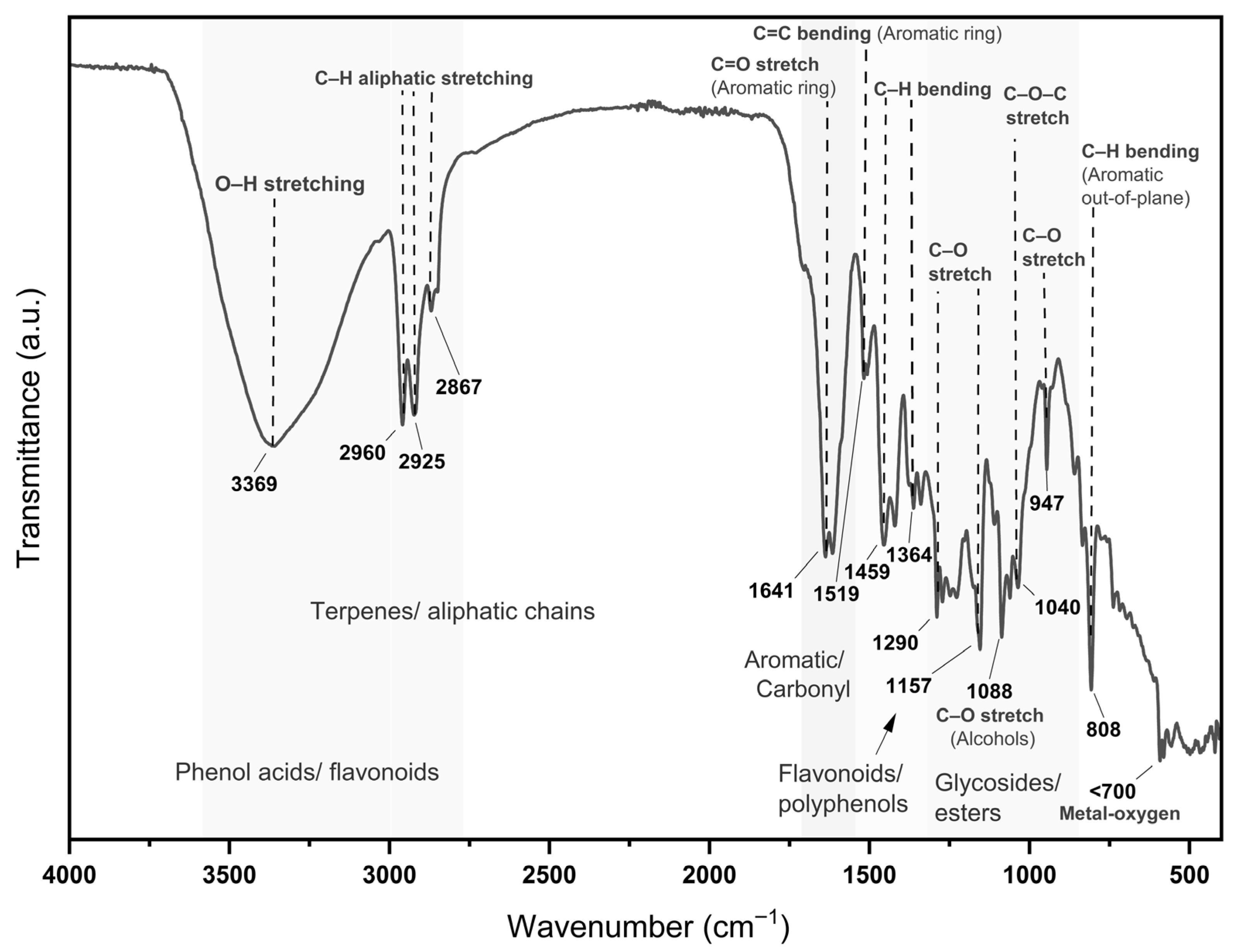
3.3. MoI Chemical Composition by FTIR
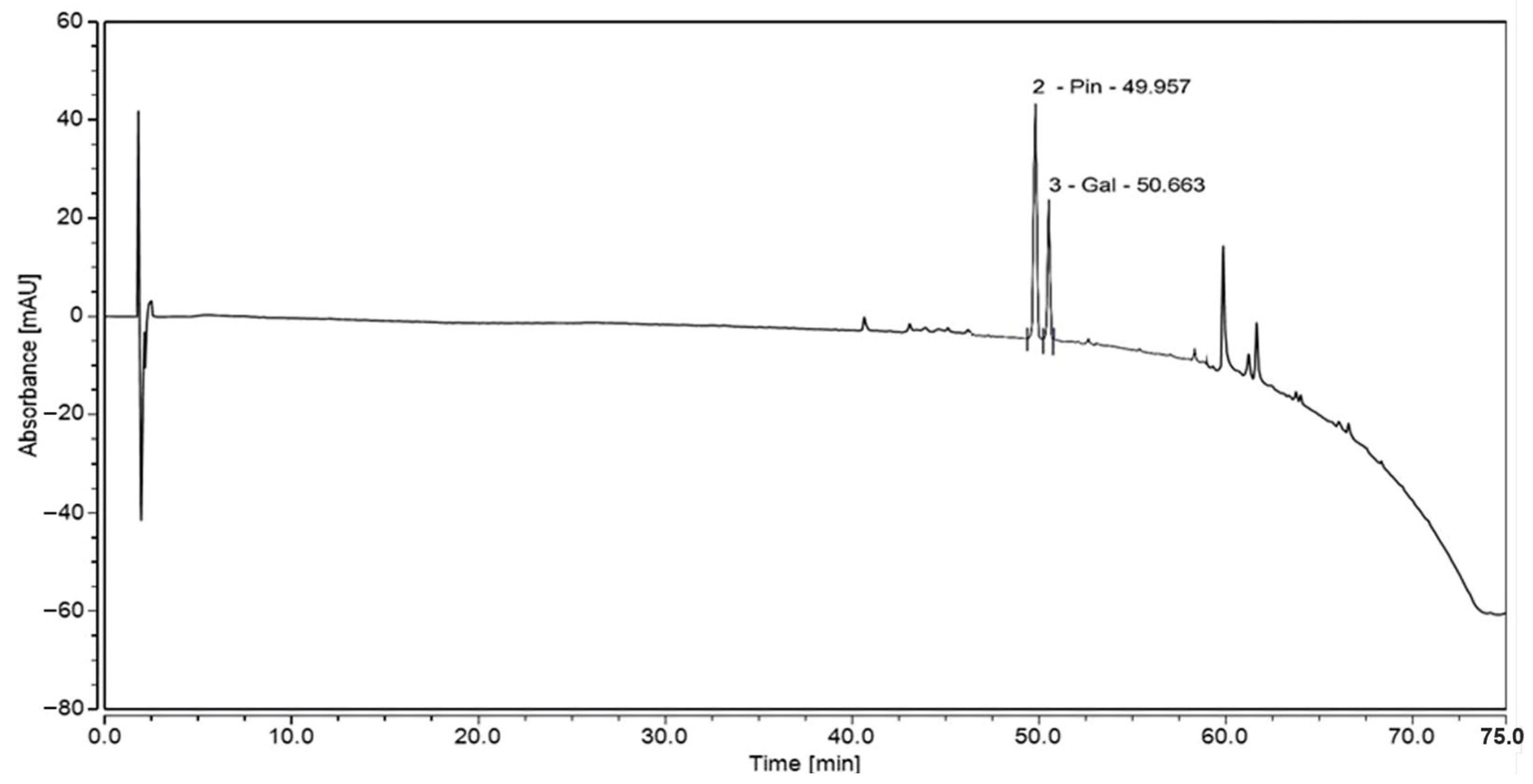
3.4. High-Performance Liquid Chromatography (HPLC) Analysis of MoI
3.5. MoI Dose–Response Curve
3.6. Cell Metabolism Assay
3.7. IC50 Calculation
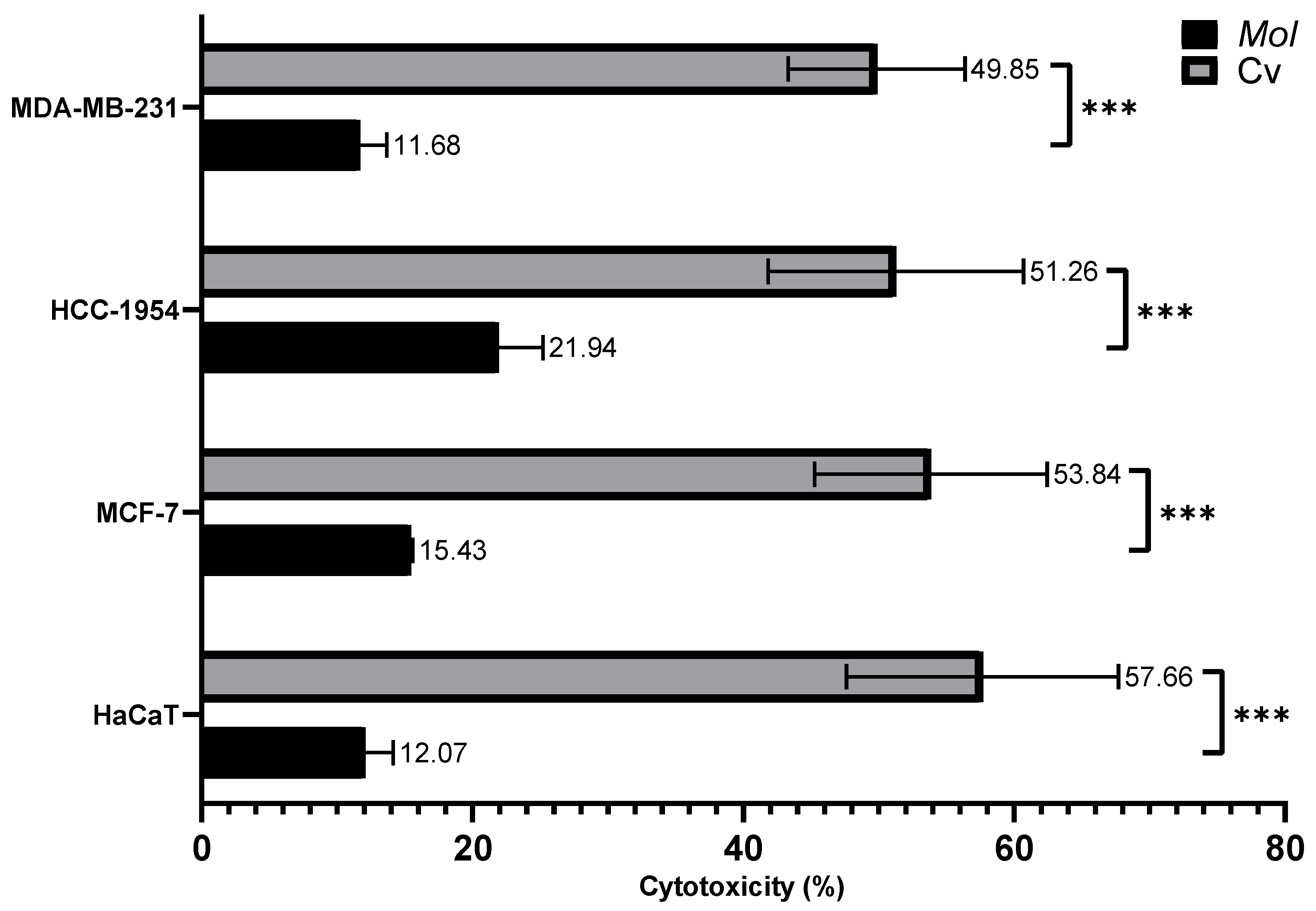
3.8. Cytotoxicity of MoI and Cv (IC50)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid |
| AOAC | Association of Official Analytical Chemists |
| ATCC | American Type Culture Collection |
| ATR | Attenuated Total Reflectance |
| BC | Breast cancer |
| Cv | Carvacrol |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl sulfoxide |
| DPPH | 1,1diphenyl-2-picrylhydrazyl |
| ER | Estrogen receptor |
| FBS | Fetal bovine serum |
| FRAP | Ferric reducing antioxidant power |
| FTIR | Fourier-transform infrared spectroscopy |
| GAE | Gallic acid equivalent |
| HER2 | Human epidermal growth factor receptor 2 |
| HER2+ | HER2-enriched |
| HPLC | High-performance liquid chromatography |
| IC50 | Half maximal inhibitory concentration |
| LDH | Lactate dehydrogenase |
| MoI | Mexican oregano infusion |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| Na2CO3 | Sodium carbonate |
| PBS | Phosphate-buffered saline |
| PR | Progesterone receptor |
| RPMI | Roswell Park Memorial Institute |
| TN | Triple negative |
| Trolox | 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid |
| USDA | U.S. Department of Agriculture |
References
- Cilibrasi, C.; Papanastasopoulos, P.; Samuels, M.; Giamas, G. Reconstituting Immune Surveillance in Breast Cancer: Molecular Pathophysiology and Current Immunotherapy Strategies. Int. J. Mol. Sci. 2021, 22, 12015. [Google Scholar] [CrossRef]
- Global Cancer Observatory Breast Cancer. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/20-Breast-fact-sheet.pdf (accessed on 9 April 2025).
- Global Cancer Observatory Mexico: All Cancers. Available online: https://gco.iarc.fr/today/data/factsheets/populations/484-mexico-fact-sheets.pdf (accessed on 9 September 2022).
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Nolan, E.; Lindeman, G.J.; Visvader, J.E. Deciphering breast cancer: From biology to the clinic. Cell 2023, 186, 1708–1728. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.R.M.R.; Kucuk, O.; Khuri, F.R.; Shin, D.M. Perspectives for cancer prevention with natural compounds. J. Clin. Oncol. 2009, 27, 2712–2725. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Nasr, S.; Nakisa, A.; Jandaghian, S.; Kouhi, M.; Sadeghi, E.; Varshosaz, J. A Systematic Review and Meta-Analysis on the Effect of Flavonoids on Insulin-like Growth Factor and Insulin-like Growth Factor Binding Protein and Incidence of Breast Cancer. Curr. Med. Chem. 2023, 30, 1657–1666. [Google Scholar] [CrossRef]
- Ho, V.W.-T.; Tan, H.-Y.; Guo, W.; Li, S.; Wang, N.; Meng, W.; So, T.-H.; Yu, E.C.-L.; Feng, Y. Efficacy and Safety of Chinese Herbal Medicine on Treatment of Breast Cancer: A Meta-analysis of Randomized Controlled Trials. Am. J. Chin. Med. 2021, 49, 1557–1575. [Google Scholar] [CrossRef]
- Martínez-Natarén, D.A.; Parra-Tabla, V.; Dzib, G.; Acosta-Arriola, V.; Canul-Puc, K.A.; Calvo-Irabién, L.M. Essential oil yield variation within and among wild populations of mexican oregano (lippia graveolens h.b.k.-verbenaceae), and its relation to climatic and edaphic conditions. J. Essent. Oil-Bear. Plants 2012, 15, 589–601. [Google Scholar] [CrossRef]
- Nieto-Ramírez, M.I.; Feregrino-Pérez, A.A.; Becerra, H.A.; Parra-Pacheco, B.; Oviedo-Olvera, M.V.; García-Trejo, J.F. Response of Phenolic Compounds in Lippia graveolens Kunth Irrigated with Aquaculture Wastewater and Steiner Solution. Int. J. Plant Biol. 2023, 14, 483–492. [Google Scholar] [CrossRef]
- Arigesavan, K.; Sudhandiran, G. Carvacrol exhibits anti-oxidant and anti-inflammatory effects against 1, 2-dimethyl hydrazine plus dextran sodium sulfate induced inflammation associated carcinogenicity in the colon of Fischer 344 rats. Biochem. Biophys. Res. Commun. 2015, 461, 314–320. [Google Scholar] [CrossRef]
- Lim, W.; Ham, J.; Bazer, F.W.; Song, G. Carvacrol induces mitochondria-mediated apoptosis via disruption of calcium homeostasis in human choriocarcinoma cells. J. Cell. Physiol. 2019, 234, 1803–1815. [Google Scholar] [CrossRef]
- Khan, F.; Khan, I.; Farooqui, A.; Ansari, I.A. Carvacrol Induces Reactive Oxygen Species (ROS)-mediated Apoptosis Along with Cell Cycle Arrest at G0/G1 in Human Prostate Cancer Cells. Nutr. Cancer 2017, 69, 1075–1087. [Google Scholar] [CrossRef]
- NMX-F-083-1986; Alimentos–Determinación de Humedad en Productos Alimenticios. Declaratoria de Vigencia Publicada en el Diario Oficial de la Federación el 14 de Julio de 1986. Normas Mexicanas: Mexico City, Mexico, 1986.
- Association of Official Analytical Chemists (AOAC). Official Methods 923.03. In Ash for Flour; AOAC: San Diego, CA, USA, 1923; Volume 7, p. 132. [Google Scholar]
- NOM-086-SSA1-1994; Bienes y Servicios. Alimentos y Bebidas no Alcohólicas con Modificaciones en su Composición. Especificaciones Nutrimentales. DOF-Diario Oficial de la Federación NORMA Oficial Mexicana: Mexico City, Mexico. Available online: https://dof.gob.mx/nota_detalle.php?codigo=4890075&fecha=26/06/1996#gsc.tab=0 (accessed on 7 June 2025).
- Association of Official Analytical Chemists (AOAC). Oficial Methods 928.08. In Nitrogen in Meat—Kjeldahl Method; AOAC: San Diego, CA, USA, 2006; p. 18. [Google Scholar]
- AOAC 2003.06-2006; Crude Fat in Feeds, Cereal Grains, and Forages. AOAC: San Diego, CA, USA, 2006. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&cPath=1&products_id=1552 (accessed on 16 November 2023).
- AOAC International. Method of Analysis for Nutrition Labeling (General Factors: Protein × 4, CHO × 4, and Fat × 9); AOAC: San Diego, CA, USA, 1993. [Google Scholar]
- Nelder, J.A.; Wedderburn, R.W.M. Generalized Linear Models. J. R. Stat. Society. Ser. A (Gen.) 1972, 135, 370. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Armentano, M.F.; Carmosino, M.; Bufo, S.A.; De Feo, V.; Camele, I. Cytotoxic activity of Origanum vulgare L. on Hepatocellular carcinoma cell line HepG2 and evaluation of its biological activity. Molecules 2017, 22, 1435. [Google Scholar] [CrossRef] [PubMed]
- Oniga, I.; Pușcaș, C.; Silaghi-Dumitrescu, R.; Olah, N.-K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. vulgare: Chemical composition and biological studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- USDA Spices, Oregano, Dried. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171328/nutrients (accessed on 20 March 2025).
- Zhang, X.-L.; Guo, Y.-S.; Wang, C.-H.; Li, G.-Q.; Xu, J.-J.; Chung, H.Y.; Ye, W.-C.; Li, Y.-L.; Wang, G.-C. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chem. 2014, 152, 300–306. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Zengin, G.; Aladag, M.O.; Ozparlak, H.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Aumeeruddy, M.Z. HPLC-MS/MS chemical characterization and biological properties of Origanum onites extracts: A recent insight. Int. J. Environ. Health Res. 2019, 29, 607–621. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Burton, D.; Parra, F.; López, J.; Muñoz, P.; Escobar, H.; Parra, C. Antioxidant and antibacterial capacities of Origanum vulgare L. Essential oil from the arid andean region of chile and its chemical characterization by gc-ms. Metabolites 2020, 10, 414. [Google Scholar] [CrossRef]
- Kogiannou, D.A.; Kalogeropoulos, N.; Kefalas, P.; Polissiou, M.G.; Kaliora, A.C. Herbal infusions; their phenolic profile, antioxidant and anti-inflammatory effects in HT29 and PC3 cells. Food Chem. Toxicol. 2013, 61, 152–159. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Berdowska, I.; Zieliński, B.; Fecka, I.; Kulbacka, J.; Saczko, J.; Gamian, A. Cytotoxic impact of phenolics from Lamiaceae species on human breast cancer cells. Food Chem. 2013, 141, 1313–1321. [Google Scholar] [CrossRef]
- Marin-Tinoco, R.I.; Ortega-Ramírez, A.T.; Esteban-Mendez, M.; Silva-Marrufo, O.; Barragan-Ledesma, L.E.; Valenzuela-Núñez, L.M.; Briceño-Contreras, E.A.; Sariñana-Navarrete, M.A.; Camacho-Luis, A.; Navarrete-Molina, C. Antioxidant and Antibacterial Activity of Mexican Oregano Essential Oil, Extracted from Plants Occurring Naturally in Semiarid Areas and Cultivated in the Field and Greenhouse in Northern Mexico. Molecules 2023, 28, 6547. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Hernández, I.; Aguilar, C.N.; Martínez-Ávila, G.C.G.; Torres-León, C.; Ilina, A.; Flores-Gallegos, A.C.; Verma, D.K.; Chávez-González, M.L. Mexican Oregano (Lippia graveolens Kunth) as Source of Bioactive Compounds: A Review. Molecules 2021, 26, 5156. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Millán, M.D.J.; Gutiérrez-Grijalva, E.P.; Contreras-Angulo, L.; Muy-Rangel, M.D.; López-Martínez, L.X.; Heredia, J.B. Spray-Dried Microencapsulation of Oregano (Lippia graveolens) Polyphenols with Maltodextrin Enhances Their Stability during In Vitro Digestion. J. Chem. 2022, 2022, 8740141. [Google Scholar] [CrossRef]
- Cortés-Chitala, M.D.C.; Flores-Martínez, H.; Orozco-Ávila, I.; León-Campos, C.; Suárez-Jacobo, Á.; Estarrón-Espinosa, M.; López-Muraira, I. Identification and Quantification of Phenolic Compounds from Mexican Oregano (Lippia graveolens HBK) Hydroethanolic Extracts and Evaluation of Its Antioxidant Capacity. Molecules 2021, 26, 702. [Google Scholar] [CrossRef]
- Anvarbatcha, R.; Kunnathodi, F.; Islam, M. Induction of G0/G1 phase cell cycle arrest and apoptosis by thymol through ROS generation and caspase-9/-3 activation in breast and colorectal cancer cell lines. J. Cancer Res. Ther. 2023, 19, 1915–1924. [Google Scholar] [CrossRef]
- Vahitha, V.; Lali, G.; Prasad, S.; Karuppiah, P.; Karunakaran, G.; AlSalhi, M.S. Unveiling the therapeutic potential of thymol from Nigella sativa L. seed: Selective anticancer action against human breast cancer cells (MCF-7) through down-regulation of Cyclin D1 and proliferative cell nuclear antigen (PCNA) expressions. Mol. Biol. Rep. 2024, 51, 61. [Google Scholar] [CrossRef]
- Song, W.; Yan, C.Y.; Zhou, Q.Q.; Zhen, L.L. Galangin potentiates human breast cancer to apoptosis induced by TRAIL through activating AMPK. Biomed. Pharmacother. 2017, 89, 845–856. [Google Scholar] [CrossRef]
- Liu, D.; You, P.; Luo, Y.; Yang, M.; Liu, Y. Galangin Induces Apoptosis in MCF-7 Human Breast Cancer Cells Through Mitochondrial Pathway and Phosphatidylinositol 3-Kinase/Akt Inhibition. Pharmacology 2018, 102, 58–66. [Google Scholar] [CrossRef]
- Sinnarkar, S.; Suryawanshi, P.; Dilip, A.; Bhawalkar, J.; Ladke, V. Galangin promotes apoptosis by upregulating the pro-apoptotic gene BAX in triple-negative breast cancer. J. Egypt. Natl. Cancer Inst. 2024, 36, 41. [Google Scholar] [CrossRef]
- Zhu, X.; Li, R.; Wang, C.; Zhou, S.; Fan, Y.; Ma, S.; Gao, D.; Gai, N.; Yang, J. Pinocembrin Inhibits the Proliferation and Metastasis of Breast Cancer via Suppression of the PI3K/AKT Signaling Pathway. Front. Oncol. 2021, 11, 661184. [Google Scholar] [CrossRef]
- Elbe, H.; Yigitturk, G.; Cavusoglu, T.; Baygar, T.; Onal, M.O.; Ozturk, F. Comparison of ultrastructural changes and the anticarcinogenic effects of thymol and carvacrol on ovarian cancer cells: Which is more effective? Ultrastruct. Pathol. 2020, 44, 193–202. [Google Scholar] [CrossRef]
- Tuncer, E.; Unver-Saraydin, S.; Tepe, B.; Karadayi, S.; Ozer, H.; Sen, M.; Karadayi, K.; Inan, D.; Elagoz, S.; Polat, Z.; et al. Antitumor effects of Origanum acutidens extracts on human breast cancer. J. BUON 2013, 18, 77–85. [Google Scholar]
- Makrane, H.; El Messaoudi, M.; Melhaoui, A.; El Mzibri, M.; Benbacer, L.; Aziz, M. Cytotoxicity of the Aqueous Extract and Organic Fractions from Origanum majorana on Human Breast Cell Line MDA-MB-231 and Human Colon Cell Line HT-29. Adv. Pharmacol. Sci. 2018, 2018, 3297193. [Google Scholar] [CrossRef] [PubMed]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Vadgama, J.V. Curcumin and Epigallocatechin Gallate Inhibit the Cancer Stem Cell Phenotype via Down-regulation of STAT3–NFκB signaling. Anticancer. Res. 2015, 35, 39. [Google Scholar] [PubMed]
- Dayem, A.A.; Choi, H.Y.; Yang, G.-M.; Kim, K.; Saha, S.K.; Cho, S.-G. The Anti-Cancer Effect of Polyphenols against Breast Cancer and Cancer Stem Cells: Molecular Mechanisms. Nutrients 2016, 8, 581. [Google Scholar] [CrossRef]
- Al Khalily, I.A.; Megantara, S.; Aulifa, D.L. Targeting Molecular Pathways in Breast Cancer Using Plant-Derived Bioactive Compounds: A Comprehensive Review. J. Exp. Pharmacol. 2025, 17, 375–401. [Google Scholar] [CrossRef]
- Herdiana, Y.; Sriwidodo, S.; Sofian, F.F.; Wilar, G.; Diantini, A. Nanoparticle-Based Antioxidants in Stress Signaling and Programmed Cell Death in Breast Cancer Treatment. Molecules 2023, 28, 5305. [Google Scholar] [CrossRef]
- Calvo Irabien, L.M.; Carnevali Fernández-Concha, G. ¿Debemos seguir llamando orégano mexicano a Lippia graveolens Kunth?: Esclareciendo el nombre del orégano mexicano. Desde El Herb. CICY 2021, 13, 212–216. [Google Scholar]
- O’Leary, N.; Denham, S.S.; Salimena, F.; Múlgura, M.E. Species delimitation in Lippia section Goniostachyum (Verbenaceae) using the phylogenetic species concept. Bot. J. Linn. Soc. 2012, 170, 197–219. [Google Scholar] [CrossRef]
- Rzedowski, J.; Calderón de Rzedowski, G. Verbenaceae. Flora Del Bajío De Reg. Adyac. 2002, 100, 1–145. [Google Scholar]





| Nutritional Content | Per 100 g |
|---|---|
| Energy content | 209 Kcal (874.4 KJ) |
| Moisture | 7.8% |
| Ash | 6.6% |
| Protein | 6.8 g |
| Total fat | 4.2 g |
| Available carbohydrates | 36 g |
| Dietary fiber | 38.6 g |
| ABTS | DPPH | Total Phenolics | Total Flavonoids | FRAP |
|---|---|---|---|---|
| 42.837 ± 0.175 mMEQ Trolox/g | 66.303 ± 0.228 mMEQ Trolox/g | 27.765 ± 1.095 mgGAE/g | 22.343 ± 0.096 mg Eq quercetin/g | 109.85 ± 0.51 mMEQ FeSO4/g |
| Cell Line | MoI IC50 (S.E.) | Cv IC50 (S.E.) |
|---|---|---|
| HaCaT | 0.18 mg/mL (0.016) | 110 µM (19.19) |
| MCF-7 | 0.08 mg/mL (0.006) | 211 µM (18.95) |
| HCC-1954 | 0.15 mg/mL (0.010) | 123 µM (6.09) |
| MDA-MB-231 | 0.17 mg/mL (0.024) | 121 µM (10.85) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojo-Ruvalcaba, B.E.; Maldonado-González, M.; Cálix-Rodríguez, G.M.; Valdés-Miramontes, E.H.; Gómez-Leyva, J.F.; García-Cobián, T.A.; Sánchez-Hernández, P.E.; Machado-Sulbaran, A.C.; López-Roa, R.I.; Balderas-León, I.; et al. Evaluation of Antioxidant-Rich Mexican Oregano (Lippia graveolens) Infusion and Carvacrol: Impact on Metabolic Activity and Cytotoxicity in Breast Cancer Cell Lines. Nutrients 2025, 17, 3089. https://doi.org/10.3390/nu17193089
Rojo-Ruvalcaba BE, Maldonado-González M, Cálix-Rodríguez GM, Valdés-Miramontes EH, Gómez-Leyva JF, García-Cobián TA, Sánchez-Hernández PE, Machado-Sulbaran AC, López-Roa RI, Balderas-León I, et al. Evaluation of Antioxidant-Rich Mexican Oregano (Lippia graveolens) Infusion and Carvacrol: Impact on Metabolic Activity and Cytotoxicity in Breast Cancer Cell Lines. Nutrients. 2025; 17(19):3089. https://doi.org/10.3390/nu17193089
Chicago/Turabian StyleRojo-Ruvalcaba, Brian Enrique, Montserrat Maldonado-González, Gabriela María Cálix-Rodríguez, Elia Herminia Valdés-Miramontes, Juan Florencio Gómez-Leyva, Teresa Arcelia García-Cobián, Pedro Ernesto Sánchez-Hernández, Andrea Carolina Machado-Sulbaran, Rocío Ivette López-Roa, Iván Balderas-León, and et al. 2025. "Evaluation of Antioxidant-Rich Mexican Oregano (Lippia graveolens) Infusion and Carvacrol: Impact on Metabolic Activity and Cytotoxicity in Breast Cancer Cell Lines" Nutrients 17, no. 19: 3089. https://doi.org/10.3390/nu17193089
APA StyleRojo-Ruvalcaba, B. E., Maldonado-González, M., Cálix-Rodríguez, G. M., Valdés-Miramontes, E. H., Gómez-Leyva, J. F., García-Cobián, T. A., Sánchez-Hernández, P. E., Machado-Sulbaran, A. C., López-Roa, R. I., Balderas-León, I., & García-Iglesias, T. (2025). Evaluation of Antioxidant-Rich Mexican Oregano (Lippia graveolens) Infusion and Carvacrol: Impact on Metabolic Activity and Cytotoxicity in Breast Cancer Cell Lines. Nutrients, 17(19), 3089. https://doi.org/10.3390/nu17193089









