Serum and Skin Carotenoid Levels in Older Adults with and Without Metabolic Syndrome: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Tests and Measurements
2.3. MetS Group Classification
2.4. Statistical Analyses
3. Results
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2014, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, K.-H.; Kim, H.-J.; Youk, H.; Lee, H.-Y. Effective Prevention and Management Tools for Metabolic Syndrome Based on Digital Health-Based Lifestyle Interventions Using Healthcare Devices. Diagnostics 2022, 12, 1730. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Sanchez, H.; Harhay, M.O.; Harhay, M.M.; McElligott, S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J. Am. Coll. Cardiol. 2013, 62, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef]
- Ju, S.Y.; Lee, J.Y.; Kim, D.H. Association of metabolic syndrome and its components with all-cause and cardiovascular mortality in the elderly: A meta-analysis of prospective cohort studies. Medicine 2017, 96, e8491. [Google Scholar] [CrossRef]
- Guembe, M.J.; Fernandez-Lazaro, C.I.; Sayon-Orea, C.; Toledo, E.; Moreno-Iribas, C. Risk for cardiovascular disease associated with metabolic syndrome and its components: A 13-year prospective study in the RIVANA cohort. Cardiovasc. Diabetol. 2020, 19, 195. [Google Scholar] [CrossRef]
- Boudreau, D.; Malone, D.; Raebel, M.; Fishman, P.; Nichols, G.; Feldstein, A.; Boscoe, A.; Ben-Joseph, R.; Magid, D.; Okamoto, L. Health care utilization and costs by metabolic syndrome risk factors. Metab. Syndr. Relat. Disord. 2009, 7, 305–314. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, Y.; Bhupathiraju, S.N.; Rosner, B.A.; Sun, Q.; Giovannucci, E.L.; Rimm, E.B.; Manson, J.E.; Willett, W.C.; Stampfer, M.J.; et al. Fruit and Vegetable Intake and Mortality: Results From 2 Prospective Cohort Studies of US Men and Women and a Meta-Analysis of 26 Cohort Studies. Circulation 2021, 143, 1642–1654. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef]
- van Staveren, W.A.; de Groot, L.C.; Blauw, Y.H.; Van der Wielen, R.P. Assessing diets of elderly people: Problems and approaches. Am. J. Clin. Nutr. 1994, 59 (Suppl. S1), 221S–223S. [Google Scholar] [CrossRef]
- Fukushima, Y.; Taguchi, C.; Kishimoto, Y.; Kondo, K. Japanese carotenoid database with alpha- and beta-carotene, beta-cryptoxanthin, lutein, zeaxanthin, lycopene, and fucoxanthin and intake in adult women. Int. J. Vitam. Nutr. Res. 2023, 93, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Madore, M.P.; Chun, O.K. Changes in Intake and Major Food Sources of Carotenoids among U.S. Adults between 2009–2018. Metabolites 2023, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Dragsted, L.O.; Gao, Q.; Scalbert, A.; Vergères, G.; Kolehmainen, M.; Manach, C.; Brennan, L.; Afman, L.A.; Wishart, D.S.; Lacueva, C.A.; et al. Validation of biomarkers of food intake—Critical assessment of candidate biomarkers. Genes. Nutr. 2018, 13, 14. [Google Scholar] [CrossRef]
- Burrows, T.L.; Rollo, M.E.; Williams, R.; Wood, L.G.; Garg, M.L.; Jensen, M.; Collins, C.E. A Systematic Review of Technology-Based Dietary Intake Assessment Validation Studies That Include Carotenoid Biomarkers. Nutrients 2017, 9, 140. [Google Scholar] [CrossRef]
- Pennant, M.; Steur, M.; Moore, C.; Butterworth, A.; Johnson, L. Comparative validity of vitamin C and carotenoids as indicators of fruit and vegetable intake: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 1331–1340. [Google Scholar] [CrossRef]
- Ermakov, I.V.; Ermakova, M.; Sharifzadeh, M.; Gorusupudi, A.; Farnsworth, K.; Bernstein, P.S.; Stookey, J.; Evans, J.; Arana, T.; Tao-Lew, L.; et al. Optical assessment of skin carotenoid status as a biomarker of vegetable and fruit intake. Arch. Biochem. Biophys. 2018, 646, 46–54. [Google Scholar] [CrossRef]
- Ermakov, I.V.; Gellermann, W. Dermal carotenoid measurements via pressure mediated reflection spectroscopy. J. Biophotonics 2012, 5, 559–570. [Google Scholar] [CrossRef]
- Radtke, M.D.; Poe, M.; Stookey, J.; Pitts, S.J.; Moran, N.E.; Landry, M.J.; Rubin, L.P.; Stage, V.C.; Scherr, R.E. Recommendations for the Use of the Veggie Meter® for Spectroscopy-Based Skin Carotenoid Measurements in the Research Setting. Curr. Dev. Nutr. 2021, 5, nzab104. [Google Scholar] [CrossRef]
- Ford, E.S.; Mokdad, A.H.; Giles, W.H.; Brown, D.W. The metabolic syndrome and antioxidant concentrations: Findings from the Third National Health and Nutrition Examination Survey. Diabetes 2003, 52, 2346–2352. [Google Scholar] [CrossRef] [PubMed]
- Coyne, T.; Ibiebele, T.I.; Baade, P.D.; McClintock, C.S.; Shaw, J.E. Metabolic syndrome and serum carotenoids: Findings of a cross-sectional study in Queensland, Australia. Br. J. Nutr. 2009, 102, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Chen, X.; Jha, K.; Beydoun, H.A.; Zonderman, A.B.; Canas, J.A. Carotenoids, vitamin A, and their association with the metabolic syndrome: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Shroff, M.R.; Chen, X.; Beydoun, H.A.; Wang, Y.; Zonderman, A.B. Serum antioxidant status is associated with metabolic syndrome among U.S. adults in recent national surveys1–3. J. Nutr. 2011, 141, 903–913. [Google Scholar] [CrossRef]
- Liu, J.; Shi, W.-Q.; Cao, Y.; He, L.-P.; Guan, K.; Ling, W.H.; Chen, Y.-M. Higher serum carotenoid concentrations associated with a lower prevalence of the metabolic syndrome in middle-aged and elderly Chinese adults. Br. J. Nutr. 2014, 112, 2041–2048. [Google Scholar] [CrossRef]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Yano, M. High serum carotenoids associated with lower risk for the metabolic syndrome and its components among Japanese subjects: Mikkabi cohort study. Br. J. Nutr. 2015, 114, 1674–1682. [Google Scholar] [CrossRef]
- May, K.; Pitts, S.J.; Stage, V.C.; Kelley, C.J.; Burkholder, S.; Fang, X.; Zeng, A.; Lazorick, S. Use of the Veggie Meter® as a tool to objectively approximate fruit and vegetable intake among youth for evaluation of preschool and school-based interventions. J. Hum. Nutr. Diet. 2020, 33, 869–875. [Google Scholar] [CrossRef]
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S.; Blevins, S.M. Step-monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: A randomized controlled trial. J. Am. Hear. Assoc. 2014, 3, e001107. [Google Scholar] [CrossRef]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39 (Suppl. S1), S1–S266. [Google Scholar]
- Aboyans, V.; Criqui, M.H.; Abraham, P.; Allison, M.A.; Creager, M.A.; Diehm, C.; Fowkes, F.G.R.; Hiatt, W.R.; Jönsson, B.; Lacroix, P.; et al. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation 2012, 126, 2890–2909. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.W.; Montgomery, P.S.; Casanegra, A.I.; Silva-Palacios, F.; Ungvari, Z.; Csiszar, A. Association between gait characteristics and endothelial oxidative stress and inflammation in patients with symptomatic peripheral artery disease. Age 2016, 38, 34. [Google Scholar] [CrossRef]
- Zhang, Q.; Yi, N.; Liu, S.; Zheng, H.; Qiao, X.; Xiong, Q.; Liu, X.; Zhang, S.; Wen, J.; Ye, H.; et al. Easier operation and similar power of 10 g monofilament test for screening diabetic peripheral neuropathy. J. Int. Med. Res. 2018, 46, 3278–3284. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Lavery, L.A.; Vela, S.A.; Quebedeaux, T.L.; Fleischli, J.G. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch. Intern. Med. 1998, 158, 289–292. [Google Scholar] [CrossRef]
- Oyer, D.S.; Saxon, D.; Shah, A. Quantitative assessment of diabetic peripheral neuropathy with use of the clanging tuning fork test. Endocr. Pract. 2007, 13, 5–10. [Google Scholar] [CrossRef]
- Pietrobelli, A.; Rubiano, F.; St-Onge, M.-P.; Heymsfield, S.B. New bioimpedance analysis system: Improved phenotyping with whole-body analysis. Eur. J. Clin. Nutr. 2004, 58, 1479–1484. [Google Scholar] [CrossRef]
- Sluyter, J.D.; Schaaf, D.; Scragg, R.K.; Plank, L.D. Prediction of fatness by standing 8-electrode bioimpedance: A multiethnic adolescent population. Obesity 2010, 18, 183–189. [Google Scholar] [CrossRef]
- Lee, B.-L.; New, A.-L.; Ong, C.-N. Simultaneous determination of tocotrienols, tocopherols, retinol, and major carotenoids in human plasma. Clin. Chem. 2003, 49, 2056–2066. [Google Scholar] [CrossRef]
- Kopec, R.E.; Schweiggert, R.M.; Riedl, K.M.; Carle, R.; Schwartz, S.J. Comparison of high-performance liquid chromatography/tandem mass spectrometry and high-performance liquid chromatography/photo-diode array detection for the quantitation of carotenoids, retinyl esters, α-tocopherol and phylloquinone in chylomicron-rich fractions of human plasma. Rapid Commun. Mass. Spectrom. 2013, 27, 1393–1402. [Google Scholar] [CrossRef]
- Colmán-Martínez, M.; Martínez-Huélamo, M.; Miralles, E.; Estruch, R.; Lamuela-Raventós, R.M.; Saso, L. A New Method to Simultaneously Quantify the Antioxidants: Carotenes, Xanthophylls, and Vitamin A in Human Plasma. Oxidative Med. Cell. Longev. 2015, 2016, 9268531. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Martínez-Huélamo, M.; Arranz-Martinez, S.; Miralles, E.; Lamuela-Raventós, R.M. Differences in the carotenoid content of ketchups and gazpachos through HPLC/ESI(Li+)-MS/MS correlated with their antioxidant capacity. J. Sci. Food Agric. 2012, 92, 2043–2049. [Google Scholar] [CrossRef]
- Hrvolová, B.; Martínez-Huélamo, M.; Colmán-Martínez, M.; Hurtado-Barroso, S.; Lamuela-Raventós, R.M.; Kalina, J. Development of an Advanced HPLC–MS/MS Method for the Determination of Carotenoids and Fat-Soluble Vitamins in Human Plasma. Int. J. Mol. Sci. 2016, 17, 1719. [Google Scholar] [CrossRef]
- Obana, A.; Gohto, Y.; Gellermann, W.; Ermakov, I.V.; Sasano, H.; Seto, T.; Bernstein, P.S. Skin Carotenoid Index in a large Japanese population sample. Sci. Rep. 2019, 9, 9318. [Google Scholar] [CrossRef]
- Godin, G.; Bélanger-Gravel, A.; Paradis, A.-M.; Vohl, M.-C.; Pérusse, L. A simple method to assess fruit and vegetable intake among obese and non-obese individuals. Can. J. Public Health 2008, 99, 494–498. [Google Scholar] [CrossRef]
- Vézina-Im, L.-A.; Godin, G.; Couillard, C.; Perron, J.; Lemieux, S.; Robitaille, J. Validity and reliability of a brief self-reported questionnaire assessing fruit and vegetable consumption among pregnant women. BMC Public Health 2016, 16, 982. [Google Scholar] [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Zimmet, P.; Magliano, D.; Matsuzawa, Y.; Alberti, G.; Shaw, J. The metabolic syndrome: A global public health problem and a new definition. J. Atheroscler. Thromb. 2005, 12, 295–300. [Google Scholar] [CrossRef]
- Burrows, T.L.; Hutchesson, M.J.; Rollo, M.E.; Boggess, M.M.; Guest, M.; Collins, C.E. Fruit and Vegetable Intake Assessed by Food Frequency Questionnaire and Plasma Carotenoids: A Validation Study in Adults. Nutrients 2015, 7, 3240–3251. [Google Scholar] [CrossRef]
- Pitts, S.B.J.; Jahns, L.; Wu, Q.; Moran, N.E.; Bell, R.A.; Truesdale, K.P.; Laska, M.N. A non-invasive assessment of skin carotenoid status through reflection spectroscopy is a feasible, reliable and potentially valid measure of fruit and vegetable consumption in a diverse community sample. Public Health Nutr. 2018, 21, 1664–1670. [Google Scholar] [CrossRef]
- Radtke, M.D.; Pitts, S.J.; Jahns, L.; Firnhaber, G.C.; Loofbourrow, B.M.; Zeng, A.; Scherr, R.E. Criterion-Related Validity of Spectroscopy-Based Skin Carotenoid Measurements as a Proxy for Fruit and Vegetable Intake: A Systematic Review. Adv. Nutr. 2020, 11, 1282–1299. [Google Scholar] [CrossRef]
- Jilcott Pitts, S.B.; Johnson, N.S.; Wu, Q.; Firnhaber, G.C.; Preet Kaur, A.; Obasohan, J. A meta-analysis of studies examining associations between resonance Raman spectroscopy-assessed skin carotenoids and plasma carotenoids among adults and children. Nutr. Rev. 2022, 80, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Pitts, S.J.; Moran, N.E.; Laska, M.N.; Wu, Q.; Harnack, L.; Moe, S.; Carr-Manthe, P.; Gates, E.; Chang, J.; Zaidi, Y.; et al. Reflection Spectroscopy-Assessed Skin Carotenoids Are Sensitive to Change in Carotenoid Intake in a 6-Week Randomized Controlled Feeding Trial in a Racially/Ethnically Diverse Sample. J. Nutr. 2023, 153, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Pitts, S.B.J.; Wu, Q.; Moran, N.E.; Laska, M.N.; Harnack, L. Examining Potential Modifiers of Human Skin and Plasma Carotenoid Responses in a Randomized Trial of a Carotenoid-Containing Juice Intervention. J. Nutr. 2023, 153, 3287–3294. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.D. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Harari, A.; Coster, A.C.F.; Jenkins, A.; Xu, A.; Greenfield, J.R.; Harats, D.; Shaish, A.; Samocha-Bonet, D. Obesity and Insulin Resistance Are Inversely Associated with Serum and Adipose Tissue Carotenoid Concentrations in Adults. J. Nutr. 2019, 150, 38–46. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Morgan, E.H.; Graham, M.L.; Marshall, G.A.; Hanson, K.L.; Seguin-Fowler, R.A. Serum carotenoids are strongly associated with dermal carotenoids but not self-reported fruit and vegetable intake among overweight and obese women. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 104. [Google Scholar] [CrossRef]
- Seguin-Fowler, R.A.; Hanson, K.L.; Marshall, G.A.; Belarmino, E.H.; Pitts, S.B.J.; Kolodinsky, J.; Sitaker, M.; Ammerman, A. Fruit and Vegetable Intake Assessed by Repeat 24 h Recalls, but Not by A Dietary Screener, Is Associated with Skin Carotenoid Measurements in Children. Nutrients 2021, 13, 980. [Google Scholar] [CrossRef]
- Di Noia, J.; Gellermann, W. Use of the Spectroscopy-Based Veggie Meter® to Objectively Assess Fruit and Vegetable Intake in Low-Income Adults. Nutrients 2021, 13, 2270. [Google Scholar] [CrossRef]
- Nagao-Sato, S.; Baltaci, A.; Reyes, A.O.P.; Zhang, Y.; Choque, G.A.H.; Reicks, M. Skin Carotenoid Scores Assessed with Reflection Spectroscopy Are Associated with Self-Reported Fruit and Vegetable Intake Among Latino Early Adolescents. J. Acad. Nutr. Diet. 2021, 121, 1507–1514. [Google Scholar] [CrossRef]

| Variables | Non-MetS Control Group (n = 63) | MetS Group (n = 77) | p-Value |
|---|---|---|---|
| Age (years) | 68.0 ± 8.4 | 69.6 ± 7.7 | 0.278 |
| Weight (kg) | 69.3 ± 12.8 | 80.4 ± 14.8 | <0.001 |
| Height (cm) | 164.8 ± 7.8 | 166.9 ± 10.4 | 0.346 |
| Body Mass Index (kg/m2) | 25.4 ± 3.9 | 28.8 ± 4.3 | <0.001 |
| Body Fat Percentage (%) | 31.0 ± 7.5 | 34.5 ± 8.7 | 0.026 |
| High-Sensitivity C-Reactive Protein, mg/L | 1.8 ± 2.2 | 2.0 ± 2.0 | 0.316 |
| Sex, men | 15 (23.8) | 38 (49.4) | 0.003 |
| Race, white | 58 (92.1) | 72 (93.5) | 0.754 |
| Education, college graduate | 43 (68.3) | 48 (62.3) | 0.482 |
| Coronary Artery Disease | 2 (3.2) | 17 (22.1) | <0.001 |
| Cerebrovascular Disease | 0 (0) | 5 (6.5) | 0.064 |
| Peripheral Artery Disease | 3 (4.8) | 11 (14.3) | 0.089 |
| Chronic Kidney Disease | 8 (12.7) | 8 (10.4) | 0.791 |
| Peripheral Neuropathy | 24 (38.1) | 38 (49.4) | 0.231 |
| Current or Past Smoking | 0 (0) | 2 (2.6) | 0.501 |
| Hypertension | 12 (19.0) | 45 (58.4) | <0.001 |
| Dyslipidemia | 38 (60.3) | 76 (98.7) | <0.001 |
| Diabetes | 0 (0) | 9 (11.7) | 0.004 |
| Obesity | 12 (19.0) | 24 (31.2) | 0.122 |
| Arthritis | 23 (36.5) | 32 (41.6) | 0.604 |
| Chronic Obstructive Pulmonary Disease | 2 (3.2) | 11 (14.3) | 0.038 |
| Variables | Non-MetS Control Group (n = 63) | MetS Group (n = 77) | Unadjusted p-Value | Adjusted p-Value b |
|---|---|---|---|---|
| Serum Alpha-Carotene, ng/mL | 136.7 ± 143.6 | 66.0 ± 79.1 | <0.001 | 0.001 |
| Serum Beta-Carotene, ng/mL | 491.2 ± 404.3 | 301.2 ± 371.9 | <0.001 | 0.022 |
| Serum Lycopene, ng/mL | 470.1 ± 227.5 | 467.9 ± 326.2 | 0.289 | 0.693 |
| Serum Lutein, ng/mL | 183.8 ± 132.7 | 175.0 ± 130.7 | 0.443 | 0.851 |
| Serum Cryptoxanthin, ng/mL | 213.8 ± 298.8 | 160.3 ± 166.2 | 0.217 | 0.313 |
| Total Serum Carotenoids, ng/mL | 1495.6 ± 837.1 | 1170.5 ± 767.0 | 0.002 | 0.045 |
| Skin Carotenoid Score c | 306.1 ± 76.5 | 317.3 ± 92.5 | 0.831 | 0.887 |
| Fruit and Vegetable Intake, servings/day | 5.1 ± 2.6 | 4.6 ± 1.8 | 0.678 | 0.420 |
| Multivariable Linear Regression Model | Estimate | 95% CI | Partial R2 (%) | p-Value |
|---|---|---|---|---|
| Intercept | 1.325 | (−1.00, 3.653) | 0.260 | |
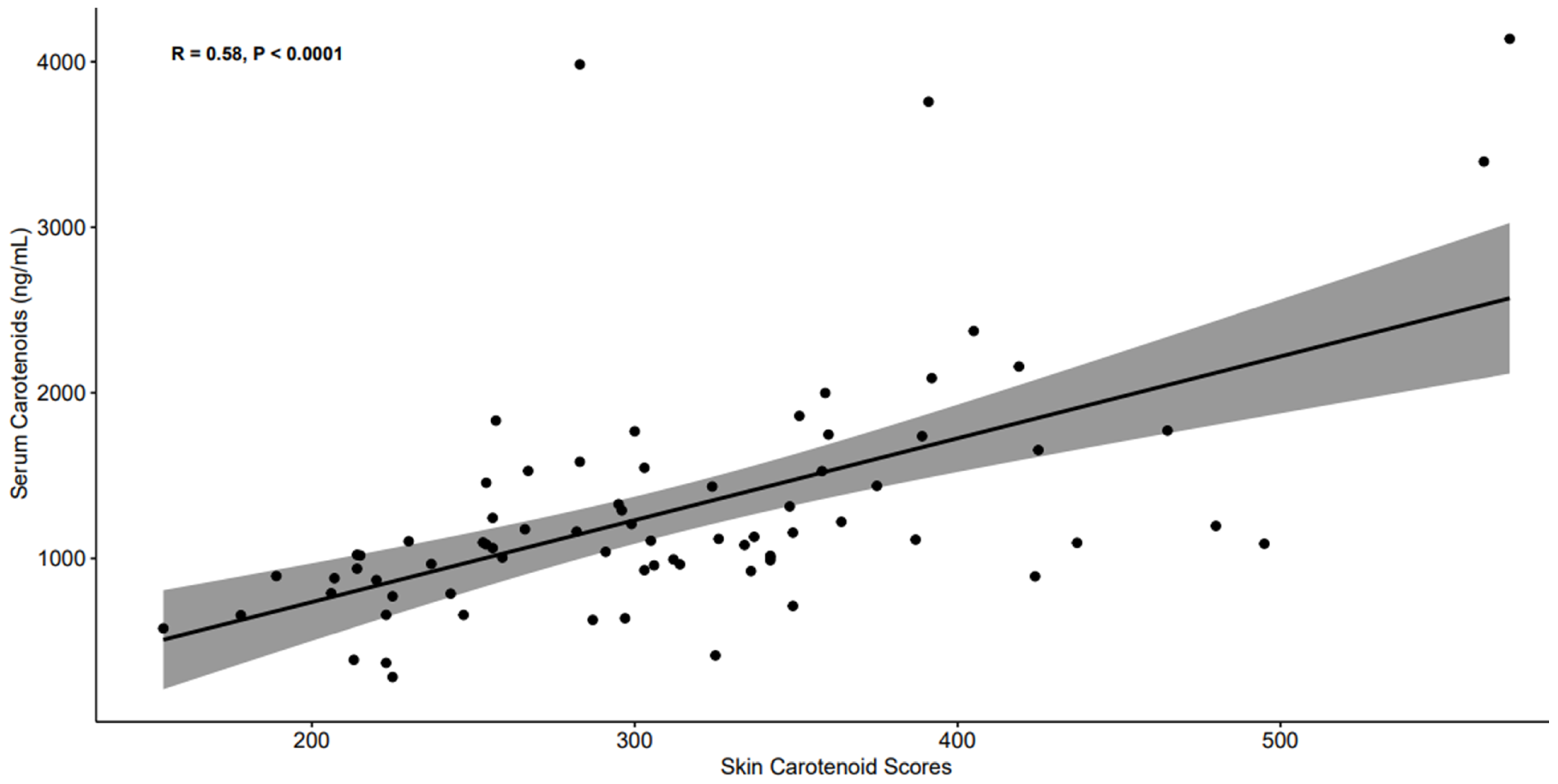
| Skin Carotenoid Score | 1.176 | (0.820, 1.531) | 40.5 | <0.001 |
| Metabolic Syndrome (MetS) | 0.024 | (−0.173, 0.220) | 0.09 | 0.809 |
| Age | −0.008 | (−0.021, 0.005) | 2.35 | 0.219 |
| Sex, men | −0.254 | (−0.458, −0.049) | 8.76 | 0.016 |
| Race, white | −0.291 | (−0.737, 0.155) | 2.59 | 0.197 |
| Education, college graduate | −0.053 | (−0.242, 0.136) | 0.49 | 0.575 |
| Coronary Artery Disease | −0.103 | (−0.367, 0.161) | 0.94 | 0.438 |
| Cerebrovascular Disease | 0.534 | (0.038, 1.009) | 6.76 | 0.028 |
| Peripheral Artery Disease | 0.073 | (−0.315, 0.462) | 0.22 | 0.709 |
| Chronic Obstructive Pulmonary Disease | −0.218 | (−0.553, 0.118) | 2.56 | 0.199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veldheer, S.; Sun, D.; Montgomery, P.S.; Wang, M.; Wu, X.; Liang, M.; George, S.; Gardner, A.W. Serum and Skin Carotenoid Levels in Older Adults with and Without Metabolic Syndrome: A Cross-Sectional Study. Nutrients 2025, 17, 3049. https://doi.org/10.3390/nu17193049
Veldheer S, Sun D, Montgomery PS, Wang M, Wu X, Liang M, George S, Gardner AW. Serum and Skin Carotenoid Levels in Older Adults with and Without Metabolic Syndrome: A Cross-Sectional Study. Nutrients. 2025; 17(19):3049. https://doi.org/10.3390/nu17193049
Chicago/Turabian StyleVeldheer, Susan, Dongxiao Sun, Polly S. Montgomery, Ming Wang, Xue Wu, Menglu Liang, Susan George, and Andrew W. Gardner. 2025. "Serum and Skin Carotenoid Levels in Older Adults with and Without Metabolic Syndrome: A Cross-Sectional Study" Nutrients 17, no. 19: 3049. https://doi.org/10.3390/nu17193049
APA StyleVeldheer, S., Sun, D., Montgomery, P. S., Wang, M., Wu, X., Liang, M., George, S., & Gardner, A. W. (2025). Serum and Skin Carotenoid Levels in Older Adults with and Without Metabolic Syndrome: A Cross-Sectional Study. Nutrients, 17(19), 3049. https://doi.org/10.3390/nu17193049





