Effects of Freeze-Dried Sake Lees and Rice Koji Extract on Osteoporosis in a Postmenopausal Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sake Lees Extract and Rice Koji Extract
2.2. Cell Cultures
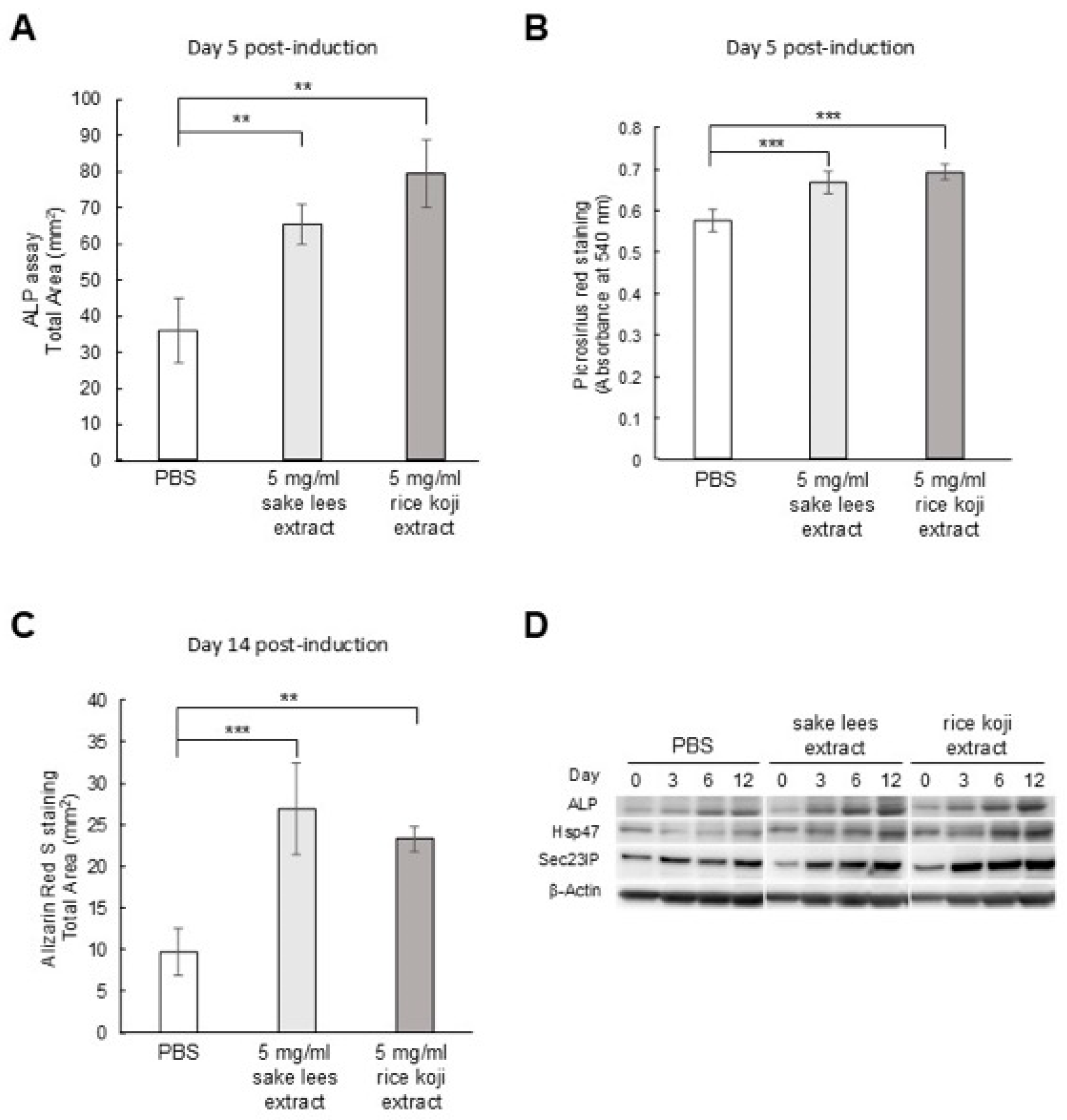
2.3. Alkaline Phosphatase (ALP) Staining Assay
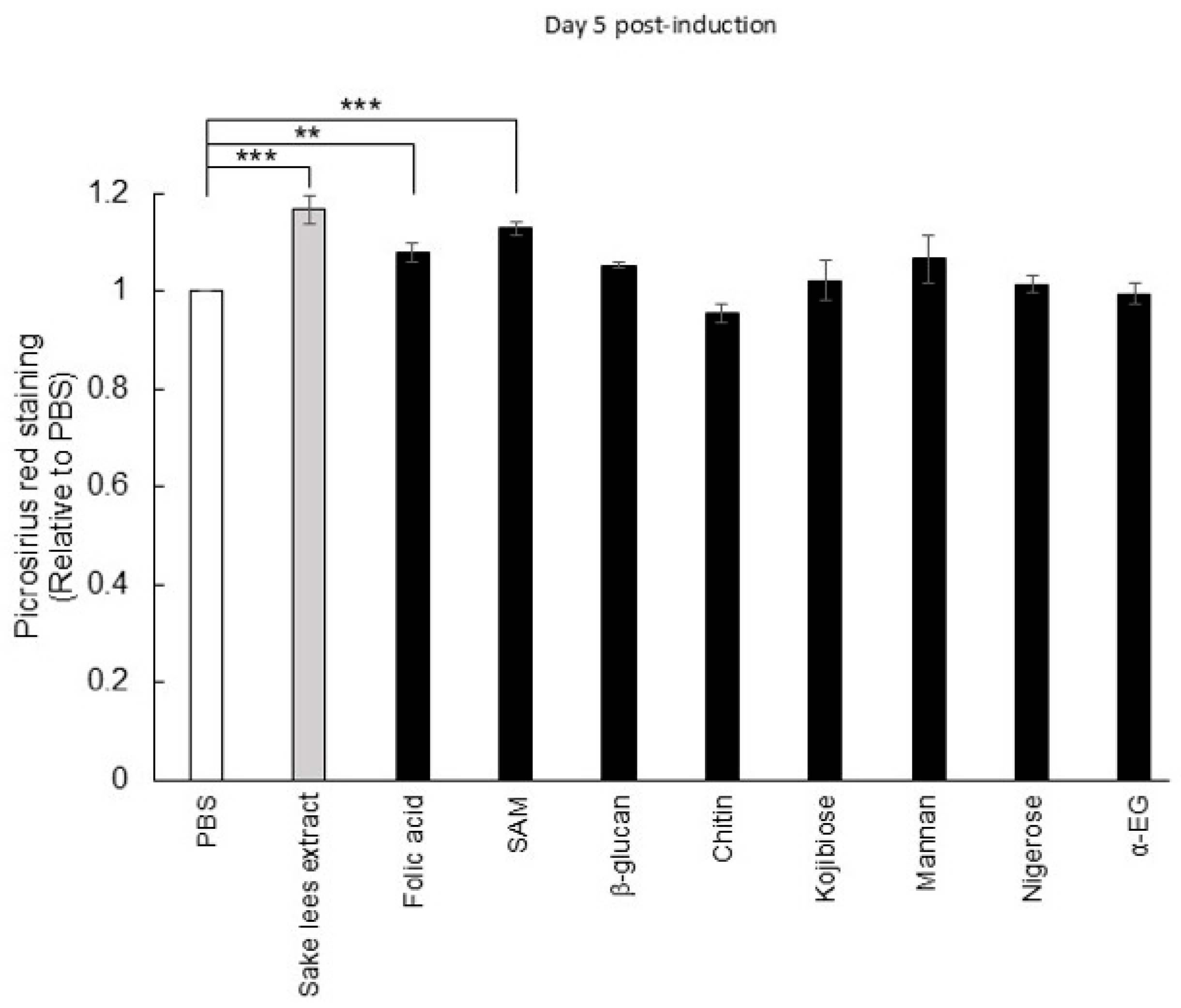
2.4. Picro-Sirius Red Staining
2.5. Alizarin Red S Staining
2.6. Western Blot Analysis
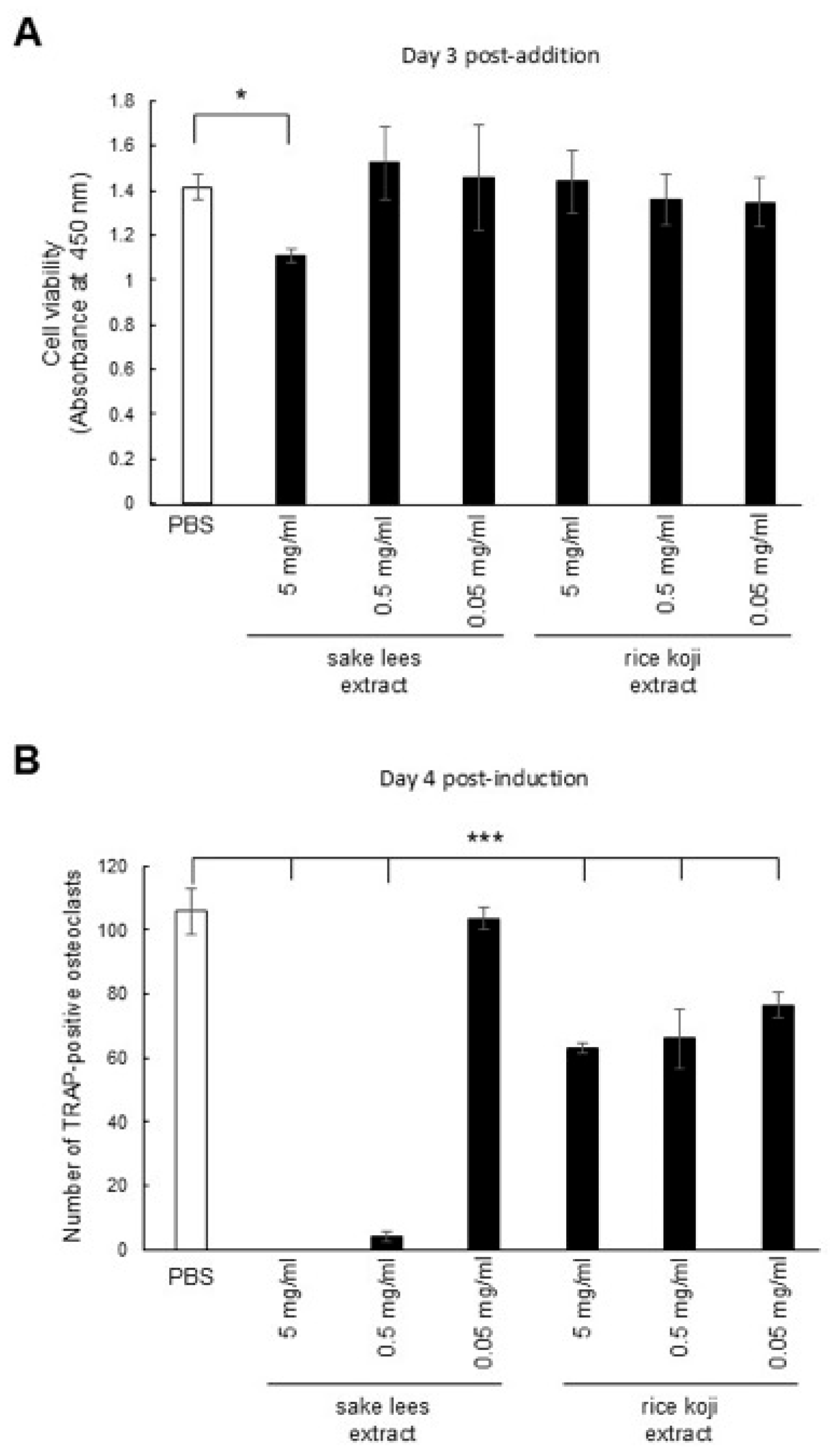
2.7. Tartrate-Resistant Acid Phosphatase (TRAP) Staining and Cell Viability Assay for Osteoclastic Cells
2.8. Animals and Experimental Design
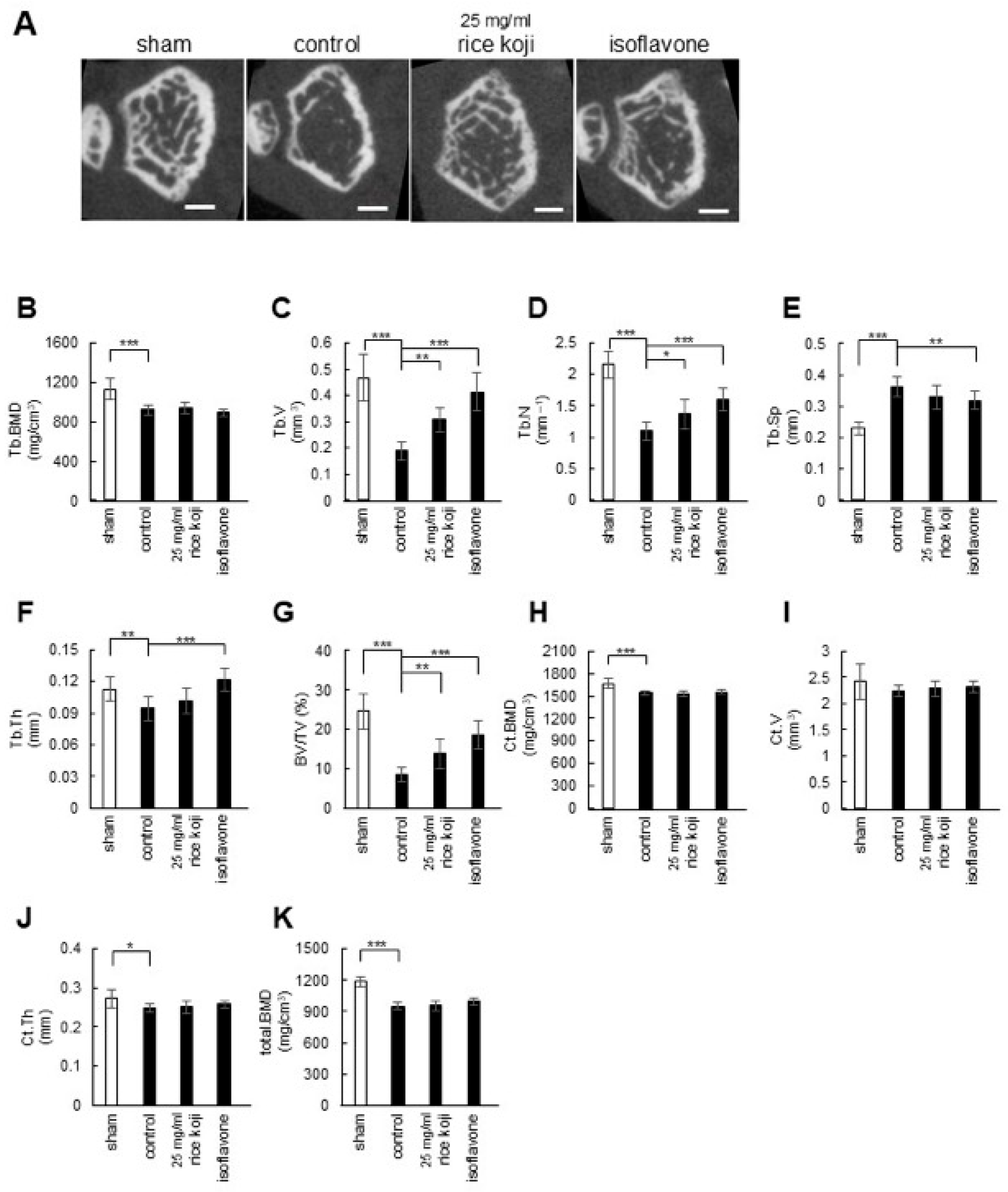
2.9. Micro-CT Analysis
2.10. Data Analysis
3. Results
3.1. Sake Lees Extract and Rice Koji Extract Promote Osteoblastic Differentiation of MC3T3-E1 Cells
3.2. Sake Lees Extract and Rice Koji Extract Inhibit Osteoclastic Differentiation of RAW264.7 Cells
3.3. Intake of Freeze-Dried Sake Lees or Rice Koji Extract Maintains Bone Volume in OVX Mice
3.4. Investigation of Putative Active Substances in Sake Lees Extract and Rice Koji Extract on Osteoblastic Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Florencio-Silva, R.; Sasso, G.R.D.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Matsuo, K.; Irie, N. Osteoclast-osteoblast communication. Arch. Biochem. Biophys. 2008, 473, 201–209. [Google Scholar] [CrossRef]
- Bolamperti, S.; Villa, I.; Rubinacci, A. Bone remodeling: An operational process ensuring survival and bone mechanical competence. Bone Res. 2022, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Coudert, A.E.; de Vernejoul, M.C.; Muraca, M.; Del Fattore, A. Osteopetrosis and its relevance for the discovery of new functions associated with the skeleton. Int. J. Endocrinol. 2015, 2015, 372156. [Google Scholar] [CrossRef]
- Amin, U.; McPartland, A.; O’Sullivan, M.; Silke, C. An overview of the management of osteoporosis in the aging female population. Womens Health (Lond). 2023, 19, 17455057231176655. [Google Scholar] [CrossRef]
- Kim, B.; Cho, Y.J.; Lim, W. Osteoporosis therapies and their mechanisms of action (Review). Exp. Ther. Med. 2021, 22, 1379. [Google Scholar] [CrossRef]
- Pouresmaeili, F.; Kamalidehghan, B.; Kamarehei, M.; Goh, Y.M. A comprehensive overview on osteoporosis and its risk factors. Ther. Clin. Risk Manag. 2018, 14, 2029–2049. [Google Scholar] [CrossRef]
- Kostecka, M. The role of healthy diet in the prevention of osteoporosis in perimenopausal period. Pak. J. Med. Sci. 2014, 30, 763–768. [Google Scholar] [CrossRef]
- Ong, A.M.; Kang, K.; Weiler, H.A.; Morin, S.N. Fermented Milk Products and Bone Health in Postmenopausal Women: A Systematic Review of Randomized Controlled Trials, Prospective Cohorts, and Case-Control Studies. Adv. Nutr. 2020, 11, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Iki, M.; Morita, A.; Kajita, E.; Kagamimori, S.; Kagawa, Y.; Yoneshima, H. Intake of fermented soybeans, natto, is associated with reduced bone loss in postmenopausal women: Japanese Population-Based Osteoporosis (JPOS) Study. J. Nutr. 2006, 136, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Ikehara, S.; Kamiya, K.; Kajita, E.; Sato, Y.; Kouda, K.; Tamaki, J.; Kagamimori, S.; Iki, M. Natto Intake is Inversely Associated with Osteoporotic Fracture Risk in Postmenopausal Japanese Women. J. Nutr. 2020, 150, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Izu, H.; Yamashita, S.; Arima, H.; Fujii, T. Nutritional characterization of sake cake (sake-kasu) after heat-drying and freeze-drying. Biosci. Biotechnol. Biochem. 2019, 83, 1477–1483. [Google Scholar] [CrossRef]
- Shibata, Y.; Takahashi, T.; Morimoto, T.; Kanai, M.; Fujii, T.; Akao, T.; Goshima, T.; Yamada, T. Quantitative stability of the folates highly accumulated in a non-Kyokai sake yeast. J. Gen. Appl. Microbiol. 2021, 67, 214–219. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- van Meurs, J.B.J.; Dhonukshe-Rutten, R.A.M.; Pluijm, S.M.F.; van der Klift, M.; de Jonge, R.; Lindemans, J.; de Groot, L.C.P.G.M.; Hofman, A.; Witteman, J.C.M.; van Leeuwen, J.P.T.M.; et al. Homocysteine Levels and the Risk of Osteoporotic Fracture. N. Engl. J. Med. 2004, 350, 2033–2041. [Google Scholar] [CrossRef]
- Zheng, Z.; Luo, H.; Xu, W.; Xue, Q. Association between dietary folate intake and bone mineral density in a diverse population: A cross-sectional study. J. Orthop. Surg. Res. 2023, 18, 684. [Google Scholar] [CrossRef]
- Saito, Y.; Wanezaki, K.; Kawato, A.; Imayasu, S. Antihypertensive effects of peptide in sake and its by-products on spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 1994, 58, 812–816. [Google Scholar] [CrossRef]
- Kawamoto, S.; Kaneoke, M.; Ohkouchi, K.; Amano, Y.; Takaoka, Y.; Kume, K.; Aki, T.; Yamashita, S.; Watanabe, K.; Kadowaki, M.; et al. Sake lees fermented with lactic acid bacteria prevents allergic rhinitis-like symptoms and IgE-mediated basophil degranulation. Biosci. Biotechnol. Biochem. 2011, 75, 140–144. [Google Scholar] [CrossRef]
- Kubo, H.; Hoshi, M.; Matsumoto, T.; Irie, M.; Oura, S.; Tsutsumi, H.; Hata, Y.; Yamamoto, Y.; Saito, K. Sake lees extract improves hepatic lipid accumulation in high fat diet-fed mice. Lipids Health Dis. 2017, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Nakatani, Y.; Kakihara, Y.; Taiyoji, M.; Saeki, M.; Takagi, R.; Yamamura, K.; Okamoto, K. Daily administration of Sake Lees (Sake Kasu) reduced psychophysical stress-induced hyperalgesia and Fos responses in the lumbar spinal dorsal horn evoked by noxious stimulation to the hindpaw in the rats. Biosci. Biotechnol. Biochem. 2020, 84, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Kakihara, Y.; Ohkura, N.; Tohma, A.; Washio, A.; Kitamura, C.; Noiri, Y.; Yamamura, K.; Saeki, M. Effects of rice fermented extracts, “Sake Lees”, on the functional activity of odontoblast-like cells (KN-3 cells). Odontology 2022, 110, 254–261. [Google Scholar] [CrossRef]
- Kurahashi, A.; Enomoto, T.; Oguro, Y.; Kojima-Nakamura, A.; Kodaira, K.; Watanabe, K.; Ozaki, N.; Goto, H.; Hirayama, M. Intake of Koji Amazake Improves Defecation Frequency in Healthy Adults. J. Fungi 2021, 7, 782. [Google Scholar] [CrossRef]
- Enomoto, T.; Kojima-Nakamura, A.; Kodaira, K.; Oguro, Y.; Kurahashi, A. Koji amazake Maintains Water Content in the Left Cheek Skin of Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Comparative Trial. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1283–1291. [Google Scholar] [CrossRef]
- Piriyaprasath, K.; Kakihara, Y.; Kurahashi, A.; Taiyoji, M.; Kodaira, K.; Aihara, K.; Hasegawa, M.; Yamamura, K.; Okamoto, K. Preventive Roles of Rice-koji Extracts and Ergothioneine on Anxiety- and Pain-like Responses under Psychophysical Stress Conditions in Male Mice. Nutrients 2023, 15, 3989. [Google Scholar] [CrossRef]
- Hamajima, H.; Tanaka, M.; Miyagawa, M.; Sakamoto, M.; Nakamura, T.; Yanagita, T.; Nishimukai, M.; Mitsutake, S.; Nakayama, J.; Nagao, K.; et al. Koji glycosylceramide commonly contained in Japanese traditional fermented foods alters cholesterol metabolism in obese mice. Biosci. Biotechnol. Biochem. 2019, 83, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthi, I.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Vet. Clin. Pathol. 2012, 41, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C.J. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Ito, S.; Nagata, K. Quality Control of Procollagen in Cells. Annu. Rev. Biochem. 2021, 90, 631–658. [Google Scholar] [CrossRef]
- Du, Y.; Fan, X.; Song, C.; Chang, W.; Xiong, J.; Deng, L.; Ji, W.K. Sec23IP recruits VPS13B/COH1 to ER exit site-Golgi interface for tubular ERGIC formation. J. Cell Biol. 2024, 223, e202402083. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Pahuja, K.B.; Ravazzola, M.; Yoon, J.; Boyadjiev, S.A.; Hammamoto, S.; Schekman, R.; Orci, L.; Kim, J. The SEC23-SEC31 interface plays critical role for export of procollagen from the endoplasmic reticulum. J. Biol. Chem. 2012, 287, 10134–10144. [Google Scholar] [CrossRef]
- Batoon, L.; Millard, S.M.; Raggatt, L.J.; Wu, A.C.; Kaur, S.; Sun, L.W.H.; Williams, K.; Sandrock, C.; Ng, P.Y.; Irvine, K.M.; et al. Osteal macrophages support osteoclast-mediated resorption and contribute to bone pathology in a postmenopausal osteoporosis mouse model. J. Bone Miner. Res. 2021, 36, 2214–2228. [Google Scholar] [CrossRef]
- Oguro, Y.; Nishiwaki, T.; Shinada, R.; Kobayashi, K.; Kurahashi, A. Metabolite profile of koji amazake and its lactic acid fermentation product by Lactobacillus sakei UONUMA. J. Biosci. Bioeng. 2017, 124, 178–183. [Google Scholar] [CrossRef]
- Kurahashi, A. Ingredients, Functionality, and Safety of the Japanese Traditional Sweet Drink Amazake. J. Fungi 2021, 7, 469. [Google Scholar] [CrossRef]
- Shibata, Y.; Yamada, T.; Morimoto, T.; Fujii, T.; Akao, T.; Goshima, T.; Takahashi, T.; Tanaka, N. Mechanism of high folate accumulation in a sake yeast other than Kyokai yeasts. J. Biosci. Bioeng. 2020, 129, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nishidono, Y.; Misaka, S.; Maejima, Y.; Shimomura, K.; Tanaka, K. Comparative analysis of functional components in Sakekasu (Sake lees). Funct. Foods Health Dis. 2024, 14, 74–86. [Google Scholar] [CrossRef]
- Kanai, M.; Mizunuma, M.; Fujii, T.; Iefuji, H. Importance and mechanisms of S-adenosylmethionine and folate accumulation in sake yeast. FEMS Yeast Res. 2023, 23, foad004. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.P.; Cates, G.A.; Ball, E.H.; Sanwal, B.D. A collagen-binding protein in the endoplasmic reticulum of myoblasts exhibits relationship with serine protease inhibitors. J. Biol. Chem. 1991, 266, 17230–17235. [Google Scholar] [CrossRef]
- Tani, K.; Mizoguchi, T.; Iwamatsu, A.; Hatsuzawa, K.; Tagaya, M. p125 Is a Novel Mammalian Sec23p-interacting Protein with Structural Similarity to Phospholipid-modifying Proteins. J. Biol. Chem. 1999, 274, 20505–20512. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Osterhoff, G.; Morgan, E.F.; Shefelbine, S.J.; Karim, L.; McNamara, L.M.; Augat, P. Bone mechanical properties and changes with osteoporosis. Injury 2016, 47 (Suppl. 2), S11–S20. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, X.; Fujita, H.; Onozuka, M.; Kubo, K.Y. Age-related changes in trabecular and cortical bone microstructure. Int. J. Endocrinol. 2013, 2013, 213234. [Google Scholar] [CrossRef]
- Klinck, J.; Boyd, S.K. The magnitude and rate of bone loss in ovariectomized mice differs among inbred strains as determined by longitudinal in vivo micro-computed tomography. Calcif. Tissue Int. 2008, 83, 70–79. [Google Scholar] [CrossRef]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cantley, L.C. Toward a better understanding of folate metabolism in health and disease. J. Exp. Med. 2019, 216, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Hu, Y.; Wang, S.C.; Li, Z.H.; Cai, G.Q.; Shen, H.; Sheng, S.H.; Chen, X.; Weng, W.Z.; Zhang, W.C.; et al. Linking the relationship between dietary folic acid intake and risk of osteoporosis among middle-aged and older people: A nationwide population-based study. Food Sci. Nutr. 2024, 12, 4110–4121. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Tu, B.P. Mechanisms and rationales of SAM homeostasis. Trends Biochem. Sci. 2025, 50, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Vaes, B.L.; Lute, C.; van der Woning, S.P.; Piek, E.; Vermeer, J.; Blom, H.J.; Mathers, J.C.; Muller, M.; de Groot, L.C.; Steegenga, W.T. Inhibition of methylation decreases osteoblast differentiation via a non-DNA-dependent methylation mechanism. Bone 2010, 46, 514–523. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, Y.; Hao, L.; Zhao, Y.; Zhang, L.; Ding, W.; Qi, Y.; Xu, Q. One-carbon metabolism supports s-adenosylmethionine and m6A methylation to control the osteogenesis of BMSCs and bone formation. J. Bone Miner. Res. 2024, 39, 1356–1370. [Google Scholar] [CrossRef]
- Izu, H.; Shobayashi, M.; Manabe, Y.; Goto, K.; Iefuji, H. Sake yeast suppresses acute alcohol-induced liver injury in mice. Biosci. Biotechnol. Biochem. 2006, 70, 2488–2493. [Google Scholar] [CrossRef]

| Component | AIN-93G | 20% Freeze-Dried Sake Lees | 40% Freeze-Dried Sake Lees |
|---|---|---|---|
| Protein (g) | 21.5 | 18.9 | 16.3 |
| Lipid (g) | 6.1 | 5.4 | 4.7 |
| Carbohydrate (g) | 54.6 | 51.6 | 48.6 |
| Energy (kcal) | 359.8 | 340.8 | 321.9 |
| Component | 25 mg/mL Rice Koji Extract |
|---|---|
| Protein (mg) | 20 |
| Lipid (mg) | 5 |
| Carbohydrate (mg) | 785 |
| Energy (kcal) | 3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáez-Chandía, J.; Castillo-Quispe, S.; Okamoto, K.; Kurahashi, A.; Kodaira, K.; Aihara, K.; Suzuki-Barrera, K.; Kaku, M.; Mikami, Y.; Terunuma, M.; et al. Effects of Freeze-Dried Sake Lees and Rice Koji Extract on Osteoporosis in a Postmenopausal Mouse Model. Nutrients 2025, 17, 3077. https://doi.org/10.3390/nu17193077
Sáez-Chandía J, Castillo-Quispe S, Okamoto K, Kurahashi A, Kodaira K, Aihara K, Suzuki-Barrera K, Kaku M, Mikami Y, Terunuma M, et al. Effects of Freeze-Dried Sake Lees and Rice Koji Extract on Osteoporosis in a Postmenopausal Mouse Model. Nutrients. 2025; 17(19):3077. https://doi.org/10.3390/nu17193077
Chicago/Turabian StyleSáez-Chandía, Jorge, Stephanny Castillo-Quispe, Keiichiro Okamoto, Atsushi Kurahashi, Kazuya Kodaira, Kotaro Aihara, Kiyoko Suzuki-Barrera, Masaru Kaku, Yoshikazu Mikami, Miho Terunuma, and et al. 2025. "Effects of Freeze-Dried Sake Lees and Rice Koji Extract on Osteoporosis in a Postmenopausal Mouse Model" Nutrients 17, no. 19: 3077. https://doi.org/10.3390/nu17193077
APA StyleSáez-Chandía, J., Castillo-Quispe, S., Okamoto, K., Kurahashi, A., Kodaira, K., Aihara, K., Suzuki-Barrera, K., Kaku, M., Mikami, Y., Terunuma, M., Yamamura, K., Hayashi, T., Saeki, M., & Kakihara, Y. (2025). Effects of Freeze-Dried Sake Lees and Rice Koji Extract on Osteoporosis in a Postmenopausal Mouse Model. Nutrients, 17(19), 3077. https://doi.org/10.3390/nu17193077






