Evaluating the Associations of Adiposity, Functional Status, and Anthropometric Measures with Nutritional Status in Chronic Hemodialysis Patients: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurements
2.3. Survey Questionnaires
2.3.1. Self-Designed Survey Questionnaire
2.3.2. Subjective Global Assessment
2.4. Anthropometric and Functional Measurements
2.4.1. Handgrip Strength Measured Using Dynamometer
2.4.2. Arm Circumference and Calf Circumference
2.4.3. Body Mass Index
2.4.4. Waist-to-Hip Ratio
2.5. Body Composition Measurements
2.6. Laboratory Tests and Adipose Tissue Distribution Indices
2.6.1. Laboratory Tests
2.6.2. Visceral Adiposity Index and Body Adiposity Index
2.7. Outcome Variables
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Patients (Table 1)
3.2. Assessment of Nutritional Status and Adiposity in the Studied Group (Table 2)
3.3. Determination of Relationship Between Nutritional Status Assessed Using Subjective Global Assessment and Indicators of Fat Tissue Distribution and Body Adiposity (Body Adiposity Index, Visceral Adiposity Index), as Well as Selected Parameters Obtained Through Bioelectrical Impedance Analysis in Groups of Studied Hemodialysis Patients (Table 3)
3.4. Diagnostic Performance of Clinical Parameters and Identification of Optimal Thresholds for Differentiating Malnutrition from Normal Nutritional Status (Table 4)
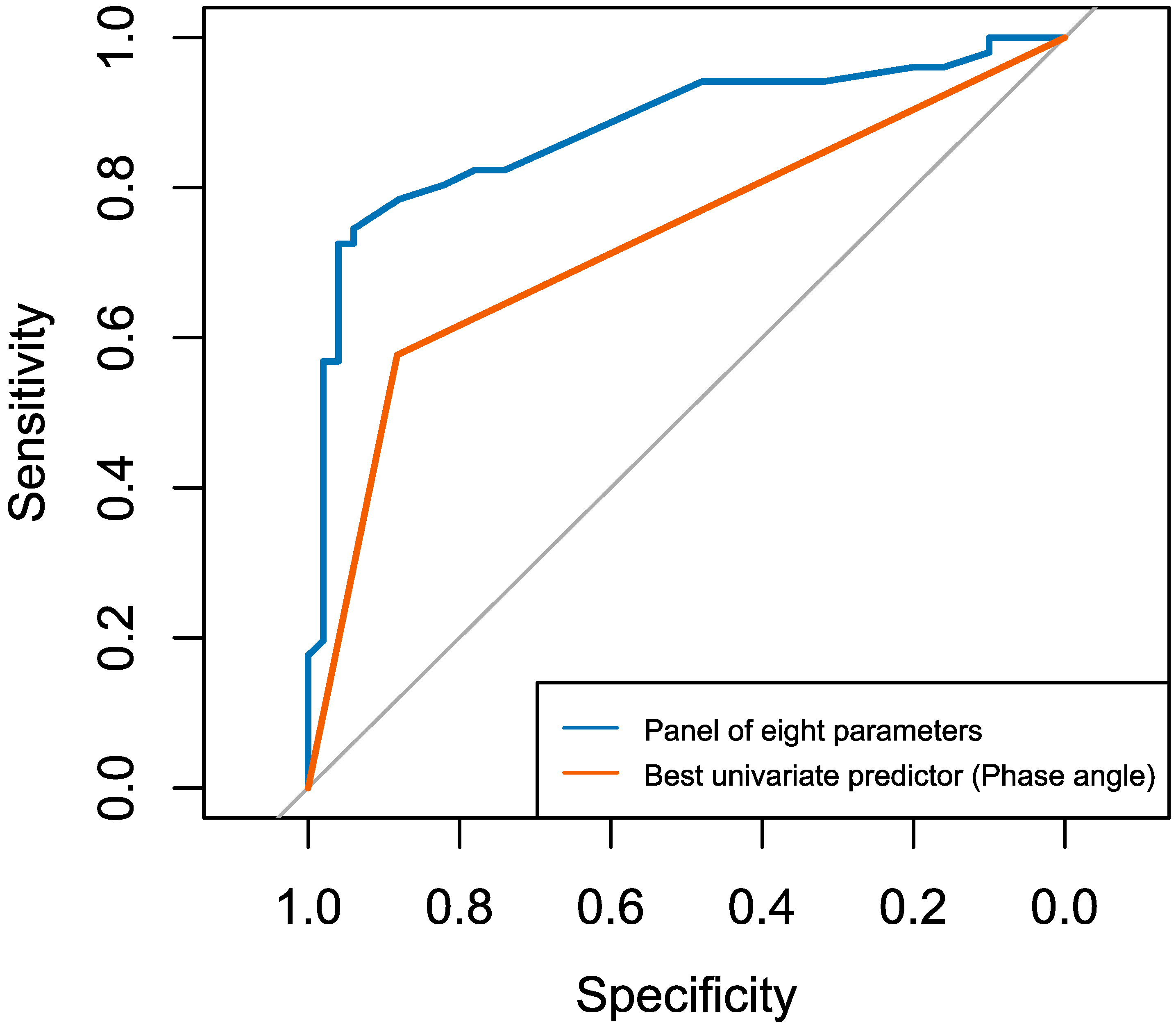
3.5. Comparative Logistic Regression Analysis: Multivariable Panel Model Versus Univariate Phase Angle Model for Predicting Malnutrition (Table 5)
3.6. Associations Between Functional, Anthropometric, and Bioelectrical Impedance Analysis-Derived Measures (Tables S1–S4)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Arm circumference |
| AIC | Akaike information criterion |
| arctan | Arcus tangent (trigonometric function) |
| AUC | Area under the receiver operating characteristic curve |
| BAI | Body adiposity index |
| BIA | Bioelectrical impedance analysis |
| BMI | Body mass index |
| C0 | Urea concentration before the hemodialysis session |
| Ct | Urea concentration after the hemodialysis session |
| CC | Calf circumference |
| CI | Confidence interval |
| CKD | Chronic kidney disease |
| COVID-19 | Coronavirus disease 2019 |
| d | Cohen’s d effect size |
| DEI | Daily energy intake |
| DPI | Daily protein intake |
| EBPG | European Best Practice Guidelines on Nutrition |
| ECW | Extracellular water |
| ECW/ICW | Extracellular to intracellular water (extracellular water/intracellular water) |
| ECW/TBW | Extracellular water/total body water |
| EWGSOP | European Working Group on Sarcopenia in Older People |
| GLIM | Global Leadership Initiative on Malnutrition |
| GNRI | Geriatric Nutritional Risk Index |
| HC | Hip circumference |
| HD | Hemodialysis |
| HDL | High-density lipoprotein |
| HGS | Handgrip strength |
| hs-CRP | High-sensitivity C-reactive protein |
| ICW | Intracellular water |
| IQR | Interquartile range |
| ISRNM | International Society of Renal Nutrition and Metabolism |
| K/DOQI | Kidney Disease Outcomes Quality Initiative |
| Kt/V | Dialysis adequacy index |
| LDL | Low-density lipoprotein |
| M | Arithmetic mean |
| MIFO | Malnutrition–inflammation–fluid overload |
| MIS | Malnutrition-Inflammation Score |
| MNA | Mini Nutritional Assessment |
| MAMC | Mid-arm muscle circumference |
| MBF | Mass of body fat (body fat mass) |
| Me | Median |
| MUNO | Metabolically unhealthy nonobese |
| n | Number of participants |
| nPCR | Normalized protein catabolic rate |
| NYHA | New York Heart Association |
| OR | Odds ratio |
| p | Probability testing |
| PA | Phase angle |
| PEM | Protein–energy malnutrition |
| PEW | Protein–energy wasting |
| R | Resistance (tissue electrical resistance) |
| ROC | Receiver operating characteristic |
| SD | Standard deviation |
| SGA | Subjective Global Assessment |
| SMM | Skeletal muscle mass |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| TG | Triglycerides |
| UFV | Ultrafiltration volume |
| URR | Urea reduction ratio |
| VAI | Visceral adiposity index |
| VIF | Variance inflation factor |
| WHR | Waist-to-hip ratio |
| Xc | Reactance |
| κ | Cohen’s kappa coefficient |
| ρ | Spearman’s rank correlation coefficient |
| τb | Kendall’s tau-b coefficient |
| χ2 | Chi-square test with Yates’ correction |
References
- Fagoonee, K.; Chinien, G.; Munnohur, N. Prevalence and risk factors of malnutrition among patients receiving hemodialysis in a tertiary level hospital in Mauritius. J. Int. Med. Res. 2024, 52, 1–9. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Małgorzewicz, S.; Ciechanowski, K.; Kozłowska, L.; Krzanowska, K.; Krzanowski, M.; Kaczkan, M.; Borek, P.; Jankowska, M.; Rutkowski, B.; Dębska-Ślizień, A. Nutrition recommendations in chronic kidney disease—The position of the working group of the Polish Nephrological Society. Forum Nefrol. 2019, 12, 240–278. [Google Scholar]
- Fouque, D.; Vennegoor, M.; ter Wee, P.; Wanner, C.; Basci, A.; Canaud, B.; Haage, P.; Konner, K.; Kooman, J.; Martin-Malo, A.; et al. EBPG Guideline on Nutrition. Nephrol. Dial. Transplant. 2007, 22, ii45–ii87. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cue-vas, A.; Fiaccadori, E.; et al. Global prevalence of protein-energy wasting in kidney disease: A meta-analysis of contemporary observational studies from the international society of renal nutrition and metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Cederholm, T.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Cuerda, C.; Cupisti, A.; Sabatino, A.; Schneider, S.; Tor-reggiani, M.; et al. Nutritional status and the risk of malnutrition in older adults with chronic kidney disease—Implications for low protein intake and nutritional care: A critical review endorsed by ERN-ERA and ESPEN. Clin. Nutr. 2023, 42, 443–457. [Google Scholar] [CrossRef]
- Lim, L.-M.; Kuo, H.-T.; Chao, Y.-L.; Shen, F.-C.; Chen, Y.-K.; Chiu, Y.-W.; Hwang, S.-J.; Hung, C.-C. Malnutrition–Inflammation Score of Patients with Chronic Kidney Disease from Early Stage to Initiation of Dialysis. Nutrients 2024, 16, 4014. [Google Scholar] [CrossRef]
- Da, J.; Long, Y.; Li, Q.; Yang, X.; Yuan, J.; Zha, Y. Resting metabolic rate and its adjustments as predictors of risk protein-energy wasting in hemodialysis patients. Biosci. Rep. 2021, 41, BSR20210010. [Google Scholar] [CrossRef]
- Bhasin, A.; Huang, L.; Shieh, M.-S.; Pekow, P.; Lindenauer, P.K.; Lagu, T. Malnutrition in hospitalized adults in the United States, 2016–2019. J. Hosp. Med. 2024, 19, 1113–1121. [Google Scholar] [CrossRef]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef]
- de van der Schueren, M.A.E.; Keller, H.; Cederholm, T.; Barazzoni, R.; Compher, C.; Correia, M.I.T.D.; Gonzalez, M.C.; Jager-Wittenaar, H.; Pirlich, M.; Steiber, A.; et al. Global Leadership Initiative on Malnutrition (GLIM): Guidance on validation of the operational criteria for the diagnosis of protein-energy malnutrition in adults. Clin. Nutr. 2020, 39, 2872–2880. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chung, C.-Y.; Wu, P.-Y.; Huang, J.-C.; Tsai, Y.-C.; Chen, S.-C.; Chiu, Y.-W.; Chang, J.-M. Associations between Metabolic Syndrome and Obesity-Related Indices and Bone Mineral Density T-Score in Hemodialysis Patients. J. Pers. Med. 2021, 11, 775. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, M.I.T.D.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Jeejeebhoy, K.N.; Keller, H.; Gramlich, L.; Allard, J.P.; Laporte, M.; Duerksen, D.R.; Payette, H.; Bernier, P.; Vesnaver, E.; Davidson, B.; et al. Nutritional assessment: Comparison of clinical assessment and objective variables for the prediction of length of hospital stay and readmission. Am. J. Clin. Nutr. 2015, 101, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Gilliland, J.; O’Connor, C.; Chesworth, B.; Madill, J. Is phase angle an appropriate indicator of malnutrition in different disease states? A systematic review. Clin. Nutr. ESPEN 2019, 29, 1–14. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Kyle, U.G.; Kondrup, J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: Phase angle and impedance ratio. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Xie, N.; Xiang, X.; Cao, T.; Xie, Y.; Liang, X.; Su, X. The value of the phase angle of bioelectrical impedance analysis to predict malnutrition in hemodialysis patients. Front. Nephrol. 2025, 5, 1478367. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Cochran, W.G. Chapter 4, The Estimation of Sample Size. In Sampling Techniques, 3rd ed.; Cochran, W.G., Ed.; Wiley: New York, NY, USA, 1977; pp. 72–88. [Google Scholar]
- Daugirdas, J.T. Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J. Am. Soc. Nephrol. 1993, 4, 1205–1213. [Google Scholar] [CrossRef]
- Lowrie, E.G.; Lew, N.L. The urea reduction ratio (URR): A simple method for evaluating hemodialysis treatment. Contemp. Dial. Nephrol. 1991, 12, 11–20. [Google Scholar]
- Steiber, A.; Leon, J.B.; Secker, D.; McCarthy, M.; McCann, L.; Serra, M.; Sehgal, A.R.; Kalantar-Zadeh, K. Multicenter study of the validity and reliability of subjective global assessment in the hemodialysis population. J. Ren. Nutr. 2007, 17, 336–342. [Google Scholar] [CrossRef]
- Chen, Z.-Q.; Luo, L.; Chen, X.-X.; Zhang, X.-Y.; Yin, S.-Q.; Xiao, G.-H.; Xu, N.; Liu, Q.; Su, C.-Y. Dietary nutrient intake and nutritional status in maintenance hemodialysis patients: A multicenter cross-sectional survey. Ren. Fail. 2024, 46, 2363589. [Google Scholar] [CrossRef]
- Silva, M.Z.C.; Antonio, K.J.; Reis, J.M.S.; Alves, L.S.; Caramori, J.C.T.; Vogt, B.P. Age, diabetes mellitus, and dialysis modality are associated with risk of poor muscle strength and physical function in hemodialysis and peritoneal dialysis patients. Kidney Res. Clin. Pract. 2021, 40, 294–303. [Google Scholar] [CrossRef]
- Martin-Alemañy, G.; Perez-Navarro, M.; Wilund, K.R.; García-Villalobos, G.; Gómez-Guerrero, I.; Cantú-Quintanilla, G.; Reyes-Caldelas, M.A.; Espinosa-Cuevas, A.; Escobedo, G.; Medeiros, M.; et al. Effect of Intradialytic Oral Nutritional Supplementation with or without Exercise Improves Muscle Mass Quality and Physical Function in Hemodialysis Patients: A Pilot Study. Nutrients 2022, 14, 2946. [Google Scholar] [CrossRef]
- Michou, V.; Davioti, M.; Syrakou, N.; Liakopoulos, V.; Deligiannis, A.; Kouidi, E. Effects of a Combined Intradialytic Exercise Training Program on Functional Capacity and Body Composition in Kidney Transplant Candidates. J. Funct. Morphol. Kinesiol. 2023, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Ben-Noach, D.; Levy, D.; Raz, M.; Anbar, R.; Schwartz, D.; Kliuk-Ben Bassat, O. Assessment of the Correlation Between Serum Phosphate Level and Muscle Strength as Measured by Handgrip Strength in Patients Treated with Hemodialysis. Can. J. Kidney Health Dis. 2024, 11, 1–9. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Nasir, K.; Sultan, S.; Qureshi, R.; Dhrolia, M.; Ahmad, A. Mini Nutritional Assessment (MNA) as a Reliable Tool for Nutritional Assessment of Hemodialysis Patients: A Single-Center Observation. Cureus 2022, 14, e21024. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, H.; Du, X.; Yin, L.; Zhang, H.; Zhou, O. Comparison of the prevalence and associated factors of cognitive frailty between elderly and middle-young patients receiving maintenance hemodialysis. Int. Urol. Nephrol. 2022, 54, 2703–2711. [Google Scholar] [CrossRef]
- Aygül, R.D.; Mercanligila, S.M.; Bardak Demirb, S. Evaluation of the relationship between dietary energy and protein intakes and anthropometric measurements in hemodialysis patients. Medicine (Baltimore) 2024, 103, e38307. [Google Scholar] [CrossRef] [PubMed]
- Pawlaczyk, W.; Rogowski, L.; Kowalska, J.; Stefańska, M.; Gołębiowski, T.; Mazanowska, O.; Gerall, C.; Krajewska, M.; Kusztal, M.; Dziubek, W. Assessment of the Nutritional Status and Quality of Life in Chronic Kidney Disease and Kidney Transplant Patients: A Comparative Analysis. Nutrients 2022, 14, 4814. [Google Scholar] [CrossRef]
- Costa de Oliveira, C.M.; Kubrusly, M.; Salani Mota, R.; da Silva, C.A.B.; Oliveira, V.N. Malnutrition in chronic kidney failure: What is the best diagnostic method to assess? J. Bras. Nefrol. 2010, 32, 55–68. [Google Scholar]
- Saitoh, M.; Ogawa, M.; Kondo, H.; Suga, K.; Takahashi, T.; Itoh, H.; Tabata, Y. Bioelectrical impedance analysis-derived phase angle as a determinant of protein-energy wasting and frailty in maintenance hemodialysis patients: Retrospective cohort study. BMC Nephrol 2020, 21, 438. [Google Scholar] [CrossRef]
- Eyre, S.; Stenberg, J.; Wallengren, O.; Keane, D.; Avesani, C.M.; Bosaeus, I.; Clyne, N.; Heimbürger, O.; Indurain, A.; Johansson, A.-C.; et al. Bioimpedance analysis in patients with chronic kidney disease. J. Ren. Care 2023, 49, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Czaja-Stolc, S.; Chatrenet, A.; Potrykus, M.; Ruszkowski, J.; Torreggiani, M.; Lichodziejewska-Niemierko, M.; Dębska-Ślizień, A.; Piccoli, G.B.; Małgorzewicz, S. Adipokines and Myokines as Markers of Malnutrition and Sarcopenia in Patients Receiving Kidney Replacement Therapy: An Observational, Cross-Sectional Study. Nutrients 2024, 16, 2480. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Pitrone, M.; Galluzzo, A. Cut-off points of the visceral adiposity index (VAI) identifying a visceral adipose dysfunction associated with cardiometabolic risk in a Caucasian Sicilian population. Lipids Health Dis. 2011, 10, 183. [Google Scholar] [CrossRef]
- Bergman, R.N.; Stefanovski, D.; Buchanan, T.A.; Sumner, A.E.; Reynolds, J.C.; Sebring, N.G.; Xiang, A.H.; Watanabe, R.M. A better index of body adiposity. Obesity (Silver Spring) 2011, 19, 1083–1089. [Google Scholar] [CrossRef]
- Takahashi, S.; Tanaka, T.; Suzuki, Y.; Yoshida, N.; Hitaka, M.; Ishii, S.; Yamazaki, K.; Masai, M.; Yamada, Y.; Ohashi, Y. Association Between Malnutrition, Low Muscle Mass, Elevated NT-ProBNP Levels, and Mortality in Hemodialysis Patients. Nutrients 2025, 17, 1896. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis—Clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- El Alami El Hassani, N.; Akrichi, M.-A.; Bajit, H.; Alem, C. Investigation of accordance between nutritional assessment tools, and bio-electrical impedance-derived phase angle, with the global leadership initiative on malnutrition criteria in hemodialysis patients. Clin. Nutr. ESPEN 2024, 62, 260–269. [Google Scholar] [CrossRef]
- Xu, Y.; Ling, S.; Liu, Z.; Luo, D.; Qi, A.; Zeng, Y. The ability of phase angle and body composition to predict risk of death in maintenance hemodialysis patients. Int. Urol. Nephrol. 2024, 56, 731–737. [Google Scholar] [CrossRef]
- Rimsevicius, L.; Gincaite, A.; Vicka, V.; Sukackiene, D.; Pavinic, J.; Miglinas, M. Malnutrition Assessment in Hemodialysis Patients: Role of Bioelectrical Impedance Analysis Phase Angle. J. Ren. Nutr. 2016, 26, 391–395. [Google Scholar] [CrossRef]
- Kim, M.; Park, Y.-W.; Im, D.W.; Jeong, Y.; Noh, H.J.; Yang, S.J.; Kang, E.; Ryu, H.; Kim, J.; Koo, J.-R.; et al. Association of Handgrip Strength and Nutritional Status in Non-Dialysis-Dependent Chronic Kidney Disease Patients: Results from the KNOW-CKD Study. Nutrients 2024, 16, 2442. [Google Scholar] [CrossRef]
- Sostisso, C.F.; Olikszechen, M.; Sato, M.N.; de Aguiar Souza Cruz Oliveira, M.; Karam, S. Handgrip strength as an instrument for assessing the risk of malnutrition and inflammation in hemodialysis patients. J. Bras. Nefrol. 2020, 42, 429–436. [Google Scholar] [CrossRef]
- Başcı, S.; Özkök, A.; Kaçar, M.; Bilgin, S.; Odabaş, A.R. Handgrip strength and serum uric acid levels are associated with dialysis-malnutrition scores in chronic hemodialysis patients. Turk. Neph. Dial. Transpl. 2017, 26, 323–332. [Google Scholar] [CrossRef]
- Saberi-Karimian, M.; Mansoori, A.; Mohammadi-Bajgiran, M.; Hosseini, Z.S.; Kiyoumarsioskouei, A.; Sadooghi Rad, E.; Mahmoudi Zo, M.; Khorasani, N.Y.; Poudineh, M.; Ghazizadeh, S. Data mining approaches for type 2 diabetes mellitus prediction using anthropometric measurements. J. Clin. Lab. Anal. 2023, 37, e24798. [Google Scholar] [CrossRef]
- Sultan, S.; Nasir, K.; Qureshi, R.; Dhrolia, M.; Ahmad, A. Assessment of the Nutritional Status of the Hemodialysis Patients by Anthropometric Measurements. Cureus 2021, 13, e18605. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Melo, D.; Miranda dos Santos, A.; Viana Hortegal Furtado, E.; Teixeira França, A.K.; Milhomem dos Santos, E.; Kruze Grande de Arruda, I.; Rodrigues de Carvalho, T.; Porto Sabino Pinho, C.; da Silva Diniz, A.; Chaves de Lemos, M.C. Visceral adiposity indicators and cardiovascular risk in hemodialytic patients. Arch. Endocrinol. Metab. 2021, 65, 811–820. [Google Scholar] [PubMed]
- de Geus, M.; Boersen, E.; Ipema, K.; Dam, M.; van Egmond, A.; den Boeft, A.; Kruizenga, H.; Visser, W. Malnutrition in hemodialysis patients? It depends on how you look at it. Clin. Nutr. ESPEN 2024, 63, 1276. [Google Scholar] [CrossRef]
- Zimmermann, S.; Mathew, A.; Schöppe, R.; Mangova, G.; Biemann, R.; Surov, A.; Meyer, H.-J.; Isermann, B. Fat tissue quantity, waist circumference or waist-to-hip ratio in patients with chronic kidney disease: A systematic review and meta-analysis. Obes. Res. Clin. Pract. 2024, 18, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Prelević, V.; Antunović, T.; Radunović, D.; Gligorović-Barhanović, N.; Gledović, B.; Ratković, M.; Bašić-Jukić, N. Malnutrition inflammation score (MIS) is stronger predictor of mortality in hemodialysis patients than waist-to-hip ratio (WHR)-4-year follow-up. Int. Urol. Nephrol. 2022, 54, 695–700. [Google Scholar] [CrossRef]
- Zhou, C.; Peng, Y.; Jiang, W.; Yuan, J.; Zha, Y. Comparison of novel visceral obesity indexes with traditional obesity measurements in predicting of metabolically unhealthy nonobese phenotype in hemodialysis patients. BMC Endocr. Disord. 2021, 21, 244. [Google Scholar] [CrossRef]
- Ruperto, M.; Barril, G. Clinical Significance of Nutritional Status, Inflammation, and Body Composition in Elderly Hemodialysis Patients—A Case–Control Study. Nutrients 2023, 15, 5036. [Google Scholar] [CrossRef]
- Marcelli, D.; Brand, K.; Ponce, P.; Milkowski, A.; Marelli, C.; Ok, E.; Merello Godino, J.-I.; Gurevich, K.; Jirka, T.; Rosenberger, J.; et al. Longitudinal Changes in Body Composition in Patients after Initiation of Hemodialysis Therapy: Results from an International Cohort. J. Ren. Nutr. 2016, 26, 72–80. [Google Scholar] [CrossRef]
- Hsiao, S.-M.; Tsai, Y.-C.; Chen, H.-M.; Lin, M.-Y.; Chiu, Y.-W.; Chen, T.-H.; Wang, S.-L.; Hsiao, P.-N.; Kung, L.-F.; Hwang, S.-J.; et al. Association of Fluid Status and Body Composition with Physical Function in Patients with Chronic Kidney Disease. PLoS ONE 2016, 11, e0165400. [Google Scholar] [CrossRef] [PubMed]
- Visser, W.J.; de Mik-van Egmond, A.M.E.; Timman, R.; Severs, D.; Hoorn, E.J. Risk Factors for Muscle Loss in Hemodialysis Patients with High Comorbidity. Nutrients 2020, 12, 2494. [Google Scholar] [CrossRef] [PubMed]

| Variable | Study Group Total n = 103 | SGA-(B+C) n = 52 (50.5%) | SGA-A n = 51 (49.5%) | SGA-(B+C) vs. SGA-A |
|---|---|---|---|---|
| M ± SD (95% CI) Me (IQR) | M ± SD (95% CI) Me (IQR) | M ± SD (95% CI) Me (IQR) | p-Value Cohen’s d | |
| Age [years] * | 61 ± 14 (59:64) 65 (51–71) | 64 ± 14 (61:68) 66 (58–75) | 58 ± 15 (54:63) 62 (50–70) | p = 0.044 d = 0.42 |
| Gender, n (%) | ||||
| Male | 64 (62.1%) | 27 (51.9%) | 37 (72.5%) | p = 0.031 |
| Female | 39 (37.9%) | 25 (48.1%) | 14 (27.5%) | |
| Education, n (%) | ||||
| Primary education | 11 (10.7%) | 8 (15.4%) | 3 (5.9%) | |
| Vocational education | 32 (31.1%) | 15 (28.8%) | 17 (33.3%) | |
| Secondary education | 37 (35.9%) | 19 (36.5%) | 18 (35.3%) | |
| Higher education | 23 (22.3%) | 10 (19.2%) | 13 (25.5%) | |
| Place of residence, n (%) | ||||
| Village | 12 (11.7%) | 8 (15.4%) | 4 (7.8%) | |
| Small town | 7 (6.8%) | 1 (1.9%) | 6 (11.8%) | |
| Medium-sized town | 13 (12.6%) | 6 (11.5%) | 7 (13.7%) | |
| Large city | 71 (68.9%) | 37 (71.2%) | 34 (66.7%) | |
| Marital status, n (%) | ||||
| Single | 17 (16.5%) | 8 (15.4%) | 9 (17.6%) | |
| Married | 59 (57.3%) | 28 (53.8%) | 31 (60.8%) | |
| Divorced | 9 (8.7%) | 3 (5.8%) | 6 (11.8%) | |
| Widow/Widower | 18 (17.5%) | 13 (25.0%) | 5 (9.8%) | |
| Social status, n (%) | ||||
| Unemployed | 6 (5.8%) | 2 (3.8%) | 4 (7.8%) | |
| Employee | 16 (15.5%) | 6 (11.5%) | 10 (19.6%) | |
| Retiree/Pensioner | 81 (78.6%) | 44 (84.6%) | 37 (72.5%) | |
| Vascular access, n (%) | ||||
| Natural arteriovenous fistula | 70 (68.0%) | 38 (73.1%) | 32 (62.7%) | |
| Permanent catheter | 33 (32.0%) | 14 (26.9%) | 19 (37.3%) | |
| Presence of residual diuresis, n (%) | 82 (79.6%) | 41 (78.8%) | 41 (80.4%) | |
| Hospitalization within the last year, n (%) | 67 (65.0%) | 33 (63.5%) | 34 (66.7%) | |
| Cause of hospitalization, n (%) ** | ||||
| COVID-19 | 7 (10.4%) | 3 (9.1%) | 4 (11.8%) | |
| Vascular access issues | 38 (56.7%) | 17 (51.5%) | 21 (61.8%) | |
| Cardiovascular diseases | 12 (17.9%) | 7 (21.2%) | 5 (14.7%) | |
| Others | 26 (38.8%) | 15 (45.5%) | 11 (32.4%) | |
| Cause of CKD/comorbidities, n (%) ** | ||||
| Diabetic kidney disease | 25 (24.3%) | 10 (19.2%) | 15 (29.4%) | |
| Hypertensive nephropathy | 52 (50.5%) | 24 (46.2%) | 28 (54.9%) | |
| Chronic glomerulonephritis | 25 (24.3%) | 15 (28.8%) | 10 (19.6%) | |
| Others | 76 (73.8%) | 35 (67.3%) | 41 (80.4%) | |
| Dialysis vintage [months] * | 65 ± 69 (51:79) 42 (15–82) | 71 ± 76 (50:92) 45 (13–103) | 59 ± 62 (41:76) 41 (19–78) | p = 0.826 d = 0.18 |
| Residual diuresis [mL] * | 616.5 ± 616.5 (496.0:737.0) 500.0 (100.0–1000.0) | 551.9 ± 593.3 (386.8:717.1) 500.0 (100.0–875.0) | 682.4 ± 638.3 (502.8:861.9) 500.0 (100.0–1000.0) | p = 0.335 d = −0.21 |
| UFV [L] | 2.2 ± 1.0 (2.0:2.4) 2.2 (1.3–3.1) | 2.1 ± 1.0 (1.9:2.4) 2.1 (1.4–2.9) | 2.2 ± 1.1 (1.9:2.5) 2.4 (1.1–3.2) | p = 0.954 d = −0.05 |
| Ideal body weight [kg] | 66.5 ± 10.6 (64.4:68.6) 67.5 (58.2–73.9) | 64.8 ± 11.7 (61.5:68.0) 64.0 (56.9–72.7) | 68.2 ± 9.2 (65.6:70.8) 69.0 (62.2–74.3) | p = 0.151 d = −0.33 |
| Height [cm] | 166.6 ± 10.4 (164.6:168.6) 167.5 (159.4–174.0) | 165.0 ± 11.3 (161.8:168.1) 164.0 (157.4–173.4) | 168.2 ± 9.2 (165.6:170.8) 169.0 (162.2–174.3) | p = 0.166 d = −0.32 |
| BMI [kg/m2] * | 27.1 ± 6.6 (25.8:28.4) 26.0 (22.7–30.4) | 25.6 ± 6.6 (23.8:27.5) 24.7 (21.0–29.1) | 28.6 ± 6.4 (26.8:30.4) 27.7 (24.1–32.7) | p = 0.012 d = −0.46 |
| % Overweight * | 18.8 ± 23.8 (14.2:23.5) 8.7 (0.0–28.7) | 15.6 ± 22.0 (9.5:21.8) 5.4 (0.0–22.6) | 22.1 ± 25.3 (15.0:29.2) 13.8 (2.3–33.6) | p = 0.083 d = −0.27 |
| BAI * | 27.7 ± 6.4 (26.5:29.0) 26.9 (23.2–31.8) | 27.4 ± 7.0 (25.5:29.4) 26.5 (23.1–31.6) | 28.0 ± 5.7 (26.4:29.6) 27.2 (23.2–31.9) | p = 0.617 d = −0.09 |
| VAI * | 2.97 ± 2.58 (2.45:3.49) 2.11 (1.25–3.91) | 2.70 ± 2.20 (2.07:3.32) 2.03 (1.05–3.71) | 3.25 ± 2.93 (2.40:4.10) 2.17 (1.26–4.25) | p = 0.249 d = −0.21 |
| WHR | 0.99 ± 0.11 (0.96:1.01) 0.97 (0.89–1.06) | 0.98 ± 0.11 (0.95:1.01) 0.95 (0.89–1.07) | 0.99 ± 0.11 (0.96:1.02) 1.00 (0.92–1.06) | p = 0.753 d = −0.09 |
| Waist circumference [cm] * | 96.7 ± 17.9 (93.2:100.2) 93.5 (82.5–110.0) | 94.1 ± 18.3 (89.0:99.2) 91.0 (80.0–110.0) | 99.4 ± 17.2 (94.5:104.2) 99.0 (86.0–111.0) | p = 0.113 d = −0.30 |
| HC [cm] * | 97.7 ± 12.0 (95.4:100.1) 97.0 (91.5–103.0) | 95.6 ± 13.1 (91.9:99.2) 94.0 (89.0–100.5) | 99.9 ± 10.3 (97.0:102.8) 97.0 (94.0–104.0) | p = 0.013 d = −0.37 |
| AC [cm] * | 28.9 ± 4.4 (28.1:29.8) 29.0 (26.0–31.0) | 27.4 ± 4.2 (26.2:28.6) 28.0 (23.8–30.0) | 30.5 ± 4.1 (29.3:31.6) 30.0 (28.0–33.0) | p = 0.001 d = −0.74 |
| CC [cm] * | 35.8 ± 4.5 (35.0:36.7) 36.0 (33.5–38.5) | 34.8 ± 4.6 (33.5:36.1) 35.3 (32.0–37.0) | 36.9 ± 4.2 (35.7:38.1) 36.5 (35.0–40.0) | p = 0.003 d = −0.48 |
| Wrist circumference [cm] * | 18.5 ± 3.2 (17.8:19.1) 18.0 (17.0–19.5) | 18.0 ± 1.9 (17.4:18.5) 18.0 (16.3–19.5) | 19.0 ± 4.1 (17.8:20.1) 18.5 (17.5–19.5) | p = 0.178 d = −0.32 |
| HGS [kg] * | 28.6 ± 11.9 (26.3:31.0) 27.3 (19.4–36.1) | 25.1 ± 13.1 (21.4:28.8) 20.9 (15.4–32.8) | 32.3 ± 9.4 (29.6:34.9) 33.2 (24.8–39.8) | p < 0.001 d = −0.63 |
| URR (%) * | 74.6 ± 6.1 (73.4:75.8) 75.2 (70.6–78.1) | 75.6 ± 6.9 (73.7:77.5) 76.8 (71.6–80.4) | 73.6 ± 5.0 (72.2:75.1) 74.3 (70.0–77.1) | p = 0.036 d = 0.32 |
| Pre-dialysis urea concentration [mmol/L] * | 19.3 ± 5.6 (18.2:20.4) 18.7 (16.0–22.0) | 18.3 ± 4.8 (17.0:19.7) 18.5 (15.7–20.9) | 20.3 ± 6.1 (18.6:22.0) 20.2 (16.4–22.6) | p = 0.067 d = −0.37 |
| Post-dialysis urea concentration [mmol/L] * | 5.0 ± 2.2 (4.6:5.4) 4.7 (3.6–6.1) | 4.7 ± 2.5 (4.0:5.3) 4.1 (3.4–5.4) | 5.4 ± 1.9 (4.8:5.9) 5.1 (4.3–6.6) | p = 0.007 d = −0.33 |
| Post-dialysis body weight [kg] * | 75.3 ± 20.2 (71.3:79.2) 74.9 (64.3–85.4) | 70.0 ± 20.4 (64.3:75.6) 69.6 (58.3–78.6) | 80.7 ± 18.7 (75.5:86.0) 78.4 (67.4–86.8) | p = 0.002 d = −0.55 |
| Post-dialysis protein [kg] | 9.7 ± 2.4 (9.3:10.2) 9.5 (8.1–11.1) | 9.0 ± 2.3 (8.3:9.6) 8.6 (7.1–10.7) | 10.5 ± 2.2 (9.9:11.2) 10.5 (8.7–12.2) | p < 0.001 d = −0.71 |
| Post-dialysis SMM [kg] | 27.3 ± 7.1 (25.9:28.7) 26.6 (22.5–31.5) | 24.8 ± 6.8 (22.9:26.7) 23.6 (19.2–29.8) | 29.8 ± 6.6 (27.9:31.7) 29.4 (24.3–34.9) | p < 0.001 d = −0.74 |
| Post-dialysis lean body mass [kg] | 50.6 ± 11.6 (48.3:52.8) 49.0 (41.4–57.6) | 47.2 ± 11.3 (44.0:50.3) 45.5 (37.7–55.6) | 54.0 ± 11.0 (50.9:57.1) 53.3 (45.2–63.0) | p = 0.005 d = −0.61 |
| Post-dialysis MBF [kg] * | 24.9 ± 14.7 (22.1:27.8) 22.4 (13.4–33.9) | 23.2 ± 14.7 (19.1:27.3) 22.1 (12.1–30.9) | 26.7 ± 14.7 (22.6:30.9) 23.3 (15.2–38.1) | p = 0.164 d = −0.24 |
| Post-dialysis fat tissue content (%) | 31.5 ± 12.7 (29.0:33.9) 31.8 (21.0–42.0) | 31.1 ± 13.1 (27.4:34.8) 30.7 (21.9–41.8) | 31.8 ± 12.4 (28.3:35.3) 31.9 (20.5–42.8) | p = 0.820 d = −0.05 |
| Post-dialysis extracellular water index | 0.391 ± 0.016 (0.388:0.394) 0.391 (0.379–0.403) | 0.398 ± 0.014 (0.394:0.402) 0.400 (0.388–0.407) | 0.384 ± 0.014 (0.380:0.388) 0.386 (0.373–0.395) | p < 0.001 d = 1.05 |
| Post-dialysis extracellular water [L] | 14.4 ± 3.3 (13.8:15.1) 14.4 (11.8–16.6) | 13.7 ± 3.4 (12.8:14.6) 13.7 (11.1–16.6) | 15.1 ± 3.0 (14.3:16.0) 14.9 (12.8–17.2) | p = 0.039 d = −0.45 |
| Post-dialysis intracellular water [L] | 22.5 ± 5.5 (21.5:23.6) 22.0 (18.8–25.7) | 20.7 ± 5.3 (19.2:22.2) 19.8 (16.3–24.7) | 24.4 ± 5.0 (23.0:25.8) 24.1 (20.2–28.3) | p < 0.001 d = −0.71 |
| Post-dialysis ECW/ICW ratio | 0.643 ± 0.043 (0.635:0.652) 0.641 (0.611–0.672) | 0.663 ± 0.039 (0.652:0.674) 0.665 (0.635–0.687) | 0.623 ± 0.037 (0.613:0.634) 0.628 (0.594–0.652) | p < 0.001 d = 1.05 |
| Post-dialysis PA [°] | 5.65 ± 1.38 (5.38:5.91) 5.60 (4.70–6.60) | 4.97 ± 1.17 (4.65:5.30) 5.00 (4.20–5.75) | 6.33 ± 1.23 (5.98:6.68) 6.10 (5.50–7.00) | p < 0.001 d = −1.13 |
| Creatinine [mg/dL] * | 8.89 ± 2.56 (8.38:9.39) 9.00 (7.20–10.50) | 7.94 ± 2.15 (7.33:8.55) 8.03 (6.26–9.40) | 9.81 ± 2.62 (9.07:10.55) 9.85 (8.46–11.70) | p < 0.001 d = −0.78 |
| Albumin [g/L] * | 38.5 ± 4.0 (37.7:39.2) 39.0 (37.0–41.0) | 37.3 ± 4.6 (36.0:38.6) 37.6 (35.8–40.0) | 39.6 ± 2.8 (38.8:40.4) 40.0 (38.0–41.6) | p = 0.001 d = −0.60 |
| Total protein [g/L] | 65.6 ± 4.6 (64.7:66.6) 65.8 (63.1–68.4) | 65.5 ± 4.6 (64.2:66.8) 65.2 (63.0–68.6) | 65.8 ± 4.6 (64.5:67.2) 65.8 (63.2–67.9) | p = 0.692 d = −0.08 |
| Transferrin [g/L] * | 1.74 ± 0.33 (1.67:1.80) 1.74 (1.49–1.92) | 1.69 ± 0.33 (1.59:1.78) 1.68 (1.45–1.91) | 1.79 ± 0.32 (1.69:1.88) 1.75 (1.55–1.93) | p = 0.161 d = −0.31 |
| Total cholesterol [mmol/L] * | 4.32 ± 1.24 (4.07:4.57) 3.99 (3.46–5.15) | 4.27 ± 1.21 (3.93:4.61) 3.86 (3.47–4.91) | 4.38 ± 1.27 (4.01:4.75) 4.07 (3.45–5.18) | p = 0.459 d = −0.09 |
| HDL [mmol/L] * | 1.11 ± 0.47 (1.02:1.20) 1.01 (0.82–1.31) | 1.12 ± 0.47 (0.99:1.26) 1.03 (0.82–1.31) | 1.10 ± 0.46 (0.97:1.23) 0.98 (0.82–1.29) | p = 0.583 d = 0.05 |
| LDL [mmol/L] * | 2.60 ± 1.04 (2.39:2.81) 2.40 (1.86–3.25) | 2.60 ± 1.07 (2.30:2.90) 2.40 (1.85–3.18) | 2.60 ± 1.02 (2.30:2.90) 2.39 (1.86–3.26) | p = 0.941 d = 0.00 |
| TG [mmol/L] * | 1.65 ± 1.06 (1.43:1.86) 1.41 (0.85–1.98) | 1.48 ± 0.89 (1.23:1.73) 1.37 (0.82–1.79) | 1.82 ± 1.20 (1.47:2.17) 1.50 (0.94–2.50) | p = 0.193 d = −0.32 |
| hs-CRP [mg/L] * | 8.8 ± 14.3 (6.0:11.7) 4.3 (1.4–9.2) | 10.7 ± 18.1 (5.6:15.8) 4.4 (2.2–9.3) | 6.9 ± 8.3 (4.5:9.3) 4.2 (1.2–9.1) | p = 0.412 d = 0.27 |
| nPCR [g/kg/day] | 1.02 ± 0.23 (0.98:1.07) 1.02 (0.87–1.16) | 1.00 ± 0.19 (0.95:1.05) 1.02 (0.85–1.14) | 1.05 ± 0.25 (0.98:1.12) 1.03 (0.89–1.20) | p = 0.406 d = −0.22 |
| Variable | SGA-(B+C), n (%) | SGA-A, n (%) | χ2; df = 1; p-Value; Kendall’s τb; Cohen’s κ | |
|---|---|---|---|---|
| n = 52 (50.5%) | n = 51 (49.5%) | |||
| HGS † | ≤Cutoff point, n = 33 (32.0%) | 22 (66.7%) | 11 (33.3%) | χ2 = 4.18; p = 0.041; τb = 0.22; κ = 0.207 |
| >Cutoff point, n = 70 (68.0%) | 30 (42.9%) | 40 (57.1%) | ||
| BMI | <23 kg/m2, n = 28 (27.2%) | 19 (67.9%) | 9 (32.1%) | χ2 = 3.74; p = 0.053; τb = 0.21; κ = 0.188 |
| ≥23 kg/m2, n = 75 (72.8%) | 33 (44.0%) | 42 (56.0%) | ||
| WHR ‡ | Visceral fat tissue within normal range, n = 26 (25.2%) | 10 (38.5%) | 16 (61.5%) | χ2 = 1.42; p = 0.233; τb = −0.14; κ = −0.121 |
| Visceral obesity, n = 77 (74.8%) | 42 (54.5%) | 35 (45.5%) | ||
| AC | ≤22 cm, n = 8 (7.8%) | 8 (100.0%) | 0 (0.0%) | χ2 = 6.49; p = 0.011; τb = 0.29; κ = 0.153 |
| >22 cm, n = 95 (92.2%) | 44 (46.3%) | 51 (53.7%) | ||
| CC | ≤31 cm, n = 12 (11.7%) | 10 (83.3%) | 2 (16.7%) | χ2 = 4.47; p = 0.035; τb = 0.24; κ = 0.152 |
| >31 cm, n = 91 (88.4%) | 42 (46.2%) | 49 (53.8%) | ||
| Post-dialysis fat tissue content | <10%, n = 6 (5.8%) | 4 (66.7%) | 2 (33.3%) | χ2 = 0.16; p = 0.692; τb = 0.08; κ = 0.037 |
| ≥10%, n = 97 (94.2%) | 48 (49.5%) | 49 (50.5%) | ||
| Post-dialysis PA | ≤5°, n = 33 (32.0%) | 27 (81.8%) | 6 (18.2%) | χ2 = 17.27; p < 0.001; τb = 0.43; κ = 0.400 |
| >5°, n = 70 (68.0%) | 25 (35.7%) | 45 (64.3%) | ||
| Variable | n = 103 | SGA and Variable | |
|---|---|---|---|
| M ± SD (95% CI) | ρ | p | |
| BAI | 27.7 ± 6.4 (26.5:29.0) | −0.08 | 0.418 |
| VAI | 2.97 ± 2.58 (2.45:3.49) | −0.14 | 0.158 |
| AC [cm] | 28.9 ± 4.4 (28.1:29.8) | −0.38 | <0.001 |
| CC [cm] | 35.8 ± 4.5 (35.0:36.7) | −0.36 | <0.001 |
| HGS [kg] | 28.6 ± 11.9 (26.3:31.0) | −0.34 | <0.001 |
| Post-dialysis body weight [kg] | 75.3 ± 20.2 (71.3:79.2) | −0.38 | <0.001 |
| Post-dialysis SMM [kg] | 27.3 ± 7.1 (25.9:28.7) | −0.39 | <0.001 |
| Post-dialysis lean body mass [kg] | 50.6 ± 11.6 (48.3:52.8) | −0.32 | 0.001 |
| Post-dialysis MBF [kg] | 24.9 ± 14.7 (22.1:27.8) | −0.21 | 0.037 |
| Post-dialysis fat tissue content (%) | 31.5 ± 12.7 (29.0:33.9) | −0.08 | 0.448 |
| Post-dialysis extracellular water index | 0.391 ± 0.016 (0.388:0.394) | 0.39 | <0.001 |
| Post-dialysis extracellular water [L] | 14.4 ± 3.3 (13.8:15.1) | −0.26 | 0.007 |
| Post-dialysis intracellular water [L] | 22.5 ± 5.5 (21.5:23.6) | −0.37 | <0.001 |
| Post-dialysis ECW/ICW ratio | 0.643 ± 0.043 (0.635:0.652) | 0.39 | <0.001 |
| Post-dialysis PA [°] | 5.65 ± 1.38 (5.38:5.91) | −0.46 | <0.001 |
| Albumin [g/L] | 38.5 ± 4.0 (37.7:39.2) | −0.32 | 0.004 |
| Parameter | Optimal Threshold | Youden’s Index | Accuracy | Sensitivity | Specificity | AUC | 95% CI | Prevalence |
|---|---|---|---|---|---|---|---|---|
| BMI | ≤23.58 kg/m2 | 0.27 | 0.63 | 0.44 | 0.82 | 0.64 | 0.49:0.78 | 0.50 |
| BAI * | ≤25.34 | 0.08 | 0.54 | 0.39 | 0.69 | 0.53 | 0.38:0.67 | 0.50 |
| VAI | ≤2.65 | 0.19 | 0.59 | 0.56 | 0.63 | 0.57 | 0.41:0.72 | 0.51 |
| Post-dialysis ECW/ICW ratio * | ≥0.66 | 0.40 | 0.70 | 0.54 | 0.86 | 0.77 | 0.63:0.88 | 0.50 |
| Post-dialysis PA * | ≤5.1° | 0.46 | 0.73 | 0.58 | 0.88 | 0.79 | 0.66:0.89 | 0.50 |
| Post-dialysis SMM | ≤21.0 kg | 0.25 | 0.62 | 0.40 | 0.84 | 0.60 | 0.44:0.75 | 0.50 |
| Post-dialysis lean body mass | ≤39.8 kg | 0.31 | 0.65 | 0.37 | 0.94 | 0.66 | 0.51:0.80 | 0.50 |
| Post-dialysis MBF | ≤35.3 kg | 0.20 | 0.60 | 0.88 | 0.31 | 0.58 | 0.42:0.72 | 0.50 |
| Albumin | ≤37.3 g/L | 0.30 | 0.65 | 0.50 | 0.80 | 0.69 | 0.53:0.82 | 0.50 |
| nPCR | ≤1.19 g/kg/day | 0.16 | 0.58 | 0.88 | 0.27 | 0.54 | 0.38:0.69 | 0.50 |
| AC | ≤25.5 cm | 0.29 | 0.64 | 0.35 | 0.94 | 0.68 | 0.53:0.82 | 0.50 |
| CC | ≤37.5 cm | 0.30 | 0.65 | 0.87 | 0.43 | 0.68 | 0.52:0.81 | 0.50 |
| HGS | ≤23.3 kg | 0.43 | 0.71 | 0.59 | 0.84 | 0.71 | 0.58:0.85 | 0.50 |
| WHR | ≤0.95 | 0.19 | 0.59 | 0.56 | 0.63 | 0.54 | 0.38:0.69 | 0.50 |
| Gender ** | — | — | 0.60 | 0.48 | 0.73 | 0.60 | 0.51:0.70 | 0.50 |
| Predictor | Multivariable Model | Univariate Model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| (Intercept) | 0.01 | 0.00–0.12 | <0.001 | 0.49 | 0.29–0.80 | 0.006 |
| PA [≤5.1° vs. >5.1°] | — | — | — | 10.23 | 3.93–30.61 | <0.001 |
| Gender [Male vs. Female] | 5.48 | 0.90–33.48 | 0.061 | — | — | — |
| Post-dialysis ECW/ICW ratio [≥0.66 vs. <0.66] | 2.41 | 0.77–7.52 | 0.142 | — | — | — |
| Post-dialysis lean body mass [≤39.8 kg vs. >39.8 kg] | 6.65 | 1.20–36.89 | 0.029 | — | — | — |
| Post-dialysis MBF [≤35.3 kg vs. >35.3 kg] | 2.93 | 0.80–10.77 | 0.099 | — | — | — |
| Albumin [≤37.3 g/L vs. >37.3 g/L] | 2.49 | 0.85–7.29 | 0.108 | — | — | — |
| nPCR [≤1.19 g/kg/day vs. >1.19 g/kg/day] | 2.47 | 0.68–8.98 | 0.173 | — | — | — |
| AC [≤25.5 cm vs. >25.5 cm] | 5.18 | 0.87–30.89 | 0.064 | — | — | — |
| HGS [≤23.3 kg vs. >23.3 kg] | 7.54 | 1.50–37.90 | 0.011 | — | — | — |
| Observations | 103 | 103 | ||||
| Pseudo-R2 | 0.460 | 0.232 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreew-Gamza, M.; Hornik, B. Evaluating the Associations of Adiposity, Functional Status, and Anthropometric Measures with Nutritional Status in Chronic Hemodialysis Patients: A Cross-Sectional Study. Nutrients 2025, 17, 3034. https://doi.org/10.3390/nu17193034
Andreew-Gamza M, Hornik B. Evaluating the Associations of Adiposity, Functional Status, and Anthropometric Measures with Nutritional Status in Chronic Hemodialysis Patients: A Cross-Sectional Study. Nutrients. 2025; 17(19):3034. https://doi.org/10.3390/nu17193034
Chicago/Turabian StyleAndreew-Gamza, Martyna, and Beata Hornik. 2025. "Evaluating the Associations of Adiposity, Functional Status, and Anthropometric Measures with Nutritional Status in Chronic Hemodialysis Patients: A Cross-Sectional Study" Nutrients 17, no. 19: 3034. https://doi.org/10.3390/nu17193034
APA StyleAndreew-Gamza, M., & Hornik, B. (2025). Evaluating the Associations of Adiposity, Functional Status, and Anthropometric Measures with Nutritional Status in Chronic Hemodialysis Patients: A Cross-Sectional Study. Nutrients, 17(19), 3034. https://doi.org/10.3390/nu17193034






