Pentadecanoic Acid (C15:0) at Naturally Occurring Circulating Concentrations Has Selective Anticancer Activities Including Targeting B-Cell Lymphomas with CCND3 Oncogenic Alterations
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Proliferation Assay
2.2. Data Analysis
3. Results
3.1. C15:0 Had Selective Inhibitory Activities Against Specific Human Cancer Cell Types, Especially B-Cell Lymphomas
3.2. Lymphomas and Liver Cancers Had the Highest Prevalence of C15:0-Responsive Cell Lines
3.3. Human Cancer Cell Lines Were More Likely to Be Responsive to C15:0 if They Had CCND3 Oncogenic Alterations
3.4. C15:0 Has Inhibitory Activities Among Additional Cancer Cell Lines as C15:0 Treatment Increased from 16 to 50 µM
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C15:0 | Pentadecanoic acid |
| EC50 | The test compound concentration at the curve inflection point or half the effective response |
| IC50 | The test compound concentration at 50% of maximal possible response |
| GI50 | The test compound concentration needed to reduce the observed growth by half (midway between the curve maximum and the time zero value) |
| CCND3 | D-cyclin type gene |
| AMPK | AMP-activated protein kinase |
| AKT | Protein kinase B |
| PPARα/δ | Peroxisome proliferator-activated receptor alpha/delta |
| mTOR | Mechanistic target of rapamycin |
| JAK-STAT | Janus kinase-signal transducer and activator of transcription |
| HDAC-6 | Histone deacetylase 6 |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| DLBCL | Diffuse large B-cell lymphoma |
| DMSO | Dimethyl sulfoxide |
| POC | Point of control |
References
- Curtin, S.C.; Tejada-Vera, B.; Bastian, B.A. Deaths: Leading causes for 2022. Natl. Vital. Stat. Rep. 2024, 73, 1–117. [Google Scholar]
- Di Martino, E.; Smith, L.; Bradley, S.H.; Hemphill, S.; Wright, J.; Renzi, C.; Bergin, R.; Emery, J.; Neal, R.D. Incidence trends for twelve cancers in younger adults—A rapid review. Br. J. Cancer 2022, 126, 1374–1386. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.L.; Jones, S.; Cikomola, J.C.; Hivert, M.F.; Misra, S. Understanding the drivers and consequences of early-onset type 2 diabetes. Lancet 2025, 405, 2327–2340. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, I.; Suffredini, J.; Virani, S.S.; Ballantyne, C.M.; Michos, E.D.; Misra, A.; Saeed, A.; Jia, X. Early-onset atherosclerotic cardiovascular disease. Eur. J. Prev. Cardiol. 2025, 32, 100–112. [Google Scholar] [CrossRef]
- Feng, S.; Wang, T.; Su, Y.; Yan, J.; Wang, Y.; Zhang, Z.; Yin, C.; Zhai, H. Global burden, risk factors, and projections of early-onset dementia: Insights from the Global Burden of Disease Study. Ageing Res. Rev. 2025, 104, 102644. [Google Scholar] [CrossRef]
- Avdic, T.; Carlsen, H.K.; Rawshani, A.; Gudbjornsdottir, S.; Mandalenakis, Z.; Eliasson, B. Risk factors for and risk of all-cause and atherosclerotic cardiovascular disease mortality in people with type 2 diabetes and peripheral artery disease: An observational, register-based cohort study. Cardiovasc. Diabetol. 2024, 23, 127. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, G.; Turazza, F.M.; Inno, A.; Canale, M.L.; Silvestris, N.; Fari, R.; Navazio, A.; Pinto, C.; Tarantini, L. Atherosclerosis and the bidirectional relationship between cancer and cardiovascular disease: From bench to bedside—Part 1. Int. J. Mol. Sci. 2024, 25, 4232. [Google Scholar] [CrossRef]
- Riley, D.R.; Hydes, T.; Hernadez, G.; Zhao, S.S.; Alam, U.; Cuthbertson, D.J. The synergistic impact of type 2 diabetes and MASLD on cardiovascular, liver, diabetes-related and cancer outcomes. Liver Intern. 2024, 44, 2538–2550. [Google Scholar] [CrossRef]
- Chu, C.S.; Cheng, S.L.; Bai, Y.M.; Su, T.P.; Tsai, S.J.; Chen, T.J.; Yang, F.C.; Chen, M.H.; Liang, C.S. Risk of dementia in different types of cancer survivors: A nationwide cohort study. Am. J. Geriatr. Psychiat. 2025, 33, 156–166. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: Could it be essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef]
- Dornan, K.; Gunenc, A.; Oomah, B.D.; Hosseinian, F. Odd chain fatty acids and odd chain phenolic lipids (alkylresorcinols) are essential for diet. J. Am. Chem. Soc. 2021, 98, 813–824. [Google Scholar] [CrossRef]
- Ciesielski, V.; Guerbette, T.; Fret, L.; Succar, M.; Launay, Y.; Dahirel, P.; Legrand, P.; Vlach, M.; Blat, S.; Rioux, V. Dietary pentadecanoic acid supplementation at weaning in essential fatty acid-deficient rats shed light on the new family of odd-chain n-8 PUFAs. J. Nutr. Biochem. 2025, 137, 109814. [Google Scholar] [CrossRef]
- Ciesielski, V.; Legrand, P.; Blat, S.; Rioux, V. New insights on pentadecanoic acid with special focus on its controversial essentiality: A mini-review. Biochimie 2024, 227, 123–129. [Google Scholar] [CrossRef]
- Ruan, M.; Xu, F.; Li, N.; Yu, J.; Teng, F.; Tang, J.; Huang, C.; Zhu, H. Free long-chain fatty acids trigger early postembryonic development in starved Caenorhabditis elegans by suppressing mTORC1. PLoS Biol. 2024, 22, e3002841. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Schork, N. Pentadecanoic acid (C15:0), an essential fatty acid, shares clinically relevant cell-based activities with leading longevity-enhancing compounds. Nutrients 2023, 15, 4607. [Google Scholar] [CrossRef]
- Fu, W.C.; Li, H.Y.; Li, T.T.; Yang, K.; Chen, J.X.; Want, S.J.; Liu, C.H.; Zhang, W. Pentadecanoic acid promotes basal and insulin-stimulated glucose uptake in C2C12 myotubes. Food Nutr. Res. 2021, 65, 10–29219. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.A.; Machate, T.; Henkel, J.; Schulze, M.B.; Klaus, S.; Piepelow, K. Heptadecanoic acid is not a key mediator in the prevention of diet-induced hepatic steatosis and insulin resistance in mice. Nutrients 2023, 15, 2052. [Google Scholar] [CrossRef] [PubMed]
- To, N.B.; Truong, V.N.P.; Ediriweera, M.K.; Cho, S.K. Effects of combined pentadecanoic acid and tamoxifen treatment on tamoxifen resistance in MCF-7/SC breast cancer cells. Int. J. Mol. Sci. 2022, 23, 11340. [Google Scholar] [CrossRef] [PubMed]
- To, N.B.; Nguyen, Y.T.; Moon, J.Y.; Ediriweera, M.K.; Cho, S.K.; To, N.B.; Nguyen, Y.T.; Moon, J.Y.; Ediriweera, M.K.; Cho, S.K. Pentadecanoic acid, an odd-chain fatty acid, suppresses the stemness of MCF-7/SC human breast cancer stem-like cells through JAK2/STAT3 signaling. Nutrients 2020, 12, 1663. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; To, N.B.; Lim, Y.; Cho, S.K. Odd-chain fatty acids as novel histone deacetylase 6 (HDAC6) inhibitors. Biochimie 2021, 186, 147–156. [Google Scholar] [CrossRef]
- Wei, W.; Wong, C.C.; Jia, Z.; Liu, W.; Liu, C.; Ji, F.; Pan, Y.; Wang, F.; Wang, G.; Zhao, L.; et al. Parabacteroides distasonis uses dietary inulin to suppress NASH via its metabolite pentadecanoic acid. Nat. Microbiol. 2023, 8, 1534–1548. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Luo, J.; Li, S.; Li, X.; Wang, W.; Lu, W.; He, Y.; Xu, X. Associations between serum pentadecanoic acid (C15:0) and heptadecanoic acid (C17:0) levels and hypertension: A cross-sectional analysis of NHANES data. Lipids Health Dis. 2025, 24, 219. [Google Scholar] [CrossRef]
- Aabis, M.; Tiwari, P.; Kumar, V.; Singh, G.; Panghal, A.; Jena, G. Pentadecanoic acid attenuates thioacetamide-induced liver fibrosis by modulating oxidative stress, inflammation, and ferroptosis pathways in rat. Nauyn-Schmied. Arch. Pharmacol. 2025, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.K.; Lee, E.; Ugalde-Nicalo, P.A.; Skonieczny, J.W.; Chun, L.F.; Newton, K.P.; Schwimmer, J.B. Pentadecanoic acid supplementation in young adults with overweight and obesity: A randomized controlled trial. J. Nutr. 2024, 154, 2763–2771. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Zhang, Q.A.; Magkos, F.; Ng, M.; Michael, N.; Wu, X.; Volchanskaya, V.S.B.; Lai, X.; Wanjaya, E.R.; Elejalde, U.; et al. Effect of an Asian-adapted Mediterranean diet and pentadecanoic acid on fatty liver disease: The TANGO randomized controlled trial. Am. J. Clin. Nutr. 2024, 119, 788–799. [Google Scholar] [CrossRef]
- Arghavani, H.; Bioldeau, J.F.; Rukdowska, I. Impact of dairy intake on circulating fatty acids and associations with blood pressure: A randomized crossover trial. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 104122. [Google Scholar] [CrossRef]
- Li, Z.; Lei, H.; Jiang, H.; Fan, Y.; Shi, J.; Li, C.; Chen, F.; Mi, B.; Ma, M.; Lin, J.; et al. Saturated fatty acid biomarkers and risk of cardiometabolic diseases: A meta-analysis of prospective studies. Front. Nutr. 2022, 9, 963471. [Google Scholar] [CrossRef]
- Trieu, K.; Bhat, S.; Dai, K.; Keander, K.; Gigante, B.; Qian, F.; Korat, A.V.A.; Sun, Q.; Pan, X.F.; Laguzzi, F.; et al. Biomarkers of dairy fat intake, incident cardiovascular disease, and all-cause mortality: A cohort study, systematic review, and meta-analysis. PLoS Med. 2021, 18, e1003763. [Google Scholar] [CrossRef]
- Huang, L.; Lin, J.S.; Aris, I.M.; Yang, G.; Chen, W.Q.; Li, L.J. Circulating saturated fatty acids and incident type 2 diabetes: A systematic review and meta-analysis. Nutrients 2019, 11, 998. [Google Scholar] [CrossRef]
- Sawh, M.C.; Wallace, M.; Shapiro, E.; Goyal, N.; Newton, K.P.; Lu, E.L.; Bross, C.; Durelle, J.; Knott, C.; Gangoiti, G.A.; et al. Dairy fat intake, plasma pentadecanoic acid, and plasma iso-heptadecanoic acid are inversely associated with liver fat in children. J. Pediatr. Gastroenterol. Nutr. 2021, 72, e90–e96. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.S.; Sharp, S.J.; Imamura, F.; Koulman, A.; Schulze, M.B.; Ye, Z.; Griffin, J.; Guevara, M.; Huerta, J.M.; Kroger, J.; et al. Association between plasma phospholipid saturated fatty acids and metabolic markers of lipid, hepatic, inflammation and glycaemic pathways in eight European countries: A cross-sectional analysis in the EPIC-Interact study. BMC Med. 2017, 15, 203. [Google Scholar] [CrossRef]
- Lu, Y.; Li, D.; Wang, L.; Zhang, H.; Jiang, F.; Zhang, R.; Xu, L.; Yang, N.; Dai, S.; Xu, X.; et al. Comprehensive investigation on associations between dietary intake and blood levels of fatty acids and colorectal cancer risk. Nutrients 2023, 15, 730. [Google Scholar] [CrossRef]
- Kruchinina, M.; Gromov, A.; Prudnikova, Y.; Shashkov, M.; Sokolova, A.; Kruchinin, V. Erythrocyte membrane fatty acids as the potential biomarkers for detection of early-stage and progression of colorectal cancer. Ann. Oncol. 2018, 29 (Suppl. 5), v52. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, L.T.; Hou, S.H.; Chen, L.N.; Zhang, C.X. Association between dietary intake of saturated fatty acid subgroups and breast cancer risk. Food Funct. 2024, 19, 2282–2294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Liang, Y.; Zhen, X.; Wang, H.; Song, L.; Xing, D.; Li, H. Analysis of serum exosome metabolites identifies potential biomarkers for human hepatocellular carcinoma. Metabolites 2024, 14, 462. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.H.; Kim, M.; Kim, M.; Kang, M.; Seo, Y.W.; Jung, K.J.; Lee, S.J.; Hong, S.; Lee, J.H. Clinical relevance of glycerophospholipid, sphingomyelin and glutathione metabolism in the pathogenesis of pharyngolaryngeal cancer in smokers: The Korean Cancer Prevention Study—II. Metabolomics 2016, 12, 164. [Google Scholar] [CrossRef]
- Teng, C.; Ren, R.; Liu, Z.; Wang, J.; Shi, S.; Kang, Y.E.; Koo, B.S.; Lu, W.; Shan, Y. C15:0 and C17:0 partially mediate the association of milk & dairy products with bladder cancer risk. J. Dairy. Sci. 2024, 107, 2586–2605. [Google Scholar]
- Venn-Watson, S.K.; Butterworth, C.N. Broader and safer clinically-relevant activities of pentadecanoic acid compared to omega-3: Evaluation of an emerging essential fatty acid across twelve primary human cell-based disease systems. PLoS ONE 2022, 17, e0268778. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Chen, Y.; Wang, K.; Luan, Y. Design, synthesis and antitumor activity study of a gemcitabine prodrug conjugated with a HDAC6 inhibitor. Bioorg Med. Chem. Lett. 2022, 72, 128881. [Google Scholar] [CrossRef]
- Zheng, J.S.; Imamura, F.; Sharp, S.J.; Koulman, A.; Griffin, J.L.; Mulligan, A.A.; Luben, R.; Khaw, K.T.; Wareham, N.J.; Forouhi, N.G. Changes in plasma phospholipid fatty acid profiles over 13 years and correlates of change: European Prospective Investigation into Cancer and Nutrition-Norfolk Study. Am. J. Clin. Nutr. 2019, 109, 1527–1534. [Google Scholar] [CrossRef]
- Venn-Watson, S. The Cellular Stability Hypothesis: Evidence of ferroptosis and accelerated aging-associated diseases as newly identified nutritional pentadecanoic acid (C15:0) deficiency syndrome. Metabolites 2024, 14, 355. [Google Scholar] [CrossRef]
- Schmitz, R.; Jhavar, S.; Xiao, W.; Liu, X.; Powell, J.; Wright, G.W.; Chan, W.C.; Jaffe, E.S.; Gascoyne, R.D.; Campo, E.; et al. Recurrent oncogenic mutations in CCND3 in aggressive lymphomas. Blood 2011, 21, 435. [Google Scholar] [CrossRef]
- Wageman, C.R.; Cavedine, L.R.; Norman, V.; Robinson, N.; Lu, T.; McBain, V.; Murphy, J.; Stehle, K.; Barner, S.M.; Croff, A.M.; et al. Drug response metrics and pharmacological profiling using the OncoPanelTM cell-based profiling service. Cancer Res. 2019, 79, 4245. [Google Scholar] [CrossRef]
- The Human Protein Atlas. Non-Cancerous Cell Lines. Available online: https://www.proteinatlas.org/humanproteome/cell+line/non-cancerous (accessed on 28 August 2025).
- Arafeh, R.; Shibue, T.; Dempster, J.M.; Hahn, W.C.; Vazquez, F. The present and future of the Cancer Dependency Map. Nat. Rev. Cancer 2025, 25, 59–73. [Google Scholar] [CrossRef]
- Do, M.C.; Yoon, T.H.; Moon, J.Y.; Go, G.M.; Cho, S.K. Anticancer effects of the Melosira nummuloides extract on hepatocellular carcinoma cells through JAK2/STAT3 and MAPK pathway inhibition. Algal. Res. 2025, 86, 103949. [Google Scholar] [CrossRef]
- Isoda, Y.; Nishizawa, Y.; Yamaguchi, S.; Hirano, J.; Yamamoto, A.; Numata, M. Antitumor activity of lipids. J. Japan Oil Chem. Soc. 1993, 42, 923–928. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Faiq, T.; Abolhasani, S. Impact of G1 phase kinetics on the acquisition of stemness in cancer cells: The critical role of cyclin D. Mol. Biol. Rep. 2025, 52, 230. [Google Scholar] [CrossRef] [PubMed]
- DepMap Portal Website. Available online: https://depmap.org/portal/gene/CCND3?tab=overview (accessed on 30 August 2025).
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Saleban, M.; Harris, E.L.; Poulter, J.A. D-Type Cyclins in Development and Disease. Genes 2023, 14, 1445. [Google Scholar] [CrossRef]
- Hua, W.; Li, Y.; Yin, H.; Du, K.X.; Zhang, X.Y.; Wu, J.Z.; Liang, J.H.; Shen, H.R.; Gau, R.; Li, J.Y.; et al. Analysis of CCND3 mutations in diffuse large B-cell lymphoma. Ann. Hematol. 2024, 103, 5729–5739. [Google Scholar] [CrossRef]
- Ketzer, F.; Abdelrasoul, H.; Vogel, M.; Marienfeld, R.; Muschen, M.; Jumaa, H.; Wirth, T.; Ushmorov, A. CCND3 is indispensable for the maintenance of B-cell acute lymphoblastic leukemia. Oncogenesis 2022, 11, 1. [Google Scholar] [CrossRef]
- Kovac, M.; Ameline, B.; Ribi, S.; Kovacova, M.; Cross, W.; Barenboim, M.; Witt, O.; Bielack, S.; Krieg, A.; Hartmann, W.; et al. The early evolutionary landscape of osteosarcoma provides clues for targeted treatment strategies. J. Pathol. 2021, 254, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, B.; Michgehl, U.; Rohde, J.; Erdmann, T.; Berning, P.; Reutter, K.; Rohde, M.; Borkhardt, A.; Burmeister, T.; Dave, S.; et al. Clinical relevance of molecular characteristics in Burkitt lymphoma differs according to age. Nature Comm. 2022, 13, 3881. [Google Scholar] [CrossRef]
- Suehara, Y.; Kitada, R.; Kamio, S.; Ogura, K.; Iwata, S.; Kobayashi, E.; Kawai, A.; Khosaka, S. Analysis of cancer multigene panel testing for osteosarcoma in pediatric and adults using the center for cancer genomics and advanced therapeutics database in Japan. J. Orthop. Sci. 2025, 30, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Huang, R.; Lin, T.; Wang, Y.; Tu, J.; Zhang, L.; Wang, B.; Huang, J.; Zhao, Z.; Xie, X.; et al. Age-dependent molecular variations in osteosarcoma: Implications for precision oncology across pediatric, adolescent, and adult patients. Front. Oncol. 2024, 14, 1382276. [Google Scholar] [CrossRef]
- Ding, Z.Y.; Li, R.; Zhang, Q.J.; Wang, Y.; Jiang, Y.; Meng, Q.Y.; Xi, Q.L.; Wu, G.H. Prognostic role of cyclin D2/D3 in multiple human malignant neoplasms: A systematic review and meta-analysis. Cancer Med. 2019, 8, 2717–2729. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Heo, S.; Shin, J.J.; Ji, E.; Tak, H.; Ahn, S.; Lee, K.L.; Lee, E.K.; Kim, W. A miR-194/PTBP1/CCND3 axis regulates tumor growth in human hepatocellular carcinoma. J. Pathol. 2019, 249, 395–408. [Google Scholar] [CrossRef]
- Han, L.P.; Fu, T.; Lin, Y.; Miao, J.L.; Jiang, Q.F. MicroRNA-138 negatively regulates non-small cell lung cancer cells through the interactions with cyclin D3. Tumor Biol. 2016, 37, 291–298. [Google Scholar] [CrossRef]
- Tanamia, H.; Tsuda, H.; Okabe, S.; Iwai, T.; Sugihara, K.; Imoto, I.; Inazawa, J. Involvement of cyclin D2 in liver metastasis of colorectal cancer, revealed by genome-wide copy-number analysis. Lab. Invest. 2005, 85, 1118–1129. [Google Scholar] [CrossRef]
- Buschges, R.; Weber, R.G.; Actor, B.; Lichter, P.; Collins, P.; Reifenberger, G. Amplification of expression of cyclin D genes (CCND1 CCND2 and CCND3) in human malignant gliomas. Brain Pathol. 1999, 9, 435–442. [Google Scholar] [CrossRef]
- Ren, Q.; Ma, Y.; Wang, R.; Niu, T. Triacylglycerol composition of butterfat fractions determines its gastrointestinal fate and postprandial effects: Lipidomic analysis of tri-, di-, and mono-acylglycerols and free fatty acids. J. Ag. Food Chem. 2021, 69, 11033–11042. [Google Scholar]
- Hori, A.; Ishida, F.; Nakazawa, H.; Yamaura, M.; Morita, S.; Uehara, T.; Honda, T.; Hidaka, H. Serum sphingomyelin species profile is altered in hematologic malignancies. Clin. Chim. Acta 2021, 514, 29–33. [Google Scholar] [CrossRef] [PubMed]
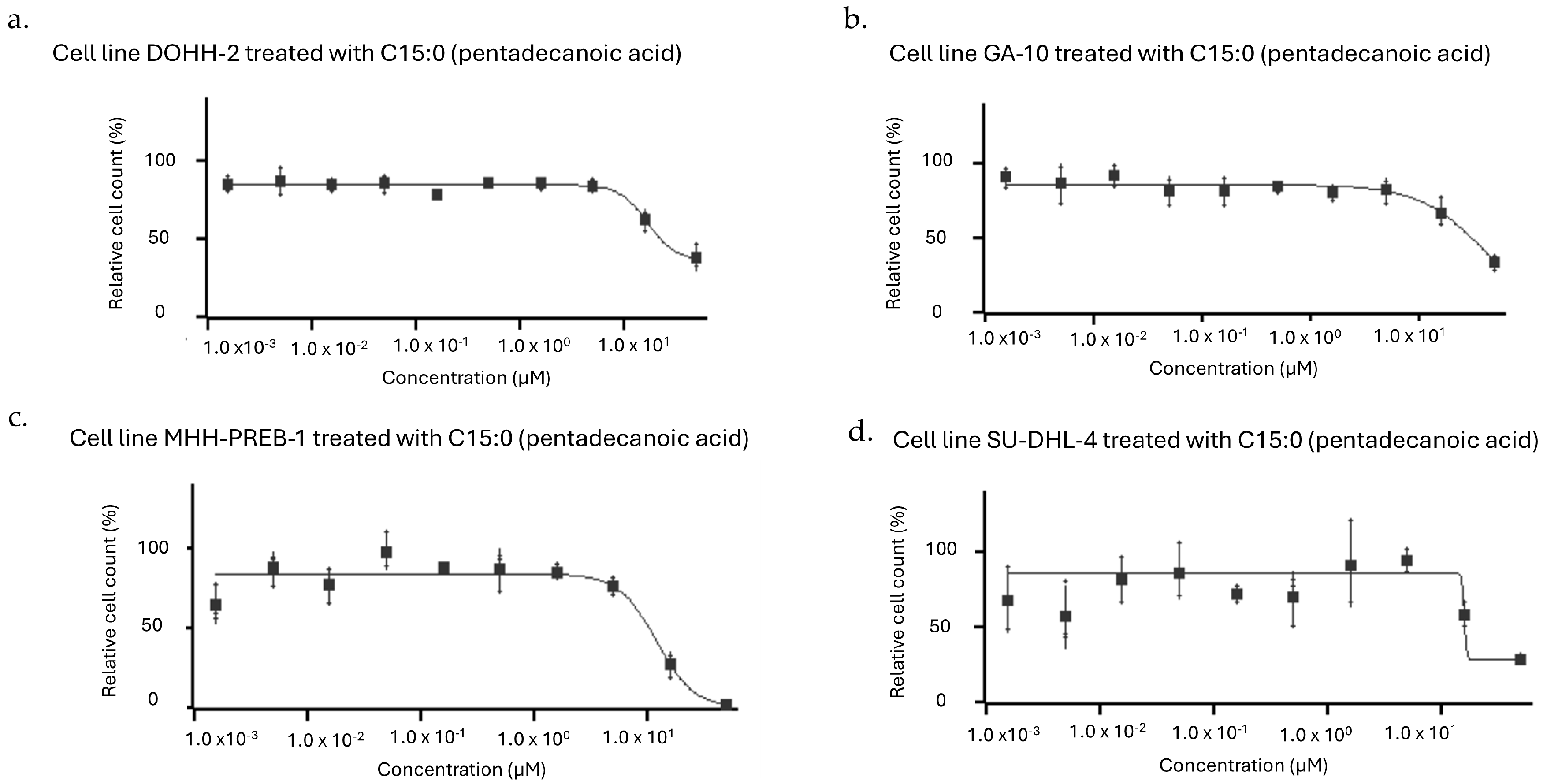


| Human Cancer Cell Line | Cancer Type | Dose-Dependent Antiproliferative Effect of C15:0 (1.5–50 µM) | ||
|---|---|---|---|---|
| Cell Count EC50 (µM) | Cell Count IC50 (µM) | Cell Count GI50 (µM) | ||
| Daudi | Lymphoma (Burkitt Lymphoma, Mature B-Cell Neoplasm) | 34 | >50 | >50 |
| DOHH-2 | Lymphoma (Diffuse Large B-Cell Lymphoma, NOS, Mature B-Cell Neoplasm) | 17 | 31 | 25 |
| GA-10 | Lymphoma (Burkitt Lymphoma, Mature B-Cell Neoplasm) | 38 | 38 | 31 |
| HLE | Hepatocellular Carcinoma | 48 | >50 | >50 |
| HLF | Hepatocellular Carcinoma | 6.2 | >50 | >50 |
| JeKo-1 | Lymphoma (Mantle Cell Lymphoma, Mature B-Cell Neoplasm) | 47 | >50 | >50 |
| MHH-PREB-1 | Lymphoma (Non-Hodgkin Lymphoma) | 12 | 12 | 11 |
| NAMALWA | Lymphoma (Burkitt Lymphoma, Mature B-Cell Neoplasm) | 18 | >50 | >50 |
| SHP-77 | Lung Cancer (Small Cell Lung Cancer Lung Neuroendocrine Tumor) | 17 | >50 | >50 |
| SU-DHL-4 | Lymphoma (Diffuse Large B-Cell Lymphoma, NOS, Mature B-Cell Neoplasm) | 16 | 16 | 16 |
| SU-DHL-10 | Lymphoma (Diffuse Large B-Cell Lymphoma, NOS, Mature B-Cell Neoplasm) | 18 | >50 | >50 |
| T47D | Breast Cancer (Breast Invasive Carcinoma) | 42 | >50 | >50 |
| ZR-75-1 | Breast Cancer (Breast Invasive Carcinoma) | 39 | >50 | >50 |
| Cell Line | C15:0 Inhibition Activities (EC, IC and GI50 ≤ 50 µM) | General Cancer Type | TP53 Loss of Function | CCND3 Gain of Function |
|---|---|---|---|---|
| DOHH-2 | Yes | Lymphoma | No | Yes |
| GA-10 | Yes | Yes | ||
| MHH-PREB-1 | Yes | Yes | ||
| SU-DHL-4 | Yes | No | ||
| SU-DHL-10 | No | Lymphoma | No | Yes |
| Daudi | Lymphoma | Yes | No | |
| HT | Lymphoma | Yes | No | |
| CALU6 | Lung | No | No | |
| Mia PaCa-2 | Pancreatic | Yes | No | |
| BxPC-3 | Pancreatic | Yes | No | |
| HuCCT1 | Liver | Yes | No | |
| HLE | Liver | Yes | No | |
| MC116 | Lymphoma | Yes | No | |
| NAMALWA | Lymphoma | Yes | No | |
| T47D | Breast | Yes | No | |
| ZR-75-1 | Breast | No | No | |
| SHP-77 | Lung | Yes | No | |
| HLF | Liver | Yes | No |
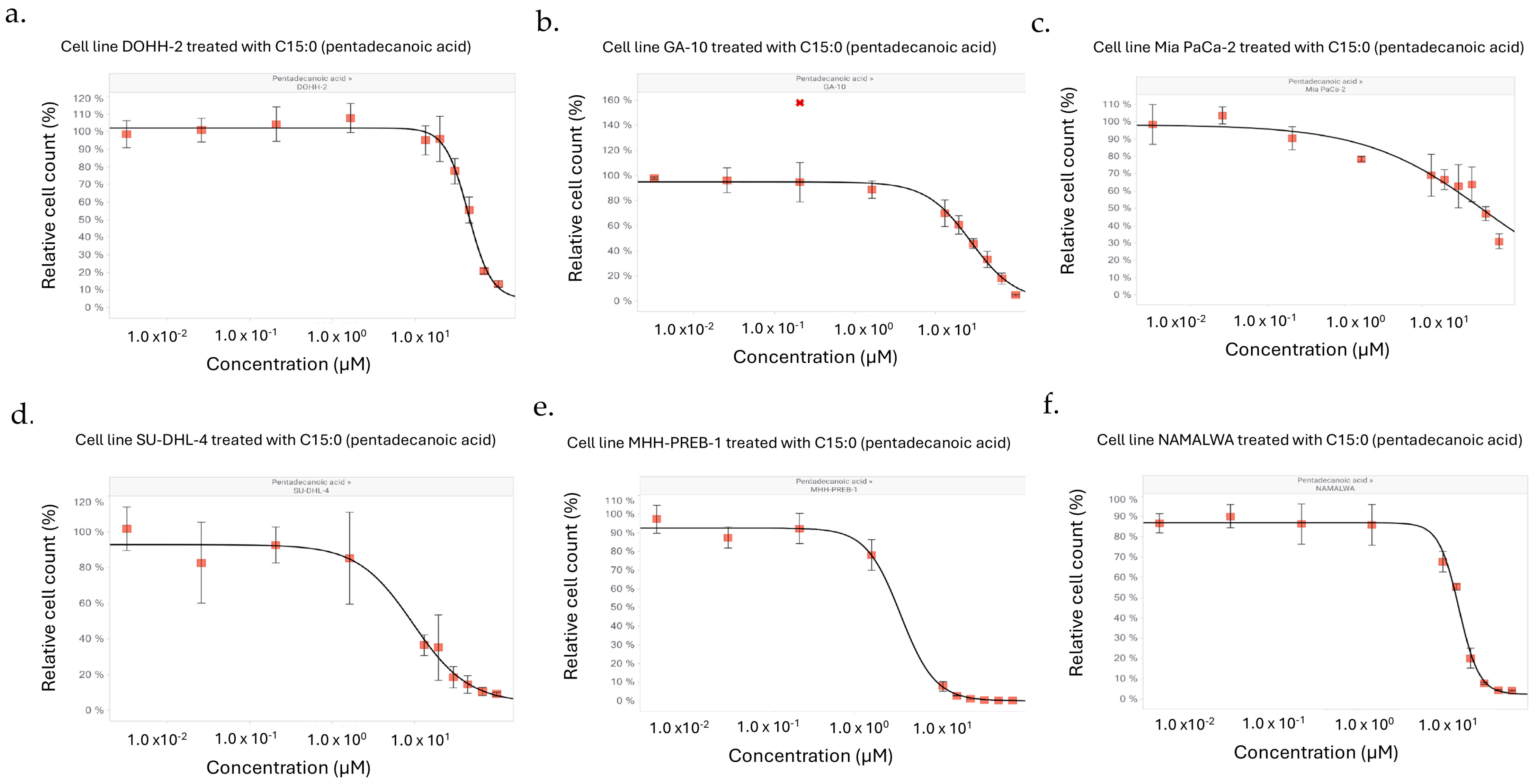
| Human Cancer Cell Line | Cancer Type | Dose-Dependent Antiproliferative Effect of C15:0 (3.2 nM–100 µM) | ||
|---|---|---|---|---|
| Cell Count EC50 (µM) | Cell Count IC50 (µM) | Cell Count GI50 (µM) | ||
| DOHH-2 | Lymphoma (Diffuse Large B-Cell Lymphoma, NOS, Mature B-Cell Neoplasm) | 44 | 45 | 38 |
| GA-10 | Lymphoma (Burkitt Lymphoma, Mature B-Cell Neoplasm) | 28 | 28 | 24 |
| MHH-PREB-1 | Lymphoma (Non-Hodgkin Lymphoma) | 0.4 | 0.4 | 0.4 |
| NAMALWA | Lymphoma (Burkitt Lymphoma, Mature B-Cell Neoplasm) | 21 | 22 | 20 |
| SU-DHL-4 | Lymphoma (Diffuse Large B-Cell Lymphoma, NOS, Mature B-Cell Neoplasm) | 9.7 | 10.4 | 6.0 |
| Mia PaCa-2 | Pancreatic (Pancreatic Adenocarcinoma) | 62 | 63 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venn-Watson, S. Pentadecanoic Acid (C15:0) at Naturally Occurring Circulating Concentrations Has Selective Anticancer Activities Including Targeting B-Cell Lymphomas with CCND3 Oncogenic Alterations. Nutrients 2025, 17, 3082. https://doi.org/10.3390/nu17193082
Venn-Watson S. Pentadecanoic Acid (C15:0) at Naturally Occurring Circulating Concentrations Has Selective Anticancer Activities Including Targeting B-Cell Lymphomas with CCND3 Oncogenic Alterations. Nutrients. 2025; 17(19):3082. https://doi.org/10.3390/nu17193082
Chicago/Turabian StyleVenn-Watson, Stephanie. 2025. "Pentadecanoic Acid (C15:0) at Naturally Occurring Circulating Concentrations Has Selective Anticancer Activities Including Targeting B-Cell Lymphomas with CCND3 Oncogenic Alterations" Nutrients 17, no. 19: 3082. https://doi.org/10.3390/nu17193082
APA StyleVenn-Watson, S. (2025). Pentadecanoic Acid (C15:0) at Naturally Occurring Circulating Concentrations Has Selective Anticancer Activities Including Targeting B-Cell Lymphomas with CCND3 Oncogenic Alterations. Nutrients, 17(19), 3082. https://doi.org/10.3390/nu17193082






